Analysis of Syngas Production from Biogas via the Tri-Reforming Process
Abstract
:1. Introduction
2. Modeling
2.1. Chemical Reaction
2.2. Process Simulation
3. Results and Discussion
4. Conclusions
- (1)
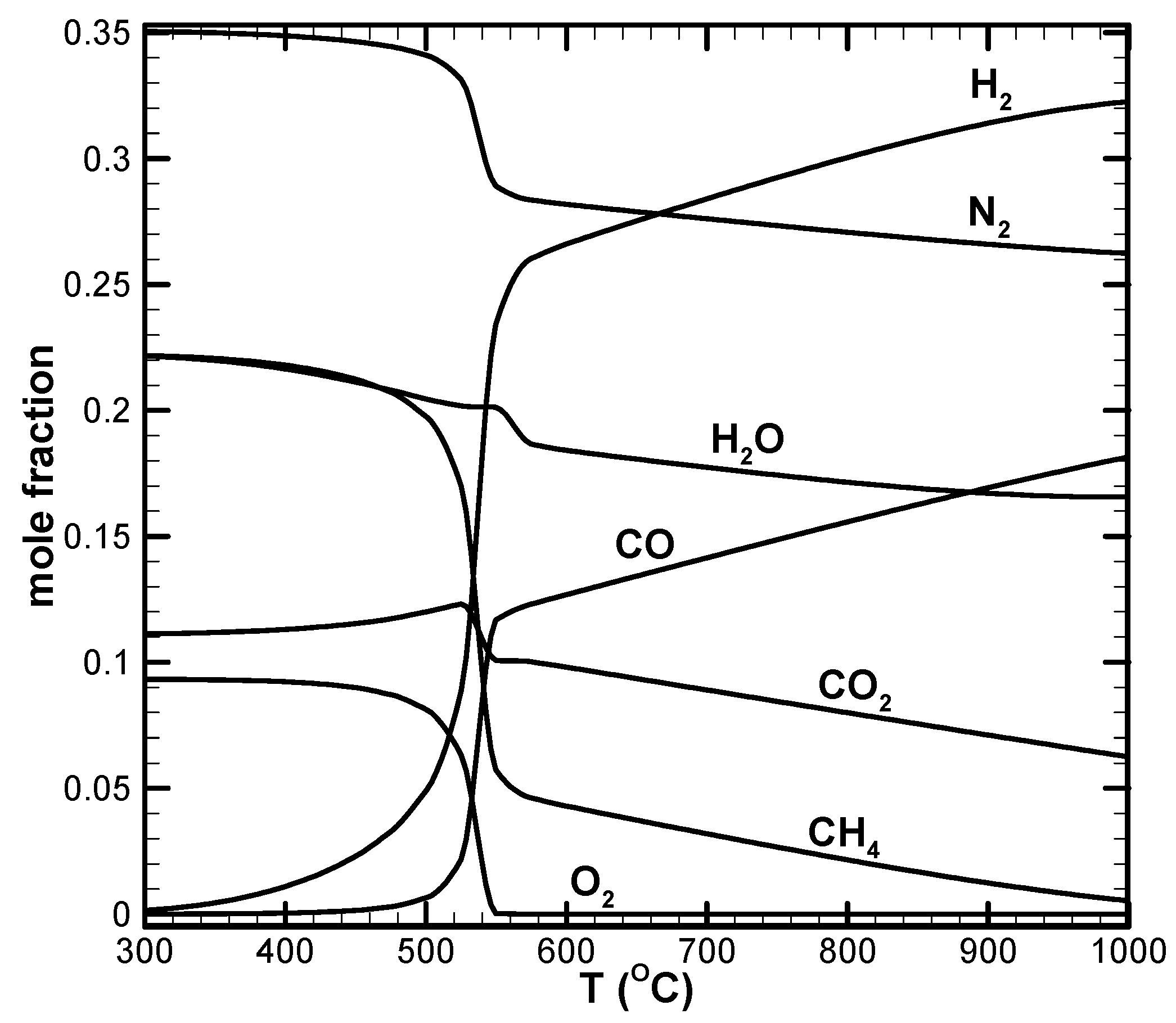
