Effect of Wall Boundary Layer Thickness on Power Performance of a Recirculation Microbial Fuel Cell
Abstract
:1. Introduction
2. Results and Discussion
2.1. Voltage Output of MFCs
2.2. Internal Resistance of MFCs
2.3. The Mechanism of Hydrodynamic Boundary Layer Effects in Recirculation Mode MFCs
2.4. Applications
3. Materials and Methods
3.1. Reactor Construction
3.2. Inoculation and Operational Conditions
3.3. Electrochemical Analysis
3.4. Reynolds Number (Re)
3.5. Flat Plate Boundary Layer Thickness (δ) and Shear Rate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Repp, S.; Harputlu, E.; Gurgen, S.; Castellano, M.; Kremer, N.; Pompe, N.; Wörner, J.; Hoffmann, A.; Thomann, R.; Emen, F.M. Synergetic effects of Fe3+ doped spinel Li4Ti5O12 nanoparticles on reduced graphene oxide for high surface electrode hybrid supercapacitors. Nanoscale 2018, 10, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.G.; Ocakoglu, K.; Erdem, E. High-capacitance hybrid supercapacitor based on multi-colored fluorescent carbon-dots. Sci. Rep. 2017, 7, 11222. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Chen, W.-J.; Huang, R.-Y. Influence of growth curve phase on electricity performance of microbial fuel cell by escherichia coli. Int. J. Hydrogen Energy 2010, 35, 7217–7223. [Google Scholar] [CrossRef]
- Khan, M.D.; Khan, N.; Sultana, S.; Joshi, R.; Ahmed, S.; Yu, E.; Scott, K.; Ahmad, A.; Khan, M.Z. Bioelectrochemical conversion of waste to energy using microbial fuel cell technology. Process Biochem. 2017, 57, 141–158. [Google Scholar] [CrossRef]
- Singh, H.M.; Pathak, A.K.; Chopra, K.; Tyagi, V.; Anand, S.; Kothari, R. Microbial fuel cells: A sustainable solution for bioelectricity generation and wastewater treatment. Biofuels 2018, 1–21. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Wang, Z.-J.; Zheng, Y.; Xiao, Y.; Yang, Z.-H.; Zhao, F. Light intensity affects the performance of photo microbial fuel cells with Desmodesmus sp. A8 as cathodic microorganism. Appl. Energy 2014, 116, 86–90. [Google Scholar] [CrossRef]
