Thermal Behavior of Coal Used in Rotary Kiln and Its Combustion Intensification
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Equipment
2.2. Methods
3. Results and Discussion
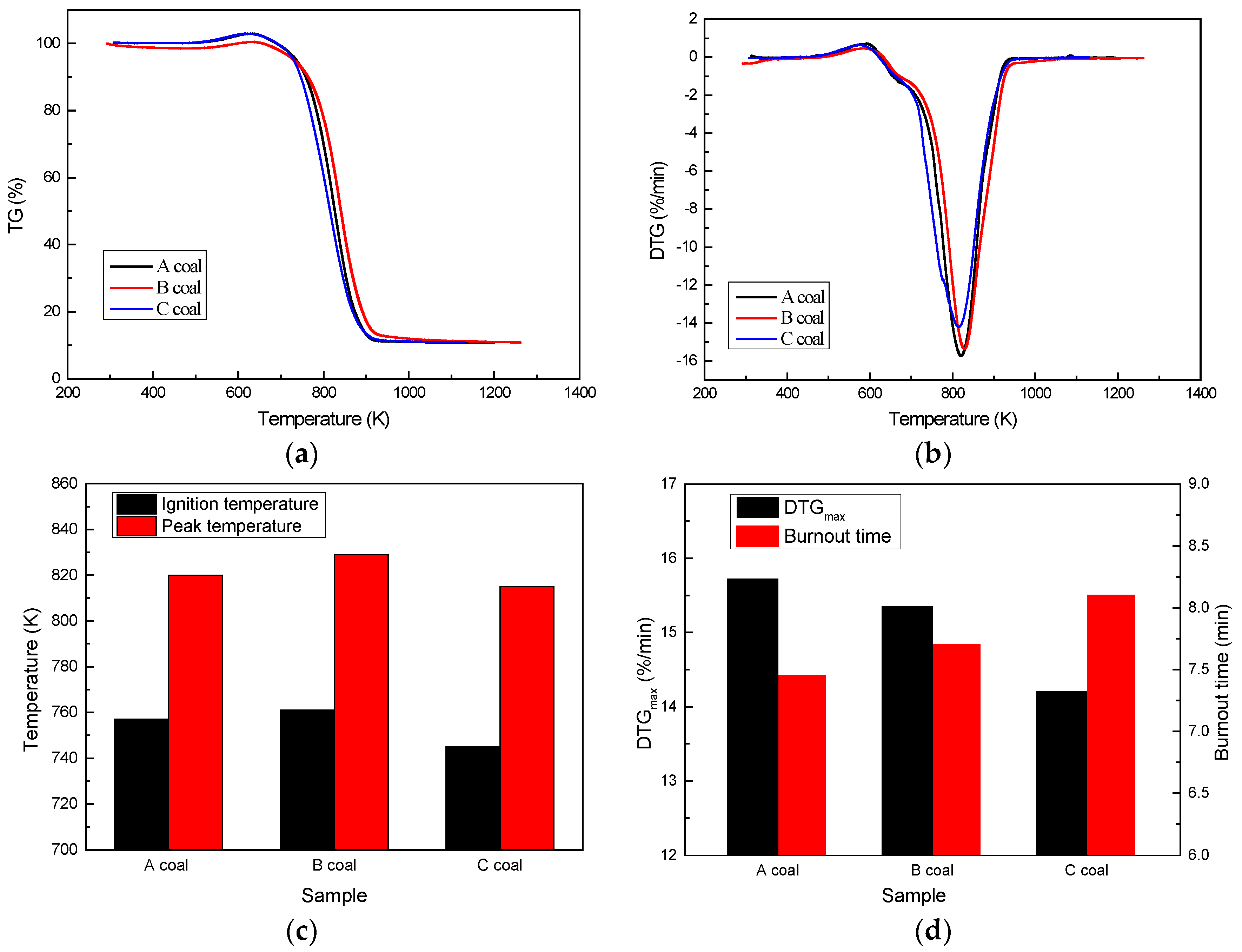
3.1. Thermal Behavior
3.1.1. Pyrolysis Behavior
3.1.2. Combustion Behavior