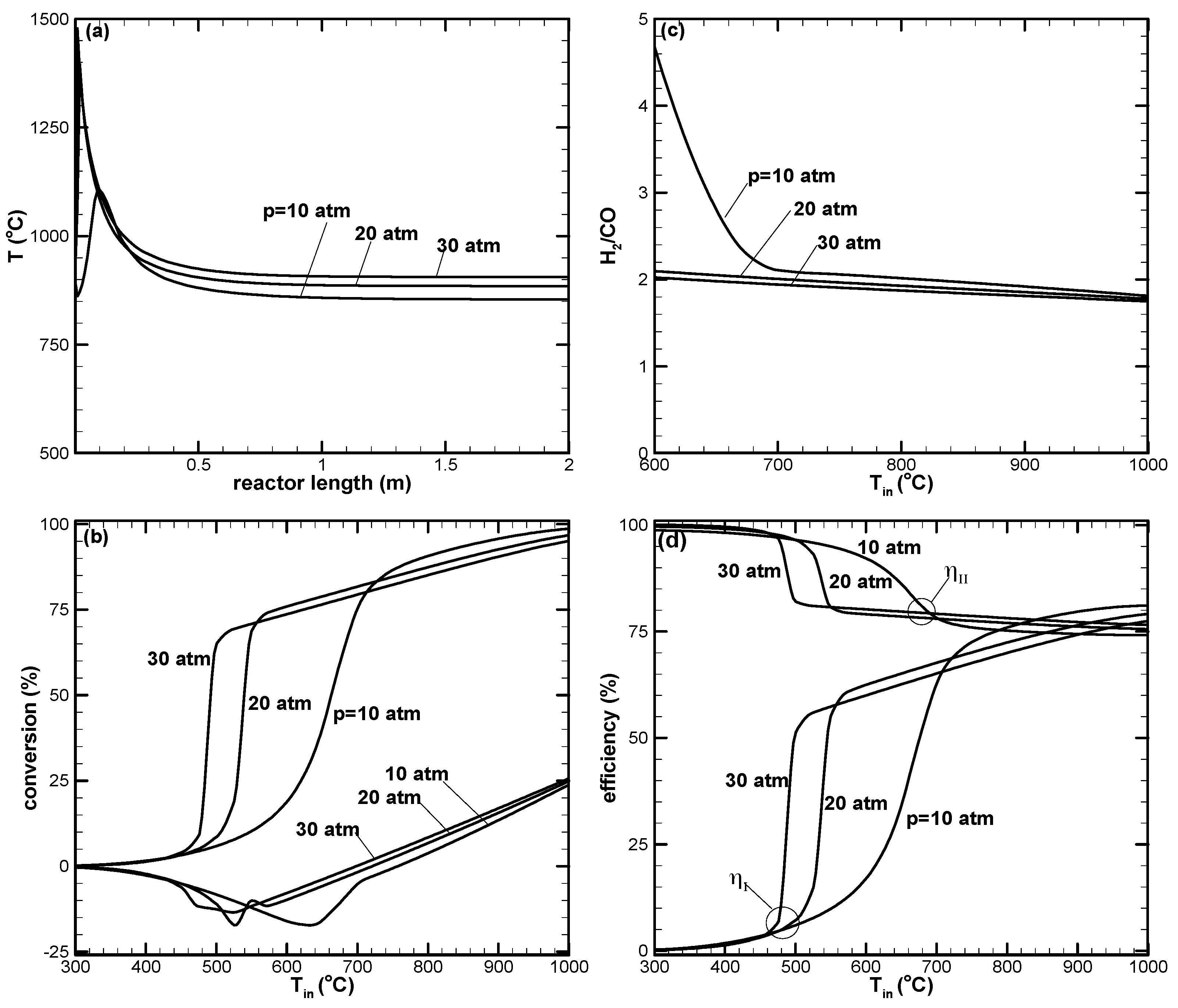
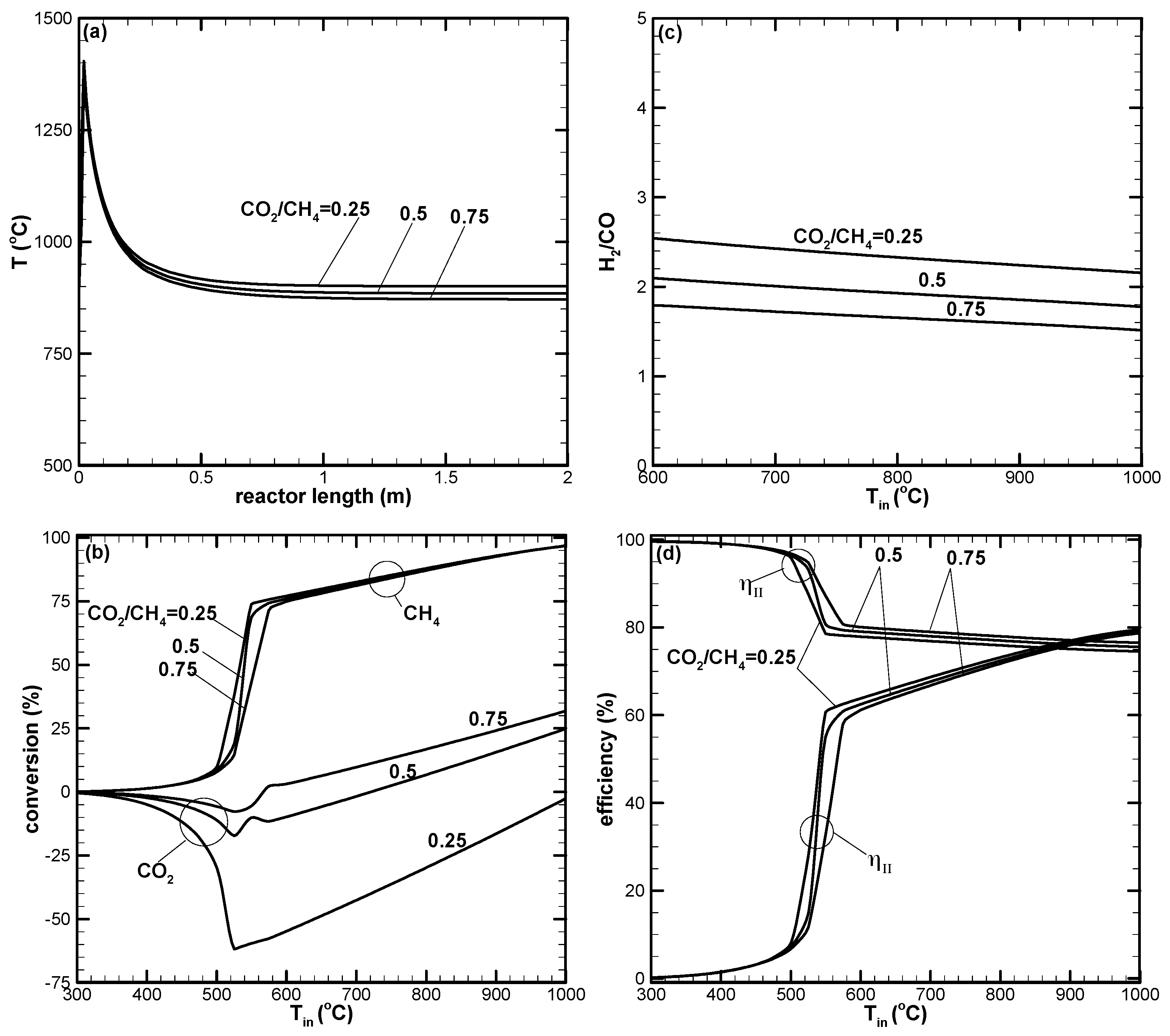
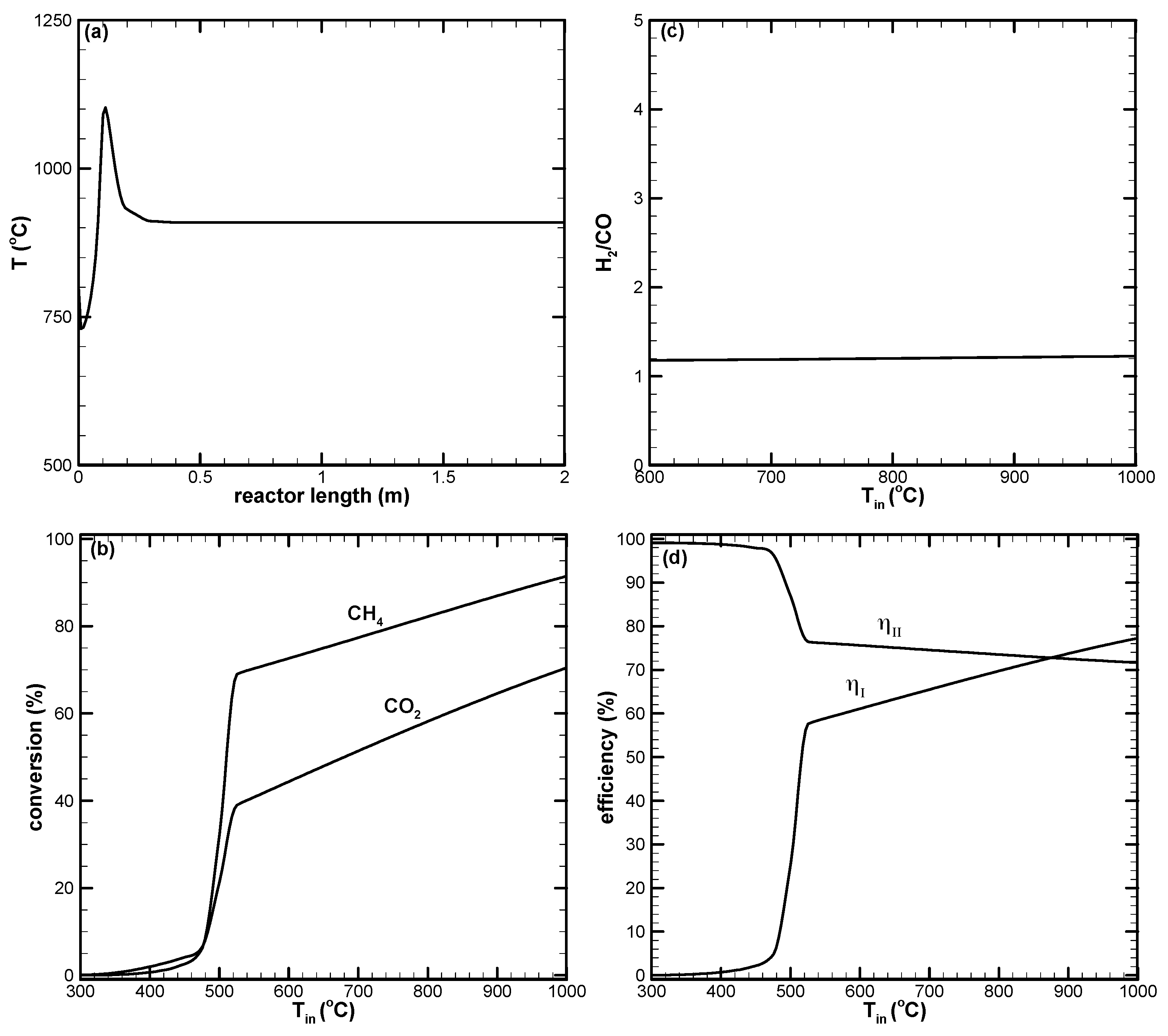
- There appears to be a limiting space velocity for the reaction. Beyond this limiting value, the