Three-Stage Anaerobic Sequencing Batch Reactor (ASBR) for Maximum Methane Production: Effects of COD Loading Rate and Reactor Volumetric Ratio
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Sludge Preparation
2.2. Substrate Preparation
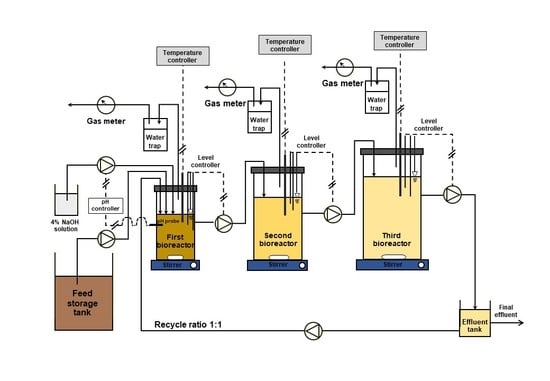
2.3. ASBR Setup and Operation
2.4. Measurements and Analytical Methods
2.5. Calculation
3. Results and Discussion
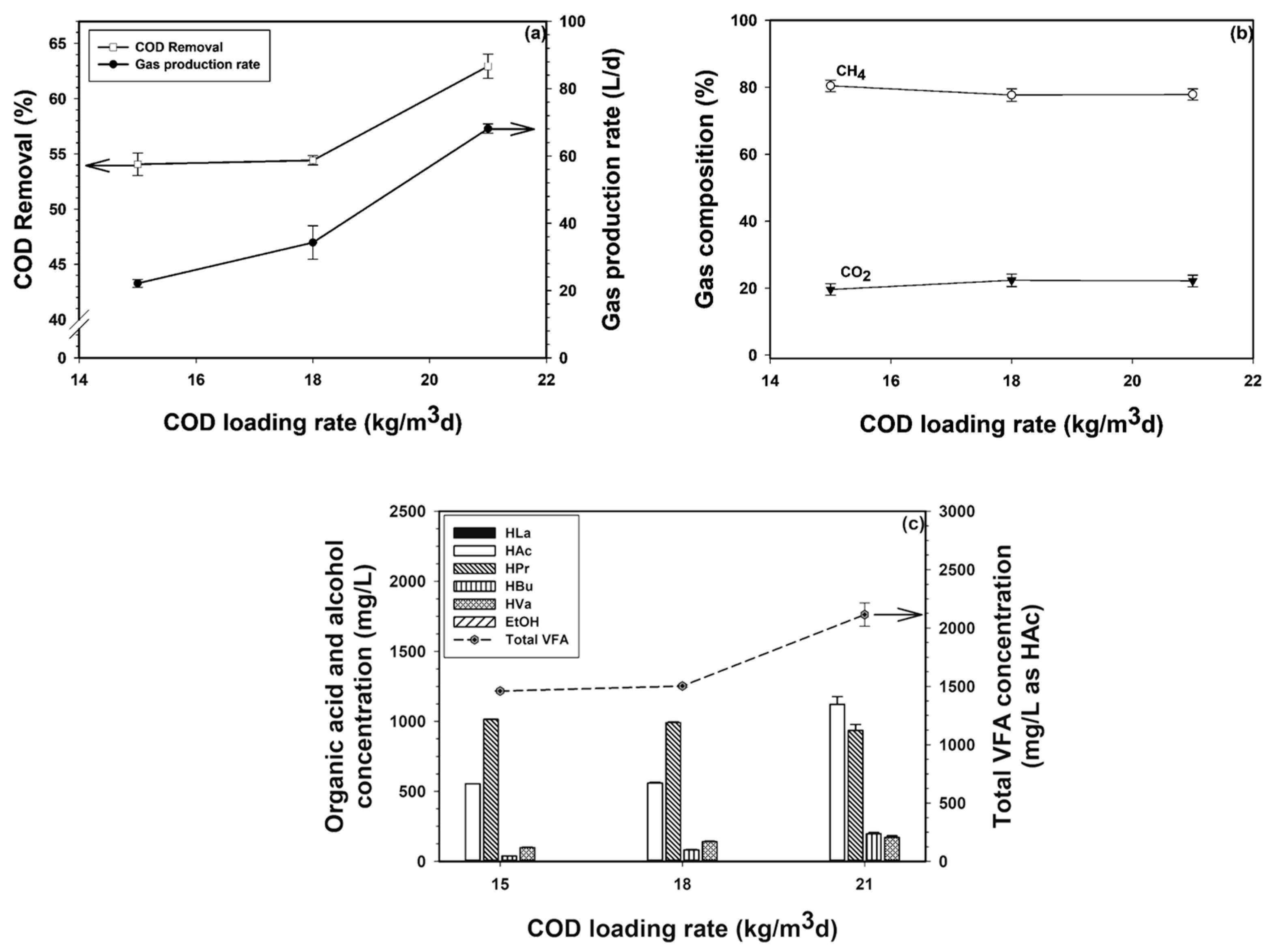
3.1. Part I: Effect of COD Loading Rate
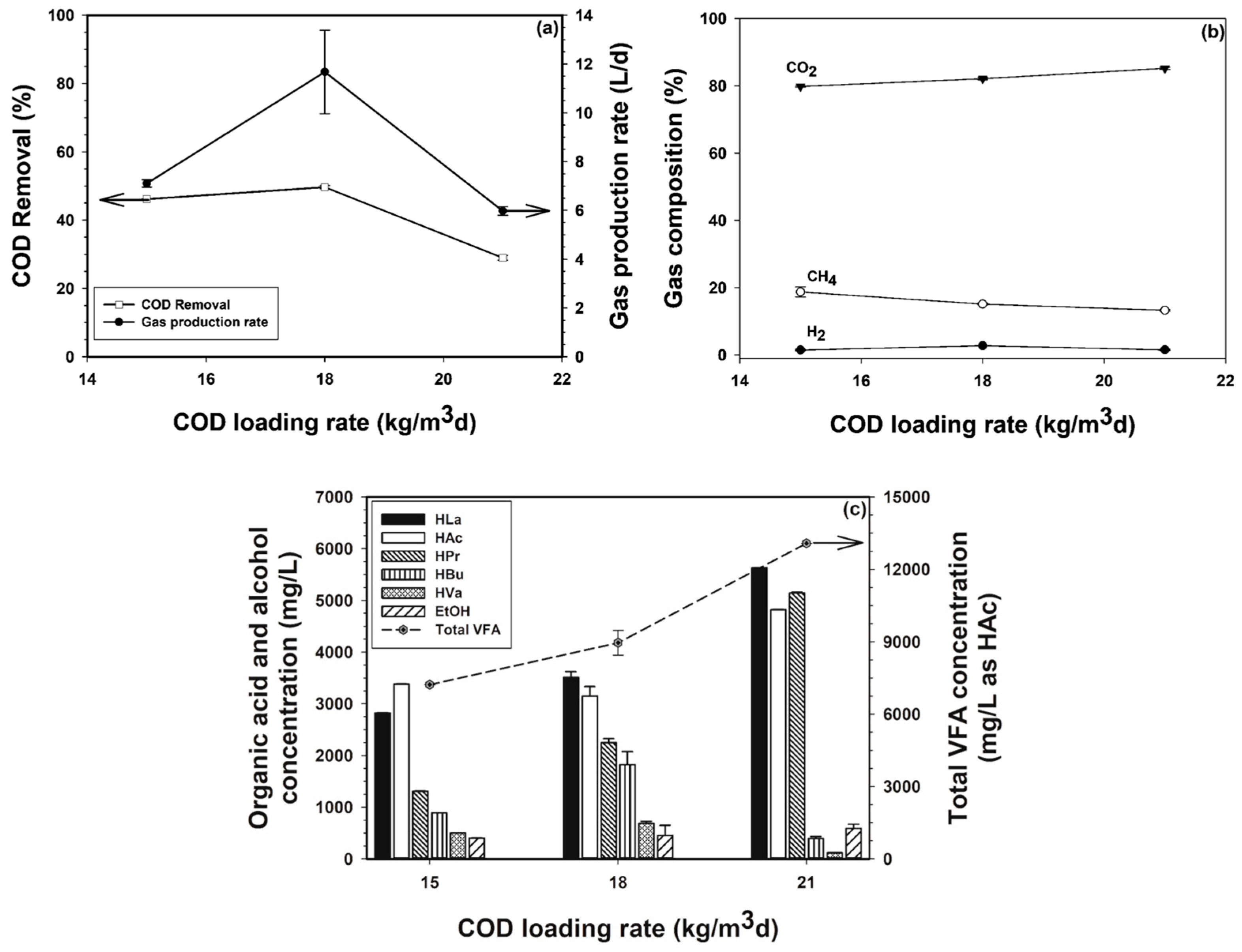
3.1.1. Process Performance of the First Bioreactor
3.1.2. Process Performance of the Second Bioreactor
3.1.3. Process Performance of the Third Bioreactor
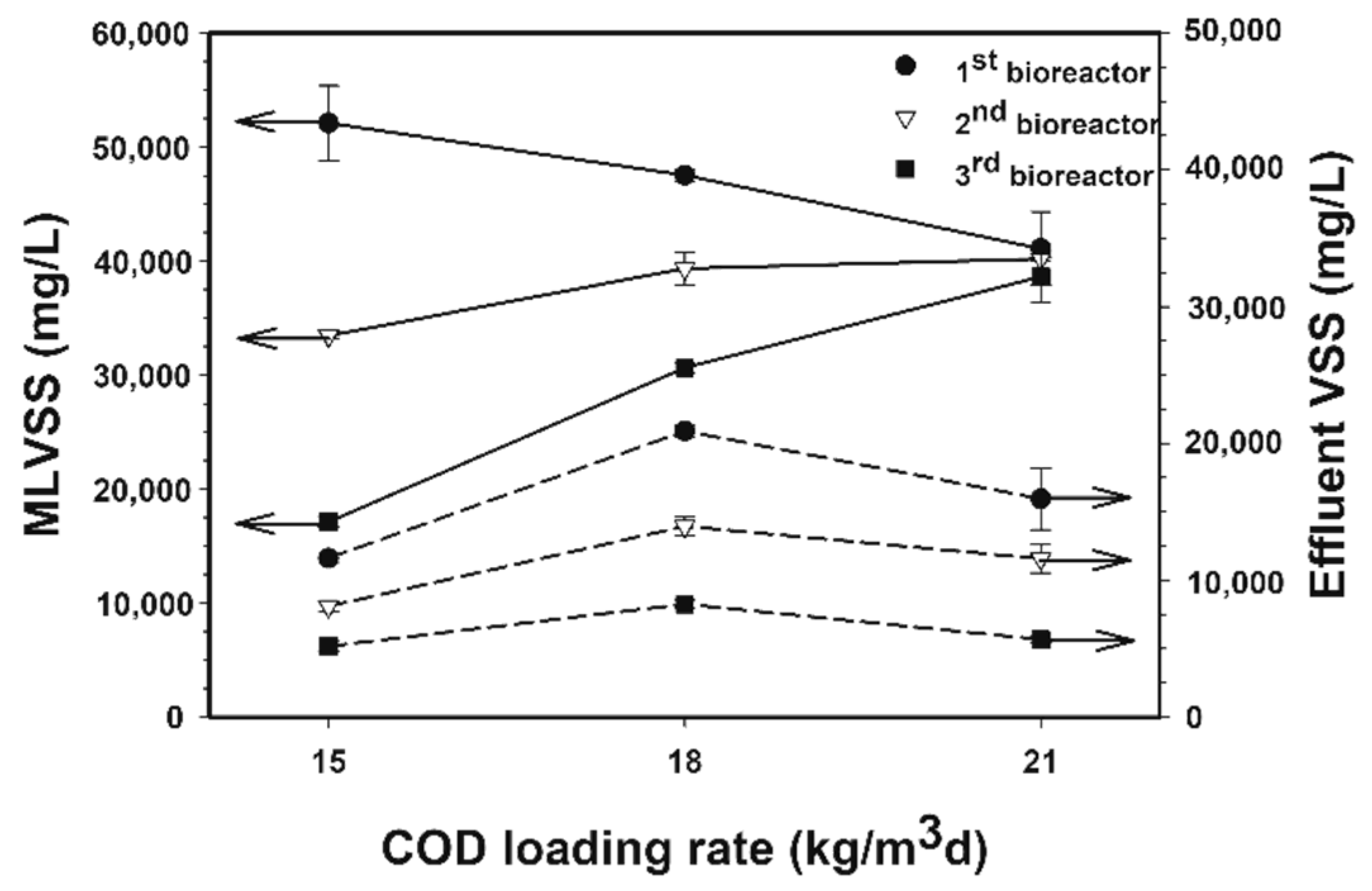
3.1.4. Microbial Concentrations and Microbial Washout Levels
3.1.5. Micronutrients
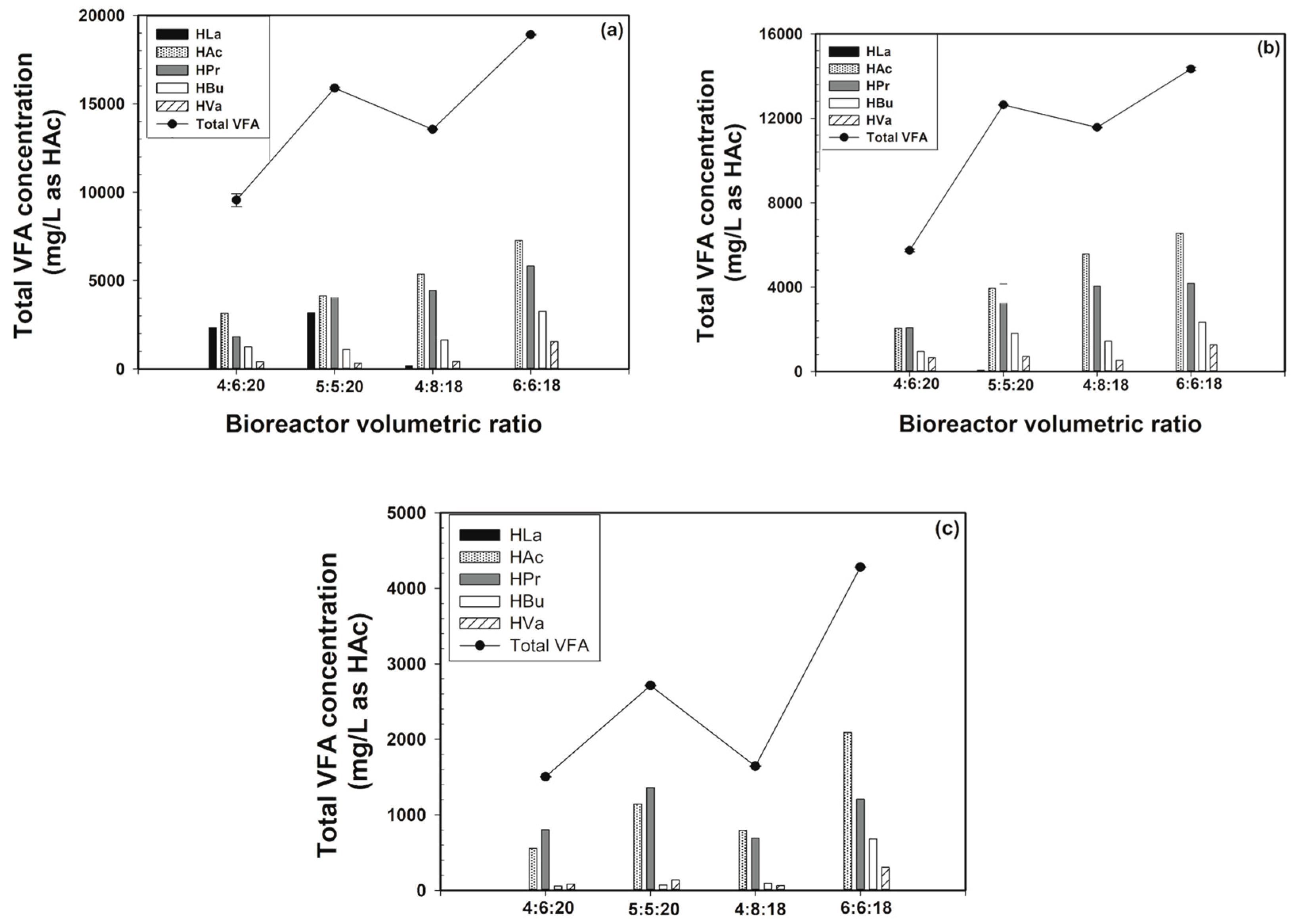
3.2. Part II: Effect of the Bioreactor Volumetric Ratio
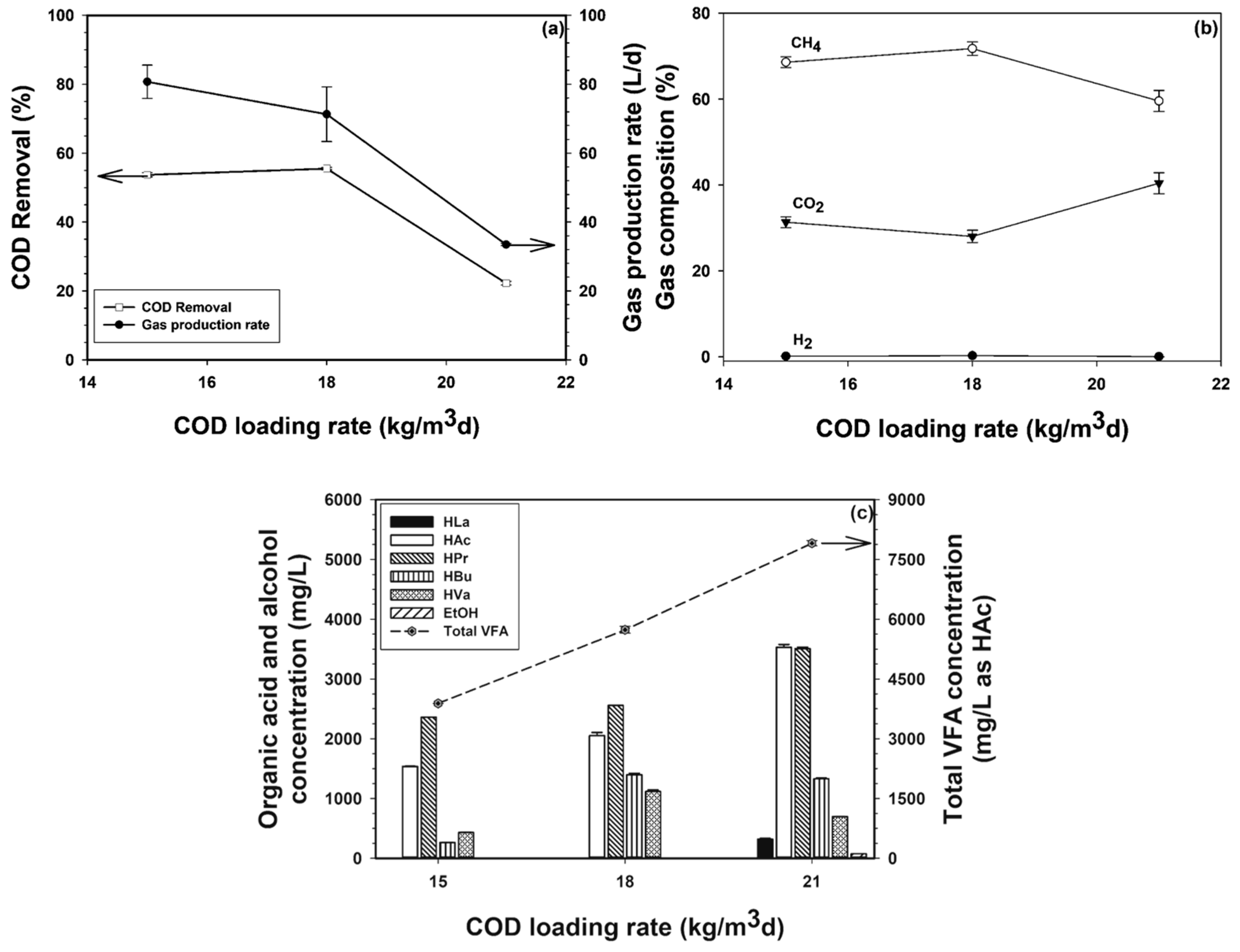
3.2.1. Overall COD Removal and Gas Production Rate
3.2.2. Gas Composition of Mixed Gas
3.2.3. Overall H2 and CH4 Production Rates
3.2.4. Overall H2 and CH4 Yields
3.2.5. Specific Energy Production Rate and Yield of Mixed Gas
3.2.6. VFA Levels
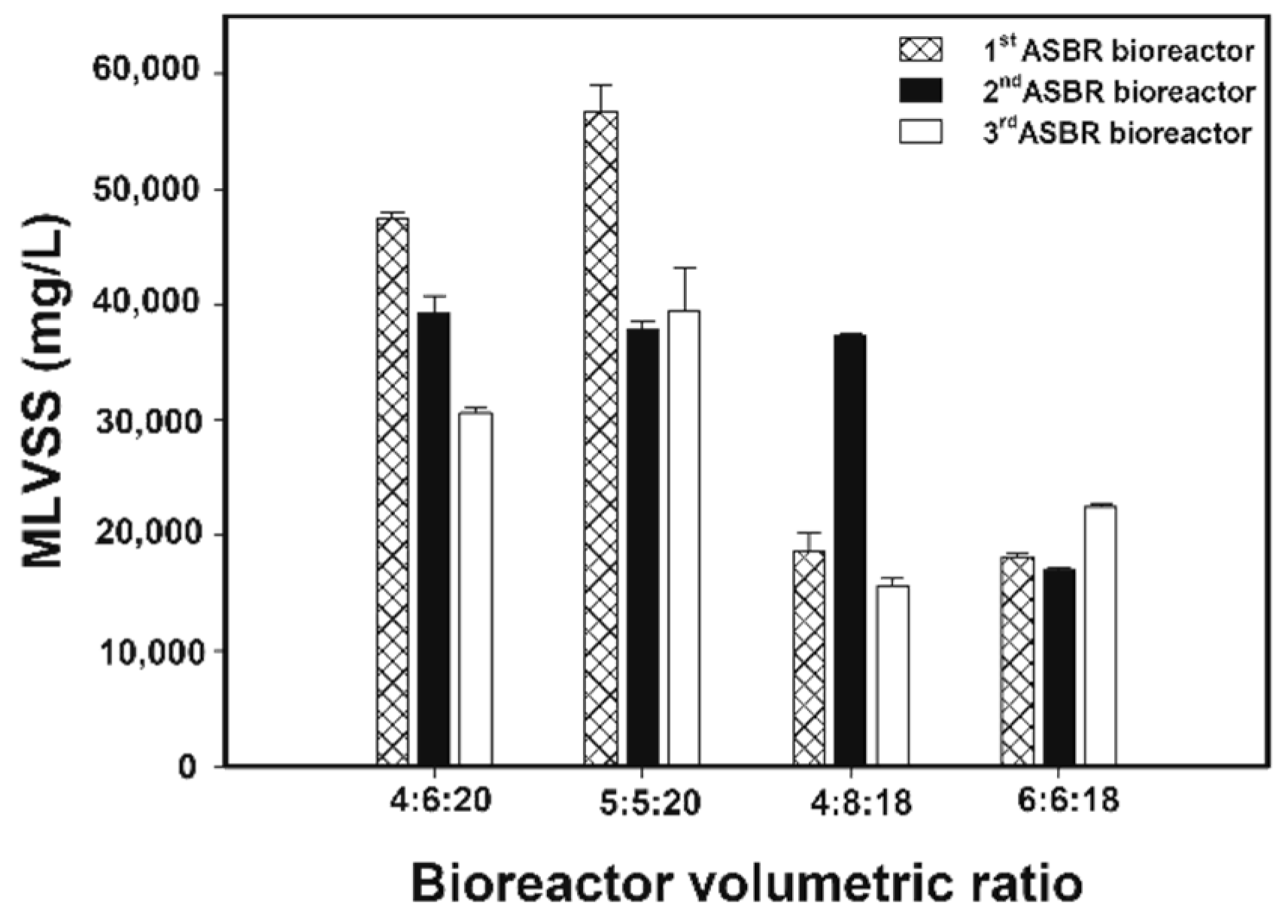
3.2.7. Microbial Concentrations
3.2.8. Comparison of Process Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| ASBR | Anaerobic sequencing batch reactor |
| COD | Chemical oxygen demand |
| CSTR | Complete stirred tank reactor |
| Co, Co2+ | Cobalt |
| Cu, Cu2+ | Copper |
| Fe, Fe2+ | Iron |
| H2S | Hydrogen sulfide |
| M2+ | Micronutrients (divalent cations) |
| MLVSS | Mixed liquor volatile suspended solids |
| Mn, Mn2+ | Manganese |
| MS | Metal sulfides |
| Mo, Mo2+ | Molybdenum |
| Ni, Ni2+ | Nickle |
| N | Nitrogen |
| P | Phosphorous |
| SHPR | Specific hydrogen production rate |
| SMPR | Specific methane production rate |
| TCD | Thermal conductivity detector |