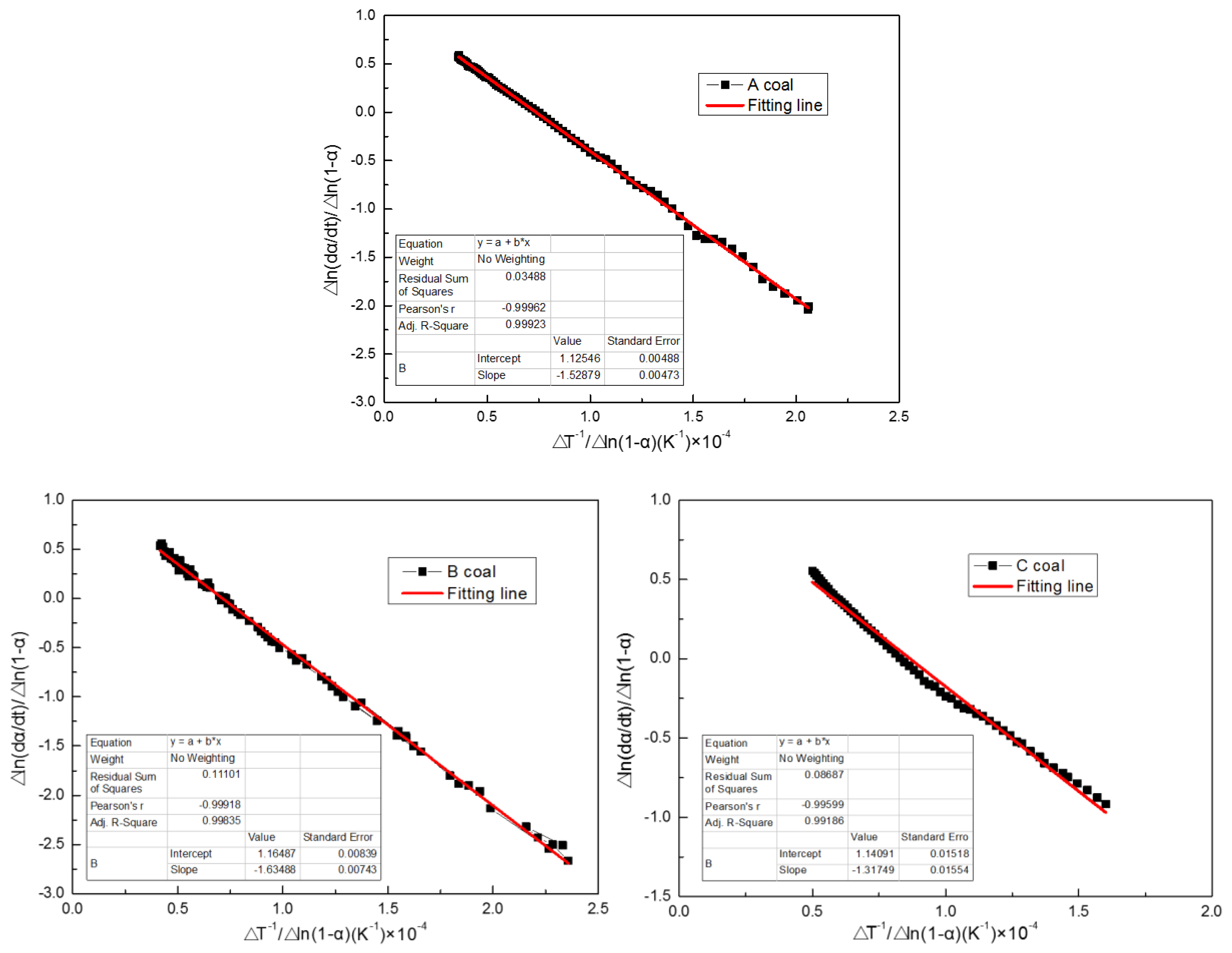
3.1.3. Combustion Kinetics
3.2. Combustion Intensification
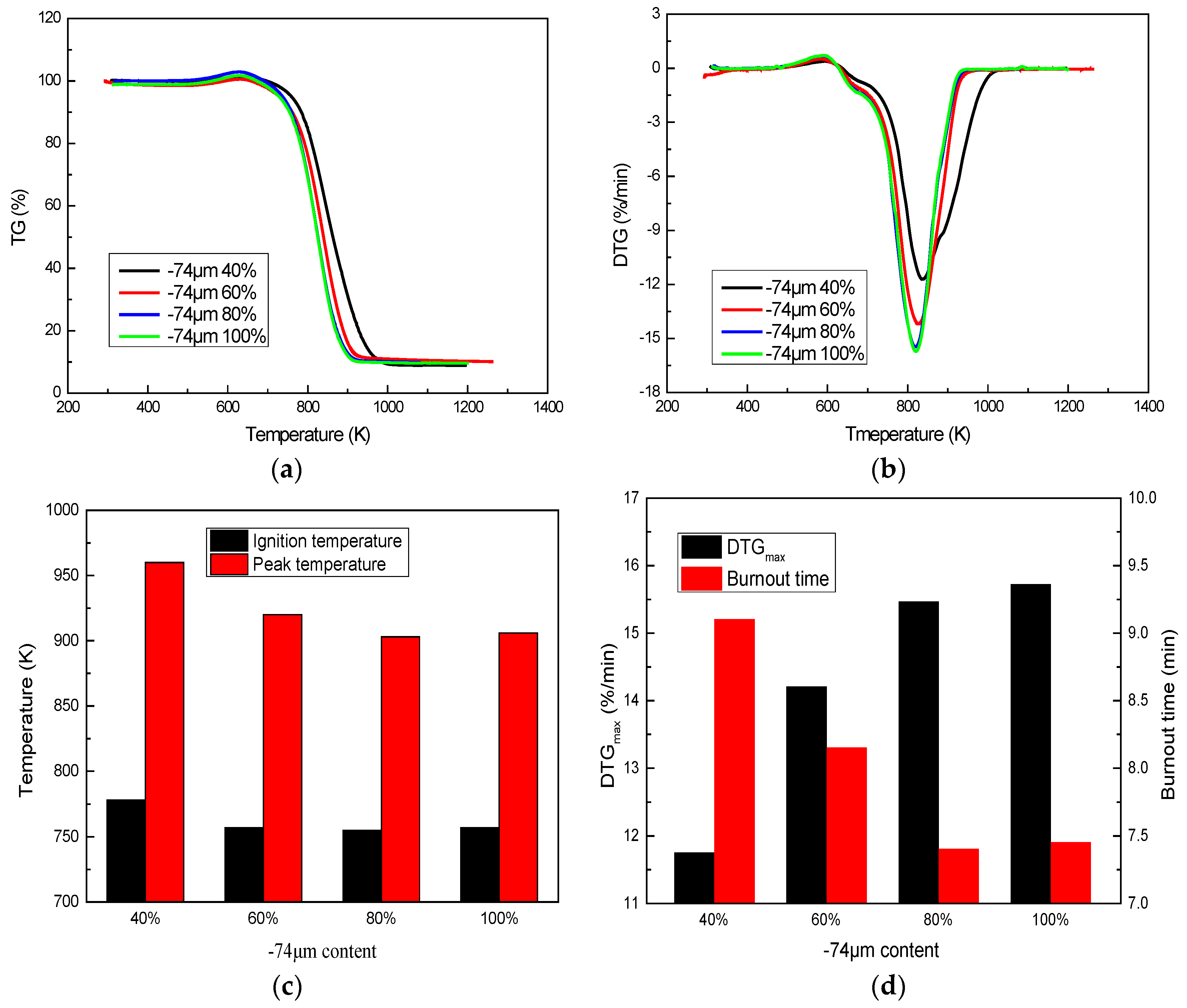
3.2.1. Effect of Coal Size
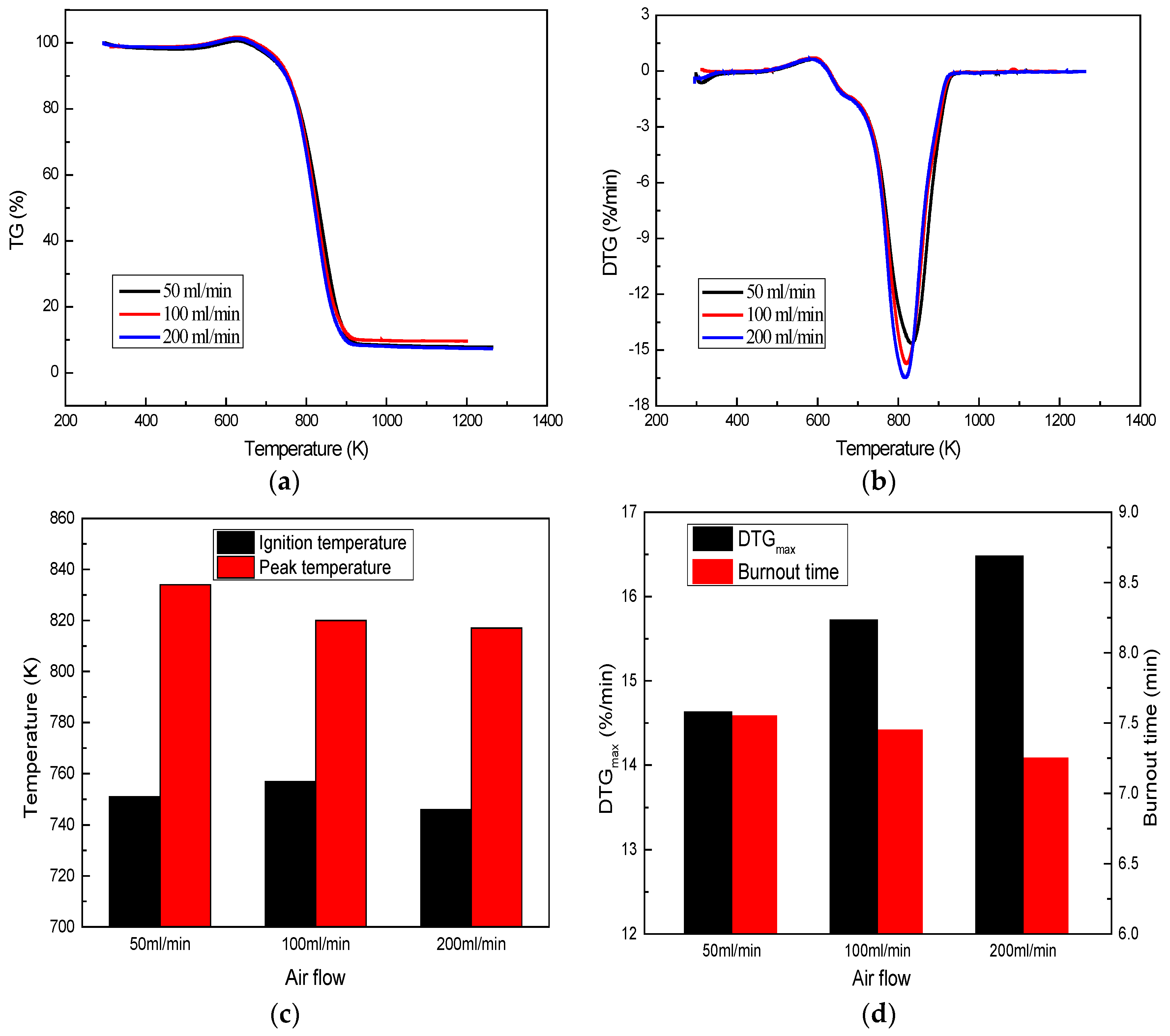
3.2.2. Effect of Air Flow
3.2.3. Effect of Oxygen Content
3.2.4. Effect of Heating Rate
4. Conclusions
- (1)
- Three coals have a similar trend of pyrolysis that occurs at about 670 K and this process continuously proceeds along with their combustion. The three coals also have comparable combustion behaviors of 745–761 K ignition temperature, 815–829 K peak temperature, 14.20–15.72%/min DTGmax, and 7.45–8.10 min burnout time.
- (2)
- Combustion kinetic parameters provide quantitative observation into coal combustion behavior. Low activation energy (Eα) and reaction order (n) make coal, especially C coal, have a simple combustion mechanism, great reactivity, easy ignition, and a low peak temperature in the combustion state.