- Wang, C.-T.; Chen, Y.-M.; Qi, Z.-Q.; Wang, Y.-T.; Yang, Y.-C. Types of simplified flow channels without flow obstacles in microbial fuel cells. Int. J. Hydrogen Energy 2014, 39, 14306–14311. [Google Scholar] [CrossRef]
- Yong, X.-Y.; Yan, Z.-Y.; Shen, H.-B.; Zhou, J.; Wu, X.-Y.; Zhang, L.-J.; Zheng, T.; Jiang, M.; Wei, P.; Jia, H.-H. An integrated aerobic-anaerobic strategy for performance enhancement of pseudomonas aeruginosa-inoculated microbial fuel cell. Bioresour. Technol. 2017, 241, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Chouler, J.; Cruz-Izquierdo, Á.; Rengaraj, S.; Scott, J.L.; Di Lorenzo, M. A screen-printed paper microbial fuel cell biosensor for detection of toxic compounds in water. Biosens. Bioelectron. 2018, 102, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Z.; Kingori, G.P.; Si, R.-W.; Zhai, D.-D.; Liao, Z.-H.; Sun, D.-Z.; Zheng, T.; Yong, Y.-C. Microbial fuel cell-based biosensors for environmental monitoring: A review. Water Sci. Technol. 2015, 71, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Lee, Y.-C.; Ou, Y.-T.; Yang, Y.-C.; Chong, W.-T.; Sangeetha, T.; Yan, W.-M. Exposing effect of comb-type cathode electrode on the performance of sediment microbial fuel cells. Appl. Energy 2017, 204, 620–625. [Google Scholar] [CrossRef]
- Liu, X.-W.; Huang, Y.-X.; Sun, X.-F.; Sheng, G.-P.; Zhao, F.; Wang, S.-G.; Yu, H.-Q. Conductive carbon nanotube hydrogel as a bioanode for enhanced microbial electrocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 8158–8164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Q.; Wu, X.-Y.; Yu, Y.-Y.; Sun, D.-Z.; Jia, H.-H.; Yong, Y.-C. Facile in-situ fabrication of graphene/riboflavin electrode for microbial fuel cells. Electrochim. Acta 2017, 232, 439–444. [Google Scholar] [CrossRef]