reaction approaches the same performance. Lowering the reaction pressure could lead to higher CH4 conversion, but the syngas produced may not be suitable for further applications.
- (2)
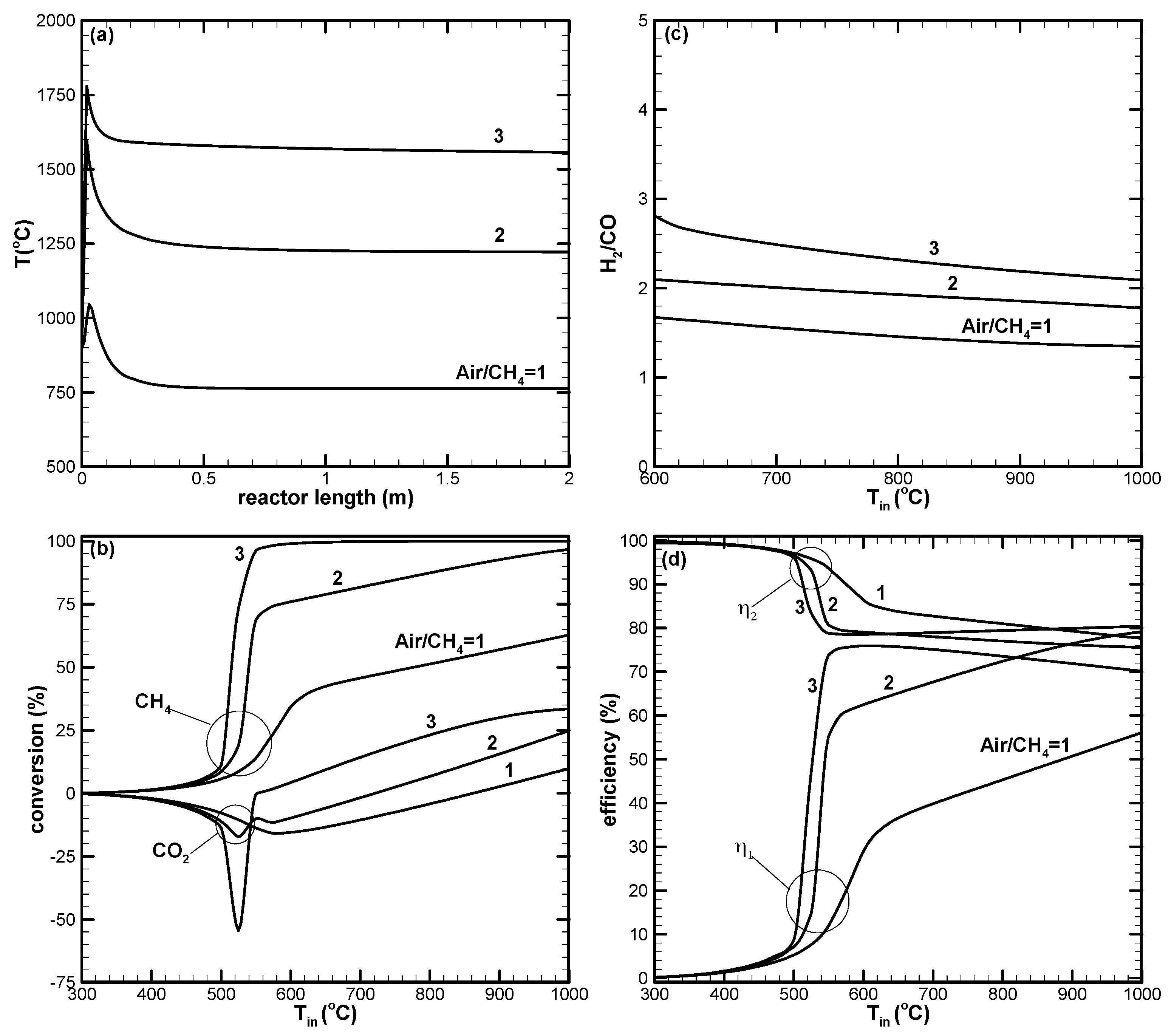
- CH4 and CO2 conversions can be enhanced by increasing the amount of air in the reactant. Higher amounts of air could result in decreased H2 yield due to the reverse water–gas shift reaction, which is favorable at high reaction temperatures.