| TSS | Total suspended solids |
| VFA | Volatile fatty acids |
| VSS | Volatile suspended solids |
| UASB | Upflow anaerobic sludge blanket |
| Zn, Zn2+ | Zinc |
References
- Lin, R.; Cheng, J.; Yang, Z.; Ding, L.; Zhang, J.; Zhou, J.; Cen, K. Enhanced energy recovery from cassava ethanol wastewater through sequential dark hydrogen, photo hydrogen and methane fermentation combined with ammonium removal. Bioresour. Technol. 2016, 214, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Stamatelatou, K.; Antonopoulou, G.; Lyberatos, G. Production of biogas via anaerobic digestion. Handb. Biofuels Prod. Process. Technol. 2011, 266–304. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lay, C.-H.; Sen, B.; Chu, C.-Y.; Kumar, G.; Chen, C.-C.; Chang, J.-S. Fermentative hydrogen production from wastewaters: A review and prognosis. Int. J. Hydrogen Energy 2012, 37, 15632–15642. [Google Scholar] [CrossRef]
- Demirel, B. The impacts of engineered nanomaterials (ENMs) on anaerobic digestion processes. Process Biochem. 2016, 51, 308–313. [Google Scholar] [CrossRef]
- Sreethawong, T.; Niyamapa, T.; Neramitsuk, H.; Rangsunvigit, P.; Leethochawalit, M.; Chavadej, S. Hydrogen production from glucose-containing wastewater using an anaerobic sequencing batch reactor: Effects of COD loading rate, nitrogen content, and organic acid composition. Chem. Eng. J. 2010, 160, 322–332. [Google Scholar] [CrossRef]
- Seneesrisakul, K.; Sutabutr, T.; Chavadej, S. The effect of temperature on the methanogenic activity in relation to micronutrient availability. Energies 2018, 11, 1057. [Google Scholar] [CrossRef]
- Papurello, D.; Iafrate, C.; Lanzini, A.; Santarelli, M. Trace compounds impact on SOFC performance: Experimental and modelling approach. Appl. Energy 2017, 208, 637–654. [Google Scholar] [CrossRef]
