Microbial Biodiesel Production by Direct Transesterification of Rhodotorula glutinis Biomass
Abstract
:1. Introduction
2. Results and Discussion
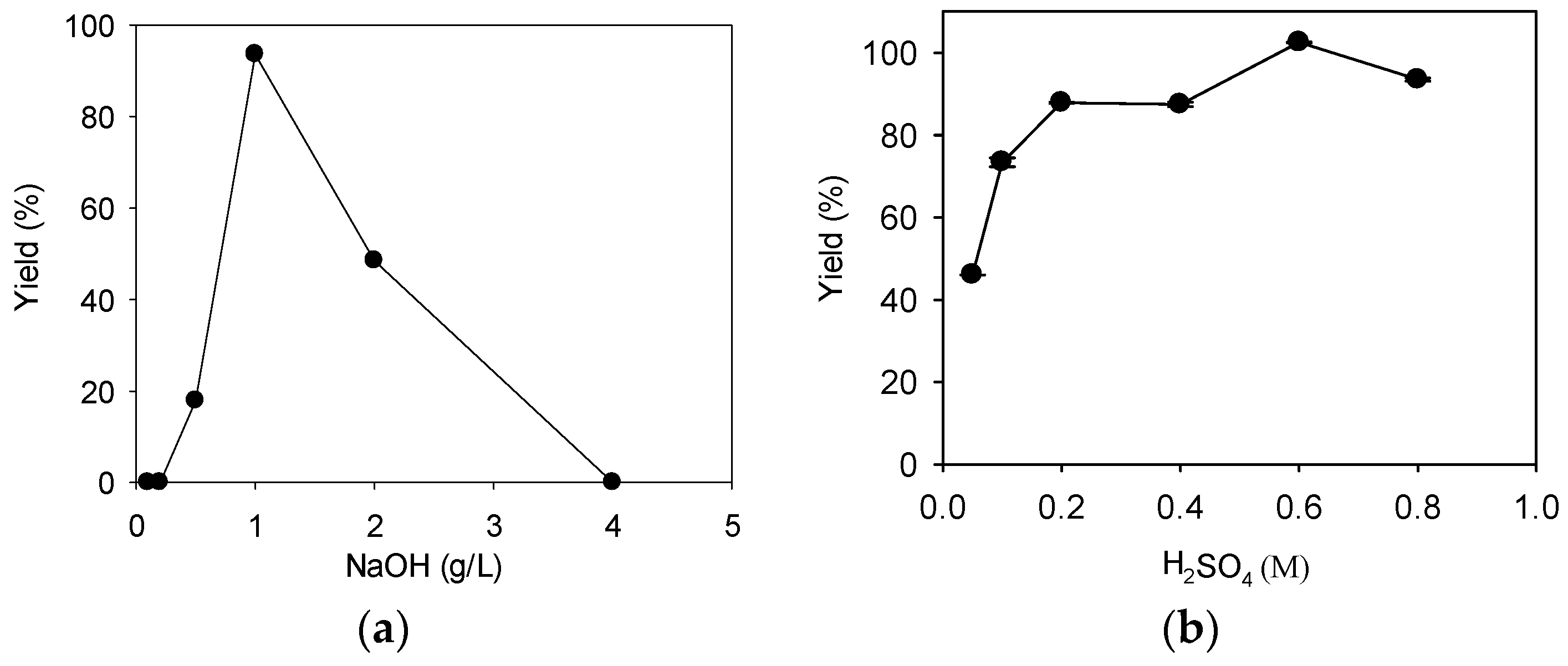
2.1. Effect of Catalyst Concentration on Transesterification
2.2. Effect of Reaction Time and Temperature on Transesterification
2.3. Effect of Methanol Loading on Transesterification
2.4. Effect of Moisture on Transesterification
1 g of biomass)
2.5. Comparison of Yields and Compositions of FAME Derived from Different Transesterification Methods
3. Materials and Methods
3.1. Microorganism and Medium
3.2. Production of Biomass
3.3. Total Lipid Analysis
3.4. Direct Transesterification
3.5. Analysis of Fatty Acid Methyl Esters
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sitepu, I.R.; Garay, L.A.; Sestric, R.; Levin, D.; Block, D.E.; German, J.B.; Boundy-Mills, K.L. Oleaginous yeasts for biodiesel: Current and future trends in biology and production. Biotechnol. Adv. 2014, 32, 1336–1360. [Google Scholar] [CrossRef] [PubMed]
- Canakci, M.; Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Demirbas, A. Importance of biodiesel as transportation fuel. Energy Policy 2007, 35, 4661–4670. [Google Scholar] [CrossRef]
- Escobar, J.C.; Lora, E.S.; Venturini, O.J.; Yáñez, E.E.; Castillo, E.F.; Almazan, O. Biofuels: Environment, technology and food security. Renew. Sustain. Energy Rev. 2009, 13, 1275–1287. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Dalai, A.K. Waste cooking oil-an economical source for biodiesel: A review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.-F.; Xiong, L.; Chen, X.-D.; Ma, L.-L.; Chen, Y. Single cell oil production from low-cost substrates: The possibility and potential of its industrialization. Biotechnol. Adv. 2013, 31, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel production from oleaginous microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, A.; Khan, M.R.; Islam, M.A.; Wahid, Z.A.; Pirozzi, D. Technical difficulties and solutions of direct transesterification process of microbial oil for biodiesel synthesis. Biotechnol. Lett. 2017, 39, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C.; Cohen, Z. Microbial and algal oils: Do they have a future for biodiesel or as commodity oils? Lipid Technol. 2008, 20, 155–160. [Google Scholar] [CrossRef]
- Yen, H.-W.; Zhang, Z. Effects of dissolved oxygen level on cell growth and total lipid accumulation in the cultivation of Rhodotorula glutinis. J. Biosci. Bioeng. 2011, 112, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-W.; Chang, J.-T. Growth of oleaginous Rhodotorula glutinis in an internal-loop airlift bioreactor by using lignocellulosic biomass hydrolysate as the carbon source. J. Biosci. Bioeng. 2015, 119, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula glutinis—Potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [CrossRef] [PubMed]