- Torres, C.I.; Marcus, A.K.; Lee, H.-S.; Parameswaran, P.; Krajmalnik-Brown, R.; Rittmann, B.E. A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiol. Rev. 2009, 34, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, Q.; Tong, M.; Li, S.; Li, H. Electricity generation using membrane-less microbial fuel cell during wastewater treatment. Chin. J. Chem. Eng. 2008, 16, 772–777. [Google Scholar] [CrossRef]
- Feng, C.; Liu, Y.; Li, Q.; Che, Y.; Li, N.; Wang, X. Quaternary ammonium compound in anolyte without functionalization accelerates the startup of bioelectrochemical systems using real wastewater. Electrochim. Acta 2016, 188, 801–808. [Google Scholar] [CrossRef]
- Wang, Z.; Mahadevan, G.D.; Wu, Y.; Zhao, F. Progress of air-breathing cathode in microbial fuel cells. J. Power Sources 2017, 356, 245–255. [Google Scholar] [CrossRef]
- Li, T.; Zhou, L.; Qian, Y.; Wan, L.; Du, Q.; Li, N.; Wang, X. Gravity settling of planktonic bacteria to anodes enhances current production of microbial fuel cells. Appl. Energy 2017, 198, 261–266. [Google Scholar] [CrossRef]
- He, G.; Gu, Y.; He, S.; Schröder, U.; Chen, S.; Hou, H. Effect of fiber diameter on the behavior of biofilm and anodic performance of fiber electrodes in microbial fuel cells. Bioresour. Technol. 2011, 102, 10763–10766. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Pyo, S.; Lee, J.-I.; Park, T.-J.; Gittleson, F.S.; Leung, F.C.; Kim, J.; Taylor, A.D.; Lee, H.-S.; Chae, J. A high power density miniaturized microbial fuel cell having carbon nanotube anodes. J. Power Sources 2015, 273, 823–830. [Google Scholar] [CrossRef]
- Wang, X.; Lin, H.; Wang, J.; Xie, B.; Huang, W. Influence of the biofilm formation process on the properties of biofilm electrode material. Mater. Lett. 2012, 78, 174–176. [Google Scholar] [CrossRef]
- Cabije, A.H.; Agapay, R.C.; Tampus, M.V. Carbon-nitrogen-phosphorus removal and biofilm growth characteristics in an integrated wastewater treatment system involving a rotating biological contactor. Asia-Pac. J. Chem. Eng. 2009, 4, 735–743. [Google Scholar] [CrossRef]