- Papurello, D.; Tomasi, L.; Silvestri, S.; Belcari, I.; Santarelli, M.; Smeacetto, F.; Biasioli, F. Biogas trace compound removal with ashes using proton transfer reaction time-of-flight mass spectrometry as innovative detection tool. Fuel Process. Technol. 2016, 145, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Papurello, D.; Silvestri, S.; Tomasi, L.; Belcari, I.; Biasioli, F.; Santarelli, M. Biowaste for SOFCs. Energy Procedia 2016, 101, 424–431. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels production by biomass gasification: A review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Liu, D.; Liu, D.; Zeng, R.J.; Angelidaki, I. Hydrogen and methane production from household solid waste in the two-stage fermentation process. Water Res. 2006, 40, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, R.; Ji, M.; Han, L. Hydrogen and methane production by co-digestion of waste activated sludge and food waste in the two-stage fermentation process: Substrate conversion and energy yield. Bioresour. Technol. 2013, 146, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, V.I.; Aivasidis, A. Comparison of single-and two-stage UASB reactors used for anaerobic treatment of synthetic fruit wastewater. Enzyme Microb. Technol. 2007, 42, 6–10. [Google Scholar] [CrossRef]
- Monlau, F.; Kaparaju, P.; Trably, E.; Steyer, J.P.; Carrere, H. Alkaline pretreatment to enhance one-stage CH4 and two-stage H2/CH4 production from sunflower stalks: Mass, energy and economical balances. Chem. Eng. J. 2015, 260, 377–385. [Google Scholar] [CrossRef]
- La Licata, B.; Sagnelli, F.; Boulanger, A.; Lanzini, A.; Leone, P.; Zitella, P.; Santarelli, M. Bio-hydrogen production from organic wastes in a pilot plant reactor and its use in a SOFC. Int. J. Hydrogen Energy 2011, 36, 7861–7865. [Google Scholar] [CrossRef]