- (3)
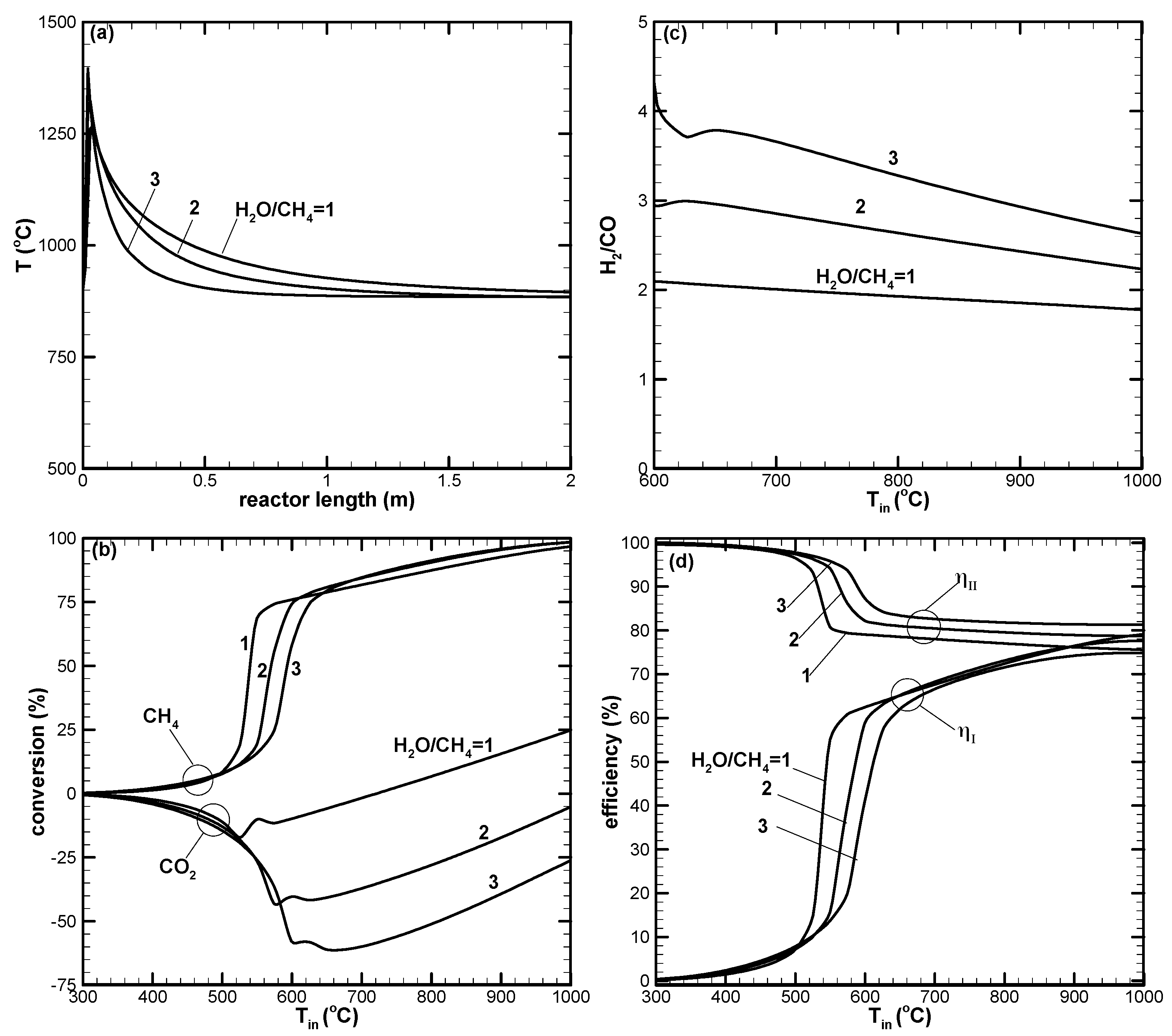
- A higher H2/CO ratio can be obtained by increasing H2O addition. However, the dry reforming reaction is suppressed, leading to low CO2 or negative conversion.