- Oliveira, V.; Simões, M.; Melo, L.; Pinto, A. Overview on the developments of microbial fuel cells. Biochem. Eng. J. 2013, 73, 53–64. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Winfield, J.; Greenman, J. Effects of flow-rate, inoculum and time on the internal resistance of microbial fuel cells. Bioresour. Technol. 2010, 101, 3520–3525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jacobson, K.S.; Torres, P.; He, Z. Effects of anolyte recirculation rates and catholytes on electricity generation in a litre-scale upflow microbial fuel cell. Energy Environ. Sci. 2010, 3, 1347–1352. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Li, J.; Kashima, H.; Liao, Q.; Regan, J.M. Step-feed strategy enhances performance of unbuffered air-cathode microbial fuel cells. RSC Adv. 2017, 7, 33961–33966. [Google Scholar] [CrossRef]
- Pham, H.T.; Boon, N.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Van Oostveldt, P.; Verbeken, K.; Rabaey, K.; Verstraete, W. High shear enrichment improves the performance of the anodophilic microbial consortium in a microbial fuel cell. Microb. Biotechnol. 2008, 1, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Wang, M.; Chang, I.S.; Ng, H.Y. Effect of shear rate on the response of microbial fuel cell toxicity sensor to Cu (II). Bioresour. Technol. 2013, 136, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Celmer, D.; Oleszkiewicz, J.; Cicek, N. Impact of shear force on the biofilm structure and performance of a membrane biofilm reactor for tertiary hydrogen-driven denitrification of municipal wastewater. Water Res. 2008, 42, 3057–3065. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Huang, Y.-S.; Sangeetha, T.; Chen, Y.-M.; Chong, W.-T.; Ong, H.-C.; Zhao, F.; Yan, W.