- Sompong, O.; Suksong, W.; Promnuan, K.; Thipmunee, M.; Mamimin, C.; Prasertsan, P. Two-stage thermophilic fermentation and mesophilic methanogenic process for biohythane production from palm oil mill effluent with methanogenic effluent recirculation for pH control. Int. J. Hydrogen Energy 2016, 41, 21702–21712. [Google Scholar] [CrossRef]
- Müller, F.; Maack, G.-C.; Buescher, W. Effects of biogas substrate recirculation on methane yield and efficiency of a liquid-manure-based biogas plant. Energies 2017, 10, 325. [Google Scholar] [CrossRef]
- Jiraprasertwong, A. Production of biogas from cassava wastewater using a three-stage upflow anaerobic sludge blanket (UASB) reactor. Renew. Energ. 2018, in press. [Google Scholar]
- Intanoo, P.; Chaimongkol, P.; Chavadej, S. Hydrogen and methane production from cassava wastewater using two-stage upflow anaerobic sludge blanket reactors (UASB) with an emphasis on maximum hydrogen production. Int. J. Hydrogen Energy 2016, 41, 6107–6114. [Google Scholar] [CrossRef]
- Sreethawong, T.; Chatsiriwatana, S.; Rangsunvigit, P.; Chavadej, S. Hydrogen production from cassava wastewater using an anaerobic sequencing batch reactor: Effects of operational parameters, COD: N ratio, and organic acid composition. Int. J. Hydrogen Energy 2010, 35, 4092–4102. [Google Scholar] [CrossRef]
- Lay, J.-J.; Lee, Y.-J.; Noike, T. Feasibility of biological hydrogen production from organic fraction of municipal solid waste. Water Res. 1999, 33, 2579–2586. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F.; Kapdan, I.K.; Oztekin, R. Biohydrogen production by dark fermentation of wheat powder solution: Effects of C/N and C/P ratio on hydrogen yield and formation rate. Int. J. Hydrogen Energy 2008, 33, 1813–1819. [Google Scholar] [CrossRef]
- Intanoo, P.; Rangsunvigit, P.; Namprohm, W.; Thamprajamchit, B.; Chavadej, J.; Chavadej, S. Hydrogen production from alcohol wastewater by an anaerobic sequencing batch reactor under thermophilic operation: Nitrogen and phosphorous uptakes and transformation. Int. J. Hydrogen Energy 2012, 37, 11104–11112. [Google Scholar] [CrossRef]