- Cheirsilp, B.; Louhasakul, Y. Industrial wastes as a promising renewable source for production of microbial lipid and direct transesterification of the lipid into biodiesel. Bioresour. Technol. 2013, 142, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, Z. Biodiesel production by direct methanolysis of oleaginous microbial biomass. J. Chem. Technol. Biotechnol. 2007, 82, 775–780. [Google Scholar] [CrossRef]
- Thliveros, P.; Uçkun Kiran, E.; Webb, C. Microbial biodiesel production by direct methanolysis of oleaginous biomass. Bioresour. Technol. 2014, 157, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.C.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Vicente, G.; Bautista, L.F.; Rodríguez, R.; Gutiérrez, F.J.; Sádaba, I.; Ruiz-Vázquez, R.M.; Torres-Martínez, S.; Garre, V. Biodiesel production from biomass of an oleaginous fungus. Biochem. Eng. J. 2009, 48, 22–27. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Ultrasonication aided in-situ transesterification of microbial lipids to biodiesel. Bioresour. Technol. 2014, 169, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.D.; Gude, V.G.; Mannarswamy, A.; Cooke, P.; Munson-McGee, S.; Nirmalakhandan, N.; Lammers, P.; Deng, S. Optimization of microwave-assisted transesterification of dry algal biomass using response surface methodology. Bioresour. Technol. 2011, 102, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Canakci, M.; Van Gerpen, J. Biodiesel production via acid catalysis. Trans. ASAE 1999, 42, 1203–1210. [Google Scholar] [CrossRef]
- Yellapu, S.K.; Kaur, R.; Tyagi, R.D. Detergent assisted ultrasonication aided in situ transesterification for biodiesel production from oleaginous yeast wet biomass. Bioresour. Technol. 2017, 224, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.-C.; Tao, J.; Xie, F.; Dai, Y.-J.; Zhao, M. Biodiesel generation from oleaginous yeast Rhodotorula glutinis with xylose assimilating capacity. Afr. J. Biotechnol. 2007, 6, 2130–2134. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]



| Method | FAME Yield (%) a | FAME Composition (%) | |||||
|---|---|---|---|---|---|---|---|
| C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | ||
| Direct acid-catalyzed | 111.0 ± 0.1 | 16.8 | 0.7 | 4.1 | 42.2 | 33.8 | 2.5 |
| Direct base-catalyzed | 102.0 ± 0.8 | 18.8 | 0.7 | 4 | 41.1 | 32.9 | 2.6 |
| Conventional acid-catalyzed b | 85.2 ± 0.9 | 18.1 | 1.6 | 3 | 33.3 | 41.1 | 4.1 |
| Conventional base-catalyzed c | 77.9 ± 0.7 | 21 | 1 | 2.5 | 30.5 | 41.2 | 3.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuan, I.-C.; Kao, W.-C.; Chen, C.-L.; Yu, C.-Y. Microbial Biodiesel Production by Direct Transesterification of Rhodotorula glutinis Biomass. Energies 2018, 11, 1036. https://doi.org/10.3390/en11051036
Kuan I-C, Kao W-C, Chen C-L, Yu C-Y. Microbial Biodiesel Production by Direct Transesterification of Rhodotorula glutinis Biomass. Energies. 2018; 11(5):1036. https://doi.org/10.3390/en11051036
Chicago/Turabian StyleKuan, I-Ching, Wei-Chen Kao, Chun-Ling Chen, and Chi-Yang Yu. 2018. "Microbial Biodiesel Production by Direct Transesterification of Rhodotorula glutinis Biomass" Energies 11, no. 5: 1036. https://doi.org/10.3390/en11051036