-M. Novel bufferless photosynthetic microbial fuel cell (PMFCs) for enhanced electrochemical performance. Bioresour. Technol. 2018, 255, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Huang, Y.-S.; Sangeetha, T.; Yan, W.-M. Assessment of recirculation batch mode operation in bufferless bio-cathode microbial fuel cells (MFCs). Appl. Energy 2018, 209, 120–126. [Google Scholar] [CrossRef]
- Jang, J.K.; Chang, I.S.; Kang, K.H.; Moon, H.; Cho, K.S.; Kim, B.H. Construction and operation of a novel mediator-and membrane-less microbial fuel cell. Process Biochem. 2004, 39, 1007–1012. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ghangrekar, M.; Shinde, V. Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour. Technol. 2007, 98, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, D.; Liao, Q.; Zhang, P.; Zhu, X.; Li, J.; Fu, Q. Enhanced biofilm distribution and cell performance of microfluidic microbial fuel cells with multiple anolyte inlets. Biosens. Bioelectron. 2016, 79, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Ter Heijne, A.; Schaetzle, O.; Gimenez, S.; Fabregat-Santiago, F.; Bisquert, J.; Strik, D.P.; Barriere, F.; Buisman, C.J.; Hamelers, H.V. Identifying charge and mass transfer resistances of an oxygen reducing biocathode. Energy Environ. Sci. 2011, 4, 5035–5043. [Google Scholar] [CrossRef] [Green Version]
- Ter Heijne, A.; Hamelers, H.V.; Saakes, M.; Buisman, C.J. Performance of non-porous graphite and titanium-based anodes in microbial fuel cells. Electrochim. Acta 2008, 53, 5697–5703. [Google Scholar] [CrossRef]
- Yunus, A.C.; Cimbala, J.M. Fluid Mechanics Fundamentals and Applications, International ed.; McGraw Hill Publication: New York, NY, USA, 2006; pp. 325–326. [Google Scholar]
- D’souza Rohan, V.D.; Rohan, G.; Satish, B. Bioelectricity production from microbial fuel using escherichia coli (glucose and brewery waste). Int. Res. J. Biol. Sci. 2013, 2, 50–54. [Google Scholar]