- Intanoo, P.; Rangsanvigit, P.; Malakul, P.; Chavadej, S. Optimization of separate hydrogen and methane production from cassava wastewater using two-stage upflow anaerobic sludge blanket reactor (UASB) system under thermophilic operation. Bioresour. Technol. 2014, 173, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Watcharanurak, T. Three-Stage ASBR System for Separate Production of H2 and CH4 from Ethanol Wastewater. Master’s Thesis, The Petroleum and Petrochemical College, Chulalongkorn University, Bangkok, Thailand, 2016. [Google Scholar]
- Association, A.P.H.; Association, A.W.W.; Federation, W.P.C.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater, 23th ed.; American Public Health Association: Washington, DC, USA, 2017; Volume 1. [Google Scholar]
- Intanoo, P.; Suttikul, T.; Leethochawalit, M.; Gulari, E.; Chavadej, S. Hydrogen production from alcohol wastewater with added fermentation residue by an anaerobic sequencing batch reactor (ASBR) under thermophilic operation. Int. J. Hydrogen Energy 2014, 39, 9611–9620. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Mohanakrishna, G.; Sarma, P.N. Integration of acidogenic and methanogenic processes for simultaneous production of biohydrogen and methane from wastewater treatment. Int. J. Hydrogen Energy 2008, 33, 2156–2166. [Google Scholar] [CrossRef]
- Balachandar, G.; Khanna, N.; Das, D. Biohydrogen production from organic wastes by dark fermentation—Chapter 6. Biohydrogen 2013, 103–144. [Google Scholar] [CrossRef]
- Ali Shah, F.; Mahmood, Q.; Maroof Shah, M.; Pervez, A.; Ahmad Asad, S. Microbial ecology of anaerobic digesters: The key players of anaerobiosis. Sci. World J. 2014, 2014, 183752. [Google Scholar] [CrossRef] [PubMed]