- (3)
- The suitable size for coal combustion in a kiln is that the content of less than 74 μm is 60% to 80%. Oxygen content and heating rate have a clear effect on coal combustion. Oxygen-enrichment and high heating rates can significantly enhance coal combustion, increase combustion intensity and peak value, and shorten burnout time.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Guo, Y.F.; Chen, F.; He, Y.; Jiang, T.; Zheng, F.Q. Combustion reaction of pulverized coal on the deposit formation in the kiln for iron ore pellet production. Energy Fuels 2016, 30, 6123–6131. [Google Scholar] [CrossRef]
- Mishra, S.; Roy, G.G. Effect of amount of carbon on the reduction efficiency of iron ore-coal composite pellets in multi-layer bed rotary hearth furnace (RHF). Metall. Mater. Trans. B 2016, 47, 2347–2356. [Google Scholar] [CrossRef]
- Yang, Y.B.; Cui, L.N.; Li, X.S.; Li, Q.; Jiang, T.; Ge, J. Novel technology on preparation of double-layered pellets for sulfur and arsenic-bearing gold concentrates. J. Cent. South Univ. 2013, 20, 2967–2973. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Mendes, Y.; Chun, T.J.; Pan, J.; Li, Q.H.; Li, J.; Qiu, G.Z. Direct reduction behaviors of composite binder magnetite pellets in coal-based grate-rotary kiln process. ISIJ Int. 2011, 51, 214–219. [Google Scholar] [CrossRef]
- Aziz, M.; Budianto, D.; Oda, T. Computational fluid dynamic analysis of co-firing of palm kernel shell and coal. Energies 2016, 9, 137. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Ramona, M.D.; Xiberta, J.; Prieto, I. Thermogravimetric analysis of the co-combustion of coal and sewage sludge. Fuel 2003, 82, 2051–2055. [Google Scholar] [CrossRef]
- Sadrmezhaad, S.K.; Ferdowsi, A.; Payab, H. Mathematical model for a straight grate iron ore pellet induration process of industrial scale. Comp. Mater. Sci. 2008, 44, 296–297. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, Z.Z.; Zhu, M.M.; Cheng, F.Q.; Zhang, D.K. Interactions of coal gangue and pine sawdust during combustion of their blends studied using differential thermogravimetric analysis. Bioresour. Technol. 2016, 214, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Kok, M.V.; Gokalp, I.P. Combustion and gasification studies of different sized coal particles using TGA-MS. Appl. Therm. Eng. 2017, 125, 1446–1455. [Google Scholar] [CrossRef]
- Li, T.; Zhan, P.T.; Lei, M.; Li, Z.Z. Understanding hydrothermal dechlorination of PVC by focusing on the operating conditions and hydrochar characteristics. Appl. Sci. 2017, 7, 256–258. [Google Scholar] [CrossRef]
- Font, R.; Fullana, A.; Conesa, J.A.; Llavador, F. Analysis of the pyrolysis and of diff combustion erent sewage sludges by TG. J. Anal. Appl. Pyrol. 2001, 58, 927–941. [Google Scholar] [CrossRef]
- Chen, W.; Wang, F.; Kanhar, A.H. Sludge acts as a catalyst for coal during the co-combustion process investigated by thermogravimetric analysis. Energies 2017, 10, 1993. [Google Scholar] [CrossRef]
- Holland, T.; Fletcher, T.H. Comprehensive model of single particle pulverized coal combustion extended to oxy-coal conditions. Energy Fuels 2017, 31, 2722–2739. [Google Scholar] [CrossRef]
- Li, F.X.; Li, S.Z.; Chou, Y.Z. Thermal analysis study of the effect of coal-burning additives on the combustion of coals. J. Therm. Anal. Calorim. 2009, 95, 633–638. [Google Scholar]
- Zhong, Q.; Yang, Y.B.; Jiang, T.; Li, Q.; Xu, B. Xylene activation of coal tar pitch binding characteristics for production of metallurgical quality briquettes from coke breeze. Fuel Process. Technol. 2016, 148, 12–18. [Google Scholar] [CrossRef]
- Zhong, Q.; Yang, Y.B.; Li, Q.; Xu, B.; Jiang, T. Coal tar pitch and molasses blended binder for production of formed coal briquettes from high volatile coal. Fuel Process. Technol. 2017, 157, 12–19. [Google Scholar] [CrossRef]
- Zhong, Q.; Yang, Y.B.; Li, Q.; Tao, J. Thermogravimetric analysis of coal used in rotary kiln of iron ore oxide pellet. In 7th International Symposium on High-Temperature Metallurgical Processing; Wiley: Nashville, TN, USA, 2016; pp. 351–358. [Google Scholar]