- Chen, Y.-M.; Wang, C.-T.; Yang, Y.-C.; Chen, W.-J. Application of aluminum-alloy mesh composite carbon cloth for the design of anode/cathode electrodes in escherichia coli microbial fuel cell. Int. J. Hydrogen Energy 2013, 38, 11131–11137. [Google Scholar] [CrossRef]
- Liu, P.; Liang, P.; Jiang, Y.; Hao, W.; Miao, B.; Wang, D.; Huang, X. Stimulated electron transfer inside electroactive biofilm by magnetite for increased performance microbial fuel cell. Appl. Energy 2018, 216, 382–388. [Google Scholar] [CrossRef]
- Zhang, F.; Merrill, M.D.; Tokash, J.C.; Saito, T.; Cheng, S.; Hickner, M.A.; Logan, B.E. Mesh optimization for microbial fuel cell cathodes constructed around stainless steel mesh current collectors. J. Power Sources 2011, 196, 1097–1102. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T. Flow control in microbial fuel cells. In Technology and Application of Microbial Fuel Cells; InTech: Rijeka, Croatia, 2014. [Google Scholar]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Schlichting, H.; Gersten, K. Boundary-Layer Theory, 7th ed.; McGraw-Hill Book Company: Columbus, OH, USA, 1989; p. 140. [Google Scholar]
- Lewandowski, Z.; Walser, G. Influence of Hydrodynamics on Biofilm Accumulation; Environmental Engineering; ASCE: Reston, VA, USA, 1991; pp. 619–624. [Google Scholar]
- Cengel, A. Heat and Mass Transfer A Practical Approach, 3rd ed.; McGraw-Hill Book Company: Columbus, OH, USA, 2006; p. 810. [Google Scholar]
- Bryers, J.D.; Characklis, W.G. Processes governing primary biofilm formation. Biotechnol. Bioeng. 1982, 24, 2451–2476. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Micheli, F. Decreased solar radiation and increased temperature combine to facilitate fouling by marine non-indigenous species. Biofouling 2013, 29, 501–512. [Google Scholar] [CrossRef] [PubMed]