- Linville, J.L.; Shen, Y.; Schoene, R.P.; Nguyen, M.; Urgun-Demirtas, M.; Snyder, S.W. Impact of trace element additives on anaerobic digestion of sewage sludge with in-situ carbon dioxide sequestration. Process Biochem. 2016, 51, 1283–1289. [Google Scholar] [CrossRef]
- Burgess, J.E.; Quarmby, J.; Stephenson, T. Micronutrient supplements for optimisation of the treatment of industrial wastewater using activated sludge. Water Res. 1999, 33, 3707–3714. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of anaerobic digestion process: A critical review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Voelklein, M.A.; O’ Shea, R.; Jacob, A.; Murphy, J.D. Role of trace elements in single and two-stage digestion of food waste at high organic loading rates. Energy 2017, 121, 185–192. [Google Scholar] [CrossRef]
- Zhang, L.; De Schryver, P.; De Gusseme, B.; De Muynck, W.; Boon, N.; Verstraete, W. Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: A review. Water Res. 2008, 42, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Narra, M.; Balasubramanian, V.; Kurchania, A.; Pathak, B.S.; Shyam, M. Enhanced biogas production from rice straw by selective micronutrients under solid state anaerobic digestion. Bioresour. Technol. 2016, 220, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xie, B.; Zhou, J.; Song, W.; Cen, K. Cogeneration of H2 and CH4 from water hyacinth by two-step anaerobic fermentation. Int. J. Hydrogen Energy 2010, 35, 3029–3035. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Meng, L.; Wang, J.; Zhang, W. Hydrogen–methane production from swine manure: Effect of pretreatment and VFAs accumulation on gas yield. Biomass Bioenergy 2009, 33, 1131–1138. [Google Scholar] [CrossRef]