- (4)
- Dry reforming of CO2 can only be found when the reaction temperature is high. This results in positive CO2 conversion and contributes to increased H2 and CO yields.
- (5)
- Higher CO2 conversion can be obtained for the low H2O addition case. However, low H2/CO with a value close to unity results.
- (6)
- The first-law efficiency increases with the increased reaction temperature because of higher H2 and CO yields. The second-law efficiency decreases with the increased temperature because of higher exergy destruction due to a more complete chemical reaction at high temperatures.
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviation
| Nomenclature | |
| dp | catalyst particle diameter, m |
| Ex | exergy, kJ |
| chemical exergy, kJ mol−1 | |
| F | reactant volumetric flow rate, m3 s−1 |
| Kj | surface adsorption equilibrium constant of species j, Pa−1 |
| surface adsorption equilibrium constant of species j in combustion reaction, Pa−1 | |
| equilibrium constant of reaction i | |
| rate constant of reaction i, mol Pa0.5kgcat s−1, or mol Pa kgcat s−1 | |
| L | length of reactor, m |
| LHV | lower heating value, kJ mol−1 |
| Ni | total molar flow rate of a stream i, mole s−1 |
| nj | molar flow rate of species j, mole s−1 |
| p | pressure, Pa |
| Q | heat transfer, W |
| R | universal gas constant, 8.314 J mol−1 K−1 |
| Rb | reactor radius, m |
| ri | kinetic rate of reaction i, mol kgcat s−1 |
| s | entropy, kJ mol−1 K−1 |
| T | temperature, K |
| W | catalyst weight, g |
| Wcomp | compressor work, W |
| Wpump | pump work, W |
| X | species conversion |
| x | mole fraction |
| Y | species yield |
| Subscript | |
| in | inlet |
| out | outlet |
| 0 | reference state |
| Greek symbols | |
| ΔH | heat of reaction, kJ/mol |
| η | efficiency |
References
- Raju, A.S.K.; Park, C.S.; Norbeck, J.M. Synthesis gas production using steam hydrogasification and steam reforming. Fuel Process. Technol. 2009, 90, 330–336. [Google Scholar] [CrossRef]
- Rathod, V.P.; Shete, J.; Bhale, P.V. Experimental investigation on biogas reforming to hydrogen rich syngas production using solar energy. Int. J. Hydrog. Energy 2016, 41, 132–138. [Google Scholar] [CrossRef]
- Su, B.; Han, W.; Jin, H. Proposal and assessment of a novel integrated CCHP system with biogas steam reforming using solar energy. Appl. Energy 2017, 206, 1–11. [Google Scholar] [CrossRef]
- Damanabi, A.T.; Bahadori, F. Improving GTL process by CO2 utilization in tri-reforming reactor and application of membranes in Fischer-Tropsch reactor. J. CO2 Util. 2017, 21, 227–237. [Google Scholar] [CrossRef]
- Dwivedi, A.; Gudi, R.; Biswas, P. An improved tri-reforming based methanol production process for enhanced CO2 valorization. Int. J. Hydrog. Energy 2017, 42, 23227–23241. [Google Scholar] [CrossRef]
- Song, C.; Pan, W. Tri-reforming of methane: A novel concept for catalytic production of industrially useful synthesis gas with desired H2/CO ratios. Catal. Today 2004, 98, 463–484. [Google Scholar] [CrossRef]
- Majewski, A.J.; Wood, J. Tri-reforming of methane over Ni@SiO2 catalyst. Int. J. Hydrog. Energy 2014, 39, 12578–12585. [Google Scholar] [CrossRef]
- Kumar, N.; Shojaee, M.; Spivey, J.J. Catalytic bi-reforming of methane: From greenhouse gases to syngas. Curr. Opin. Chem. Eng. 2015, 9, 8–15. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mondal, K.C.; Choudhary, T.V. Oxy-CO2 reforming of methane to syngas over CoOx/MgO/SA-5205 catalyst. Fuel 2006, 85, 2484–2488. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.A.; Fennell, P.S.; Shen, L.; Fan, L. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef]
- Samson, R.; LeDuy, A. Biogas production from anaerobic digestion of spirulina maxima algal biomass. Biotechnol. Bioeng. 2012, 24, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Rasi, S.; Veijanen, A.; Rintala, J. Trace compounds of biogas from different biogas production plants. Energy 2017, 32, 1370–1380. [Google Scholar] [CrossRef]