- Zhong, Q.; Zhang, J.; Yang, Y.B.; Jiang, T.; Li, Q.; Xu, B. Combustion Behavior of Coals in Rotary Kiln and Their Interaction on Co-combustion. Energy Fuels 2018, 32, 3833–3841. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Perez-maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Artemyeva, A.A.; Seames, W.S.; Pierce, D.T.; Kozliak, E.I. Molecular scale studies that inform trace element sulfide evaporation and atomization behavior during coal combustion. Fuel 2017, 188, 544–552. [Google Scholar] [CrossRef]
- Saeed, M.A.; Andrews, G.E.; Phylaktou, H.N.; Gibbs, B.M. Global kinetics of the rate of volatile release from biomasses in comparison to coal. Fuel 2016, 181, 347–357. [Google Scholar] [CrossRef]
- Rubiera, A.; Pevida, F.C.; Pis, J.J. A comparison of different methods for predicting coal devolatilisation kinetics. J. Anal. Appl. Pyrol. 2001, 58, 685–701. [Google Scholar]
- Chandrasekaran, A.; Ramachandran, S.; Subbiah, S. Determination of kinetic parameters in the pyrolysis operation and thermal behavior of Prosopis juliflora using thermogravimetric analysis. Bioresour. Technol. 2017, 233, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Mendez, A.; Fidalgo, J.M.; Guerrero, F.; Gasco, G. Characterization and pyrolysis behaviour of different paper mill waste materials. J. Anal. Appl. Pyrol. 2009, 86, 66–73. [Google Scholar] [CrossRef]
- Liu, Z.J.; Hu, W.H.; Jiang, Z.H.; Mi, B.B.; Fei, B.H. Investigating combustion behaviors of bamboo, torrefied bamboo, coal and their respective blends by thermogravimetric analysis. Renew. Energy 2016, 87, 346–352. [Google Scholar] [CrossRef]
- Dwaraoudi, S.; Devi, T.U.; Rao, S.M.; Ranjan, M. Influence of Pellet Size on Quality and Microstructure of Iron Ore Pellets. ISIJ Int. 2008, 48, 768–769. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrol. 2011, 91, 1–4. [Google Scholar] [CrossRef]
- Wang, M.Y.; Liao, B.; Liu, Y.Q.; Wang, S.B.; Qing, S.; Zhang, A.M. Numerical simulation of oxy-coal combustion in a rotary cement kiln. Appl. Therm. Eng. 2016, 103, 491–500. [Google Scholar] [CrossRef]
- Kim, G.M.; Kim, J.P.; Lisandy, K.Y.; Jeon, C.H. Experimental model development of oxygen-enriched combustion kinetics on porous coal char and non-porous graphite. Energies 2017, 10, 1436. [Google Scholar] [CrossRef]



| Samples | Proximate Analyses (wt. %, d) | Ultimate Analyses (wt. %, d) | Qnet.d (Kcal/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Volatile Matter | Fixed Carbon | Ash | C | H | N | O | S | ||
| A coal | 12.90 | 76.47 | 10.63 | 79.79 | 3.53 | 1.17 | 1.90 | 1.07 | 7794 |
| B coal | 13.15 | 75.41 | 11.44 | 78.54 | 3.47 | 1.12 | 2.34 | 1.06 | 7536 |
| C coal | 12.66 | 79.05 | 8.29 | 80.78 | 3.67 | 1.28 | 3.57 | 0.34 | 7832 |
| Sample | 1st Peak | 2nd Peak | ||
|---|---|---|---|---|
| Temperature (K) | DTGmax (%/min) | Temperature (K) | DTGmax (%/min) | |
| A coal | 307 | 0.54 | 831 | 0.84 |
| B coal | 311 | 0.40 | 798 | 1.27 |
| C coal | 306 | 0.45 | 806 | 1.02 |
| Sample | Eα, KJ/mol | n | A, min−1 | R2 |
|---|---|---|---|---|
| A coal | 127.10 | 1.12 | 4.72 × 107 | 0.99923 |
| B coal | 135.92 | 1.16 | 1.06 × 108 | 0.99835 |
| C coal | 109.54 | 1.14 | 4.24 × 106 | 0.99186 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Q.; Zhang, J.; Yang, Y.; Li, Q.; Xu, B.; Jiang, T. Thermal Behavior of Coal Used in Rotary Kiln and Its Combustion Intensification. Energies 2018, 11, 1055. https://doi.org/10.3390/en11051055
Zhong Q, Zhang J, Yang Y, Li Q, Xu B, Jiang T. Thermal Behavior of Coal Used in Rotary Kiln and Its Combustion Intensification. Energies. 2018; 11(5):1055. https://doi.org/10.3390/en11051055
Chicago/Turabian StyleZhong, Qiang, Jian Zhang, Yongbin Yang, Qian Li, Bin Xu, and Tao Jiang. 2018. "Thermal Behavior of Coal Used in Rotary Kiln and Its Combustion Intensification" Energies 11, no. 5: 1055. https://doi.org/10.3390/en11051055