- Searmsirimongkol, P.; Rangsunvigit, P.; Leethochawalit, M.; Chavadej, S. Hydrogen production from alcohol distillery wastewater containing high potassium and sulfate using an anaerobic sequencing batch reactor. Int. J. Hydrogen Energy 2011, 36, 12810–12821. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Stamatelatou, K.; Venetsaneas, N.; Kornaros, M.; Lyberatos, G. Biohydrogen and methane production from cheese whey in a two-stage anaerobic process. Ind. Eng. Chem. Res. 2008, 47, 5227–5233. [Google Scholar] [CrossRef]
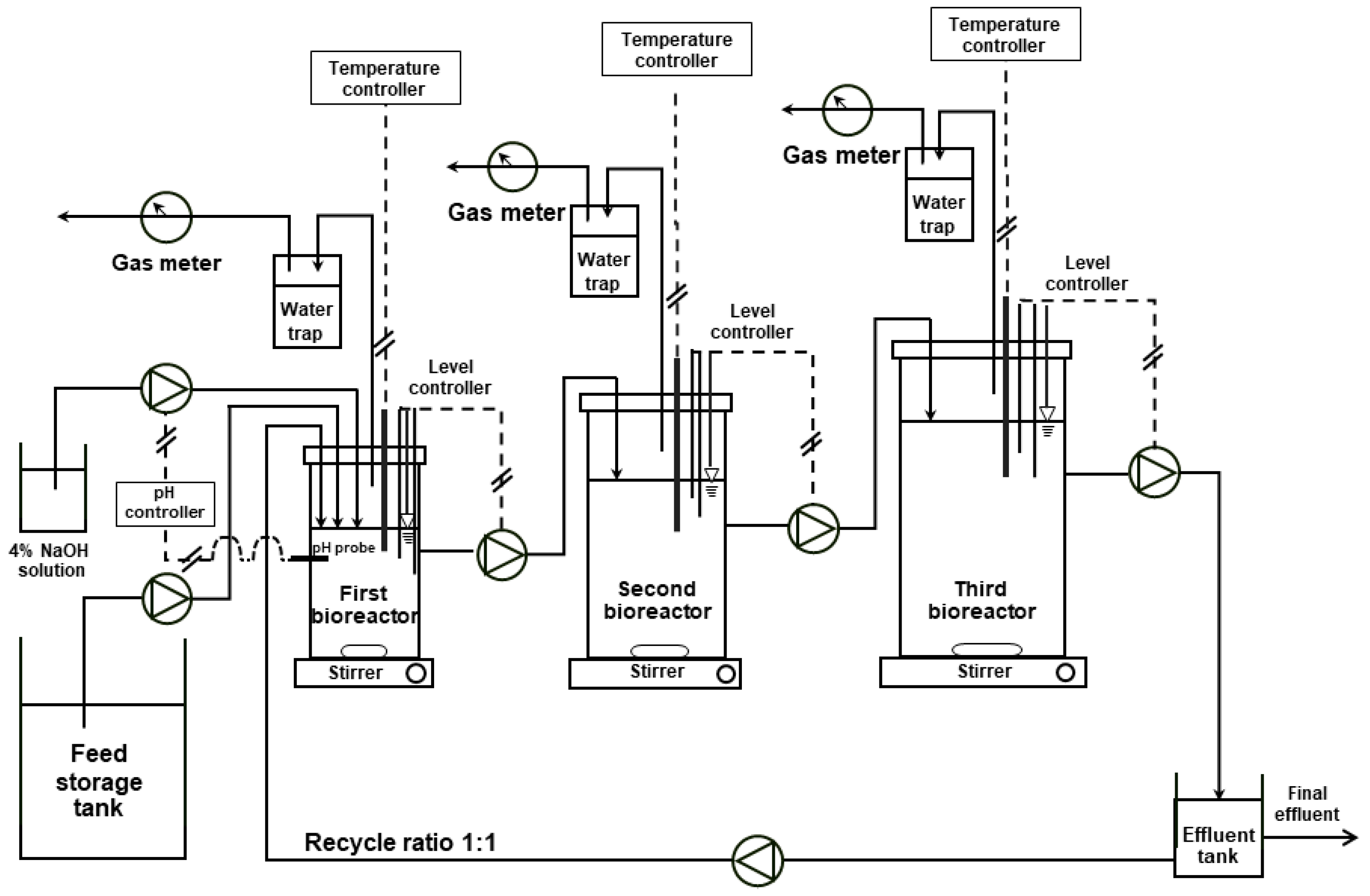

| Parameter | Units | Value |
|---|---|---|
| pH | - | 3.72 |
| Total chemical oxygen demand (COD) | mg/L | 80,000 |
| Total volatile fatty acids (VFA) | mg/L as HAc | 12,600 |
| Ethanol concentration | mg/L | 530 |
| Total suspended solids (TSS) | mg/L | 31,700 |
| Total phosphorous (P) | mg/L | 1070 |
| Total nitrogen (N) | mg/L | 825 |
| COD:N:P | by weight | 100:1.4:1.8 |
| Trace Element | Concentration (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Theoretical Values for Anaerobic Digestion [36] | Feed | COD Loading Rate of 15 kg/m3d | COD Loading Rate of 18 kg/m3d | COD Loading Rate of 21 kg/m3d | |||||||
| Effluent of | Effluent of | Effluent of | |||||||||
| 1st ASBR Unit | 2nd ASBR Unit | 3rd ASBR Unit | 1st ASBR Unit | 2nd ASBR Unit | 3rd ASBR Unit | 1st ASBR Unit | 2nd ASBR Unit | 3rd ASBR Unit | |||
| Co2+ | 0.10–5.00 | 0.11 | 0.17 | 0.13 | 0.11 | 0.19 | 0.17 | 0.15 | 0.17 | 0.17 | 0.15 |
| Cu2+ | 0.01–0.05 | 0.20 | 0.11 | 0.09 | 0.03 | 0.10 | 0.06 | 0.06 | 0.93 | 0.13 | 0.09 |
| Fe2+ | 0.10–0.40 | 36.12 | 37.97 | 7.49 | 5.32 | 47.21 | 20.94 | 7.45 | 29.76 | 25.45 | 6.36 |
| Mn2+ | 0.01–0.05 | 4.94 | 3.10 | 1.47 | 0.83 | 3.43 | 3.36 | 0.87 | 1.69 | 1.39 | 0.75 |
| Mo2+ | 0.10–0.70 | 1.20 | 0.32 | 0.32 | 0.32 | 0.74 | 0.63 | 0.21 | 0.42 | 0.21 | 0.21 |
| Ni2+ | 0.05–0.30 | 0.21 | 0.24 | 0.22 | 0.19 | 0.28 | 0.17 | 0.16 | 0.26 | 0.22 | 0.16 |
| Zn2+ | 0.10–1.00 | 1.41 | 0.44 | 0.43 | 0.27 | 1.37 | 0.30 | 0.26 | 4.40 | 0.53 | 0.25 |