- Hagman, L.; Blumenthal, A.; Eklund, M.; Svensson, N. The role of biogas solutions in sustainable biorefineries. J. Clean. Prod. 2018, 172, 3982–3989. [Google Scholar] [CrossRef]
- Meyer, A.K.P.; Ehimen, E.A.; Holm-Nielsen, J.B. Future European biogas: Animal manure, straw and grass potentials for a sustainable European biogas production. Biomass Bioenergy 2018, 111, 154–164. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels production by biomass gasification: A review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Chianese, S.; Loipersböck, J.; Malits, M.; Rauch, R.; Hofbauer, H.; Molino, A.; Musmarra, D. Hydrogen from the high temperature water gas shift reaction with an industrial Fe/Cr catalyst using biomass gasification tar rich synthesis gas. Fuel Process. Technol. 2015, 132, 39–48. [Google Scholar] [CrossRef]
- Molino, A.; Migliori, M.; Blasi, A.; Davoli, M.; Marino, T.; Chianese, S.; Catizzone, E.; Giordano, G. Municipal waste leachate conversion via catalytic supercritical water gasification process. Fuel 2017, 206, 155–161. [Google Scholar] [CrossRef]
- Chianese, S.; Fail, S.; Binder, M.; Rauch, R.; Hofbauer, H.; Molino, A.; Blasi, A.; Musmarra, D. Experimental investigations of hydrogen production from CO catalytic conversion of tar rich syngas by biomass gasification. Catal. Today 2016, 277, 182–191. [Google Scholar] [CrossRef]
- Vita, A.; Pino, L.; Cipitì, F.; Laganà, M.; Recupero, V. Biogas as renewable rawmaterial for syngas production by tri-reforming process over NiCeO2 catalysts: Optimal operative condition and effect of nickel content. Fuel Process. Technol. 2014, 127, 47–58. [Google Scholar] [CrossRef]
- Lau, C.S.; Tsolakis, A.; Wyszynski, M.L. Biogas upgrade to syn-gas (H2-CO) via dry and oxidative reforming. Int. J. Hydrog. Energy 2011, 36, 397–404. [Google Scholar] [CrossRef]
- Zhu, X.; Li, K.; Liu, J.; Li, X.; Zhu, A. Effect of CO2/CH4 ratio on biogas reforming with added O2 through an unique spark-shade plasma. Int. J. Hydrog. Energy 2014, 39, 13902–13908. [Google Scholar] [CrossRef]
- Corigliano, O.; Fragiacomo, P. Technical analysis of hydrogen-rich stream generation through CO2 reforming of biogas by using numerical modeling. Fuel 2015, 158, 538–548. [Google Scholar] [CrossRef]
- Hernández, B.; Martín, M. Optimal process operation for biogas reforming to methanol: Effects of dry reforming and biogas composition. Ind. Eng. Chem. Res. 2016, 55, 6677–6685. [Google Scholar] [CrossRef]
- Hajjaji, N.; Martinez, S.; Trably, E.; Steyer, J.; Helias, A. Life cycle assessment of hydrogen production from biogas reforming. Int. J. Hydrog. Energy 2016, 41, 6064–6075. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Benson, T. A conceptual design by integrating dimethyl ether (DME) production with tri-reforming process for CO2 emission reduction. Fuel Process. Technol. 2015, 131, 7–13. [Google Scholar] [CrossRef]
- Solovev, S.A.; Kurilets, Y.; Orlik, S.N. Tri-reforming of methane on structured Ni-containing catalysts. Theor. Exp. Chem. 2012, 48, 199–205. [Google Scholar] [CrossRef]
- Cho, W.; Song, T.; Mitso, A.; McKinnon, T.J.; Ko, G.H.; Tolsma, J.E. Optimal design and operation of a natural gas tri-reforming reactor for DME synthesis. Catal. Today 2009, 139, 261–267. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.; Green, M.L.H. Brief overview of the partial oxidation of methane to synthesis gas. Top. Catal. 2003, 22, 345–358. [Google Scholar] [CrossRef]
- Horn, R.; Williams, K.A.; Degenstein, N.J.; Bitsch-Larsen, A.; Dalle Nogare, D.; Tupy, S.A.; Schmidt, L.D. Methane catalytic partial oxidation on autothermal Rh and Pt foam catalysts: Oxidation and reforming zones, transport effects, and approach to thermodynamic equilibrium. J. Catal. 2007, 249, 380–393. [Google Scholar] [CrossRef]