| System | Substrate | Production | Optimum COD Loading Rate (kg/m3d) | Temperature (°C) | Volume of Bioreactor (L) | CH4 Yield (mL CH4/g COD Applied) | Energy Yield (kJ/g COD Applied) | Overall COD Removal (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| One-stage ASBR | Alcohol | H2 | 60 | 37 | 4 | - | 0.50 | 32 | [41] |
| One-stage ASBR | Ethanol | H2 | 50.6 | 55 | 4 | - | 0.29 | 32 | [27] |
| One-stage ASBR | Ethanol | CH4 | 6 10 | 37 55 | 2 | 231 324 | 8.60 10.90 | 93 83 | [6] |
| Two-stage UASB | Cassava | H2 & CH4 | 7 | 37 | 4 and 24 | 115 | 4.39 | 96 | [19] |
| Two-stage CSTR | Cheese whey | H2 & CH4 | NR | 35 | 3 and 15 | 310 | 0.41 | 94 | [42] |
| Two-stage UASB | Cassava | H2 & CH4 | 13 | 55 | 4 and 24 | 165 | 6.49 | 82 | [24] |
| Three-stage UASB | Cassava | H2 & CH4 | 15 | 37 | 4, 10 and 24 | 328 | 11.76 | 92.5 | [18] |
| Three-stage ASBR | Ethanol | H2 & CH4 | 18 | 37 | 5, 5 and 20 | 686 | 22.50 | 92.1 | Present work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiraprasertwong, A.; Vichaitanapat, K.; Leethochawalit, M.; Chavadej, S. Three-Stage Anaerobic Sequencing Batch Reactor (ASBR) for Maximum Methane Production: Effects of COD Loading Rate and Reactor Volumetric Ratio. Energies 2018, 11, 1543. https://doi.org/10.3390/en11061543
Jiraprasertwong A, Vichaitanapat K, Leethochawalit M, Chavadej S. Three-Stage Anaerobic Sequencing Batch Reactor (ASBR) for Maximum Methane Production: Effects of COD Loading Rate and Reactor Volumetric Ratio. Energies. 2018; 11(6):1543. https://doi.org/10.3390/en11061543
Chicago/Turabian StyleJiraprasertwong, Achiraya, Kornpong Vichaitanapat, Malinee Leethochawalit, and Sumaeth Chavadej. 2018. "Three-Stage Anaerobic Sequencing Batch Reactor (ASBR) for Maximum Methane Production: Effects of COD Loading Rate and Reactor Volumetric Ratio" Energies 11, no. 6: 1543. https://doi.org/10.3390/en11061543