- De Groote, A.M.; Froment, D. Simulation of the catalytic partial oxidation of methane to syngas. Appl. Catal. A 1996, 138, 245–264. [Google Scholar] [CrossRef]
- Scognamiglio, D.; Russo, L.; Maffettone, P.L.; Salemme, L.; Simeone, M.; Crescitelli, S. Modeling temperature profiles of a catalytic autothermal methane reformer with nickel catalyst. Ind. Eng. Chem. Res. 2009, 48, 1804–1815. [Google Scholar] [CrossRef]
- Chan, S.H.; Wang, M.H. Thermodynamic analysis of natural gas fuel processing for fuel cell applications. Int. J. Hydrog. Energy 2000, 25, 441–449. [Google Scholar] [CrossRef]
- Izquierdo, U.; García-García, I.; Gutierrez, Á.M.; Arraibi, J.R.; Barrio, V.L.; Cambra, J.F.; Arias, P.L. Catalyst deactivation and regeneration processes in biogas tri-reforming process. The effect of hydrogen sulfide addition. Catalysts 2018, 8, 12. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Trimm, D.L.; Lam, C.W. The combustion of methane on platinum-alumina fibre catalysts. I. Kinetics and mechanism. Chem. Eng. Sci. 1908, 35, 1405–1413. [Google Scholar] [CrossRef]
- De Smet, C.R.H.; de Croon, M.H.J.M.; Berger, R.J.; Marin, G.B.; Schouten, J.C. Design of adiabatic fixed-bed reactors for the partial oxidation of methane to synthesis gas. Chem. Eng. Sci. 2001, 56, 4849–4861. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Velasco, J.A.; Fernandez, C.; Lopez, L.; Cabrera, S.; Boutonnet, M.; Jaras, S. Catalytic partial oxidation of methane over nickel and ruthenium based catalysts under low O2/CH4 ratios and with addition of steam. Fuel 2015, 153, 192–201. [Google Scholar] [CrossRef]
- Ahmed, S.; Lee, S.H.E.; Ferrandon, M.S. Catalytic steam reforming of biogas e Effects of feed composition and operating conditions. Int. J. Hydrog. Energy 2015, 40, 1005–1015. [Google Scholar] [CrossRef]
- Barbieri, G.; Di Maio, F.P. Simulation of the methane steam re-forming process in a catalytic Pd-membrane reactor. Ind. Eng. Chem. Res. 1997, 36, 2121–2127. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, B.H.; Yi, J. Modified simulation of methane steam reforming in Pd-membrane/pack-bed type reactor. J. Chem. Eng. Jpn. 1999, 32, 760–769. [Google Scholar] [CrossRef]
- Lutz, A.E.; Bradshaw, R.W.; Keller, J.O.; Witmer, D.E. Thermodynamic analysis of hydrogen production by steam reforming. Int. J. Hydrog. Energy 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Simpson, A.P.; Lutz, A.E. Exergy analysis of hydrogen production via steam methane reforming. Int. J. Hydrog. Energy 2007, 32, 4811–4820. [Google Scholar] [CrossRef]
- Chein, R.; Wang, C.; Yu, C. Parametric study on catalytic tri-reforming of methane for syngas production. Energy 2017, 118, 1–17. [Google Scholar] [CrossRef]
- Arab Aboosadi, Z.; Jahanmiri, A.H.; Rahimpour, M.R. Optimization of tri-reformer reactor to produce synthesis gas for methanol production using differential evolution (DE) method. Appl. Energy 2011, 88, 2691–2701. [Google Scholar] [CrossRef]





| Parameter | Value |
|---|---|
| Reactor length, L | 2 m |
| Reactor diameter, d(=2Rb) | 10 mm |
| Inlet pressure, pin | 20 atm |
| Inlet temperature, Tin | 300~1000 °C |
| Reactant flow rate, F | 0.0723 L min−1 |
| Molar ratio of biogas CH4:CO2:Air:H2O | 1:0.5:2:1 |
| Catalyst | Ni/Al2O3 |
| Catalyst size, dp | 0.42 mm |
| Catalyst weight, W | 0.25 g |
| W/F ratio | 0.0576 ghL-1 |
| Heat-transfer condition | Adiabatic |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chein, R.-Y.; Hsu, W.-H. Analysis of Syngas Production from Biogas via the Tri-Reforming Process. Energies 2018, 11, 1075. https://doi.org/10.3390/en11051075
Chein R-Y, Hsu W-H. Analysis of Syngas Production from Biogas via the Tri-Reforming Process. Energies. 2018; 11(5):1075. https://doi.org/10.3390/en11051075
Chicago/Turabian StyleChein, Rei-Yu, and Wen-Hwai Hsu. 2018. "Analysis of Syngas Production from Biogas via the Tri-Reforming Process" Energies 11, no. 5: 1075. https://doi.org/10.3390/en11051075