- De Beer, D.; Stoodley, P.; Lewandowski, Z. Liquid flow in heterogeneous biofilms. Biotechnol. Bioeng. 1994, 44, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Yang, Y.; Li, J.; Zhu, X.; Liao, Q.; Deng, B.; Chen, R. Performance of a microfluidic microbial fuel cell based on graphite electrodes. Int. J. Hydrogen Energy 2013, 38, 15710–15715. [Google Scholar] [CrossRef]
- Sleutels, T.H.; Lodder, R.; Hamelers, H.V.; Buisman, C.J. Improved performance of porous bio-anodes in microbial electrolysis cells by enhancing mass and charge transport. Int. J. Hydrogen Energy 2009, 34, 9655–9661. [Google Scholar] [CrossRef]
- Sleutels, T.H.; Hamelers, H.V.; Buisman, C.J. Effect of mass and charge transport speed and direction in porous anodes on microbial electrolysis cell performance. Bioresour. Technol. 2011, 102, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Chen, Y.-M.; Hu, Z.-Y.; Chong, W.-T. Dynamic power response of microbial fuel cells under external electrical exciting. Int. J. Hydrogen Energy 2017, 42, 22208–22213. [Google Scholar] [CrossRef]
- Hutchinson, A.J.; Tokash, J.C.; Logan, B.E. Analysis of carbon fiber brush loading in anodes on startup and performance of microbial fuel cells. J. Power Sources 2011, 196, 9213–9219. [Google Scholar] [CrossRef]
- Van Dyke, M.; Van Dyke, M. An Album of Fluid Motion; Parabolic Press: Stanford, CA, USA, 1982. [Google Scholar]
- Chen, Y.-M.; Wang, C.-T.; Yang, Y.-C.; Wang, Y.-T. Effect of Boudary Layer Thickness on the Performance of Microbial Fuel Cell. In Proceedings of the EMChIE 2015, Tarragona, Spain, 10–12 June 2015. [Google Scholar]
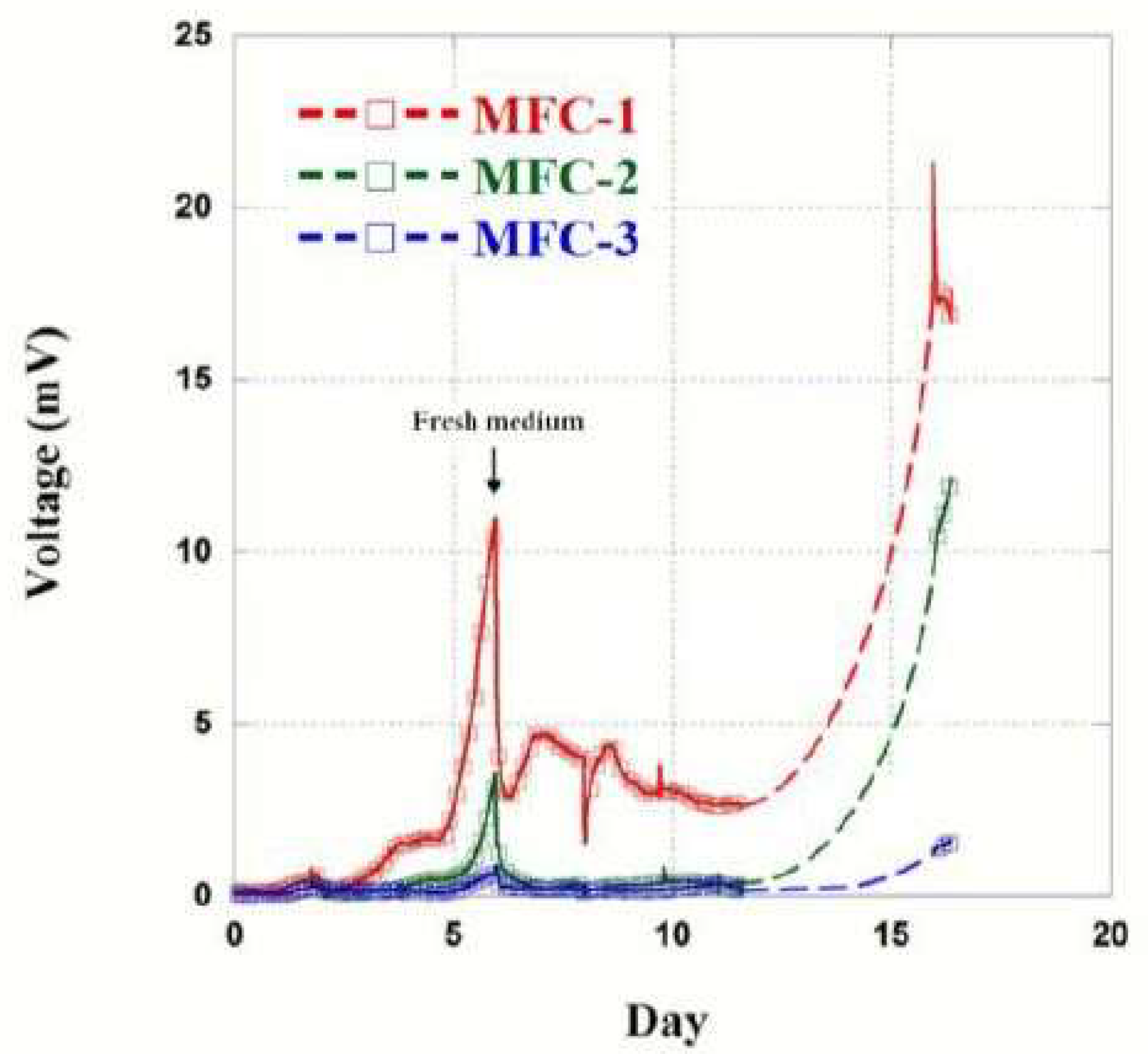
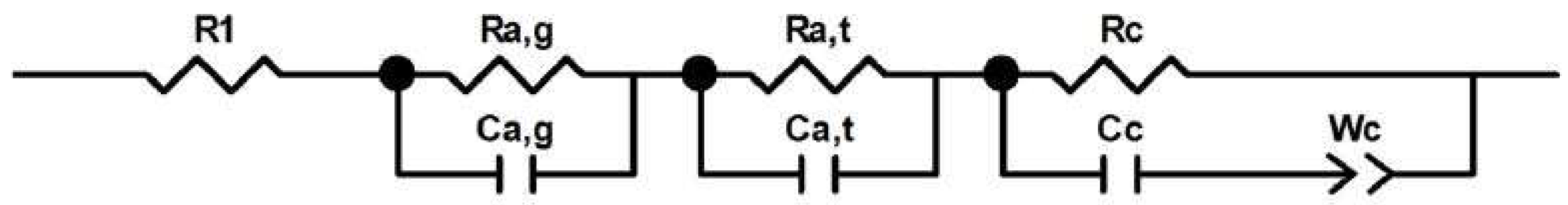
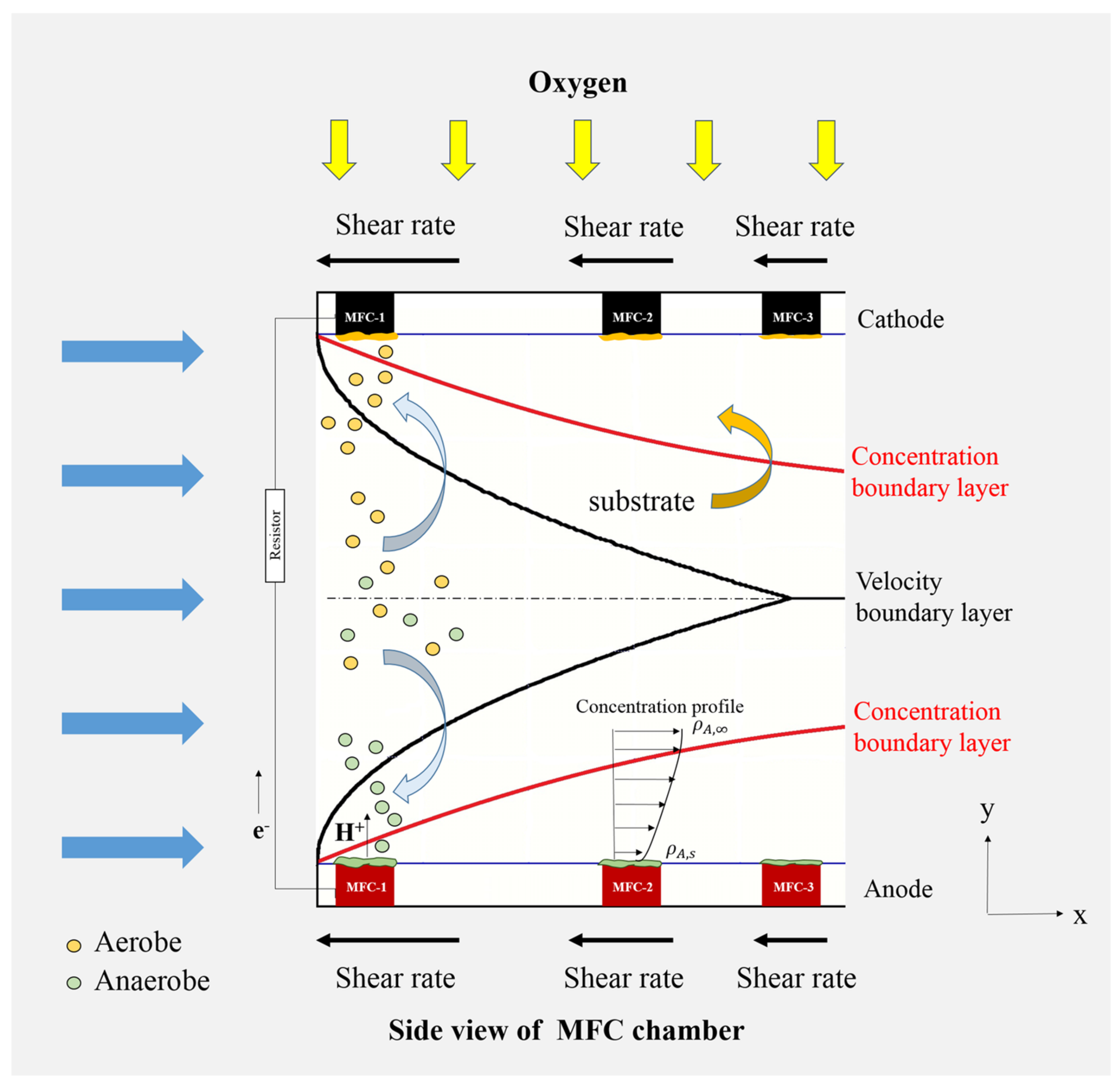

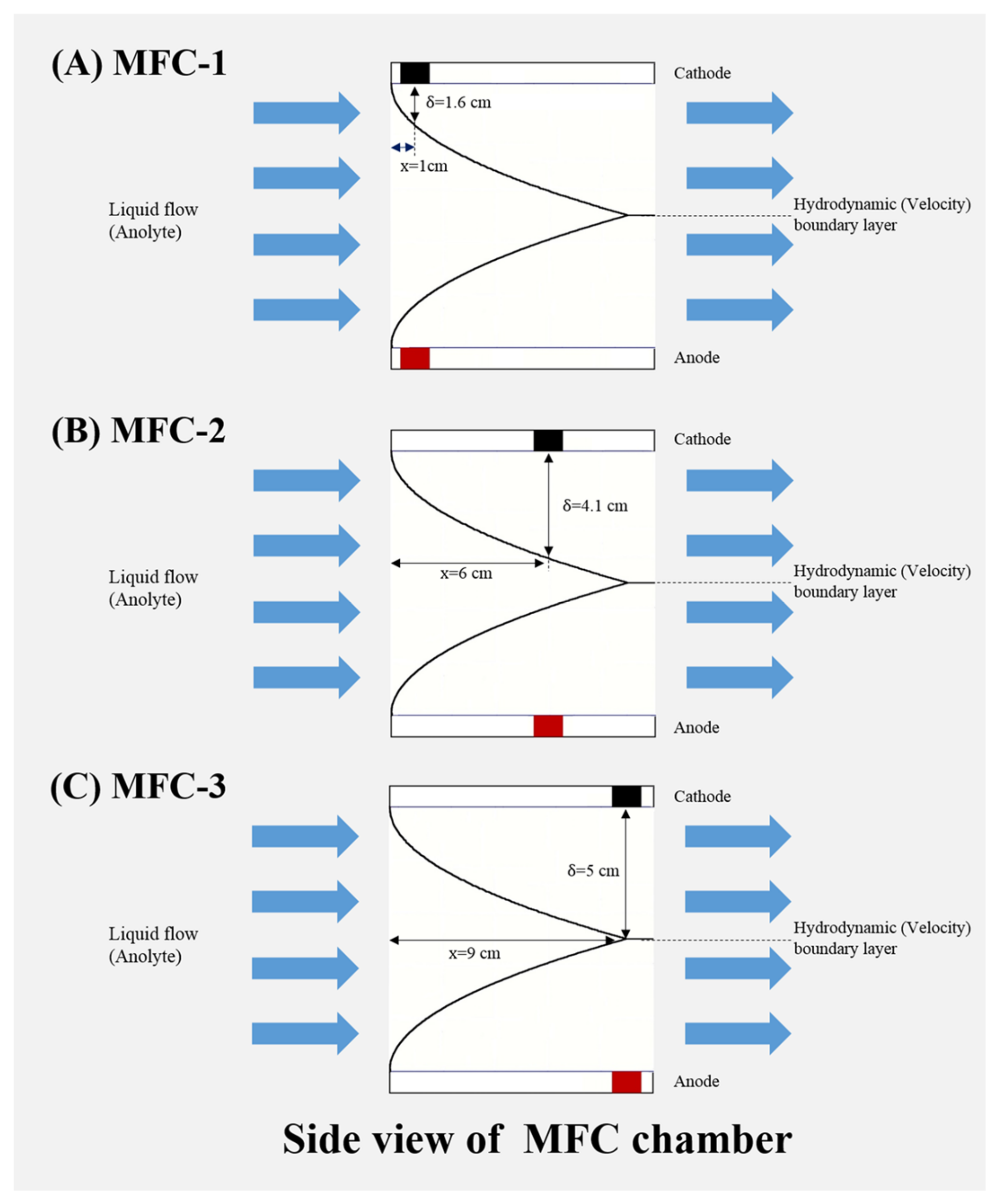
| Kinds | δ (cm) | G (1/S) | R1 (Ω) | Ra,g (Ω) | Ca,g (C) | Ra,t (Ω) | Ca,t (C) | Rc (Ω) | Cc (C) | Wc (C) |
|---|---|---|---|---|---|---|---|---|---|---|
| MFC-1 | 1.6 | 0.047 | 47.4 | 39 | 0.0015 | 12.6 | 1.5 × 10−6 | 15.8 | 1.6 × 10−6 | 0.00086 |
| MFC-2 | 4.1 | 0.02 | 45 | 45 | 0.0013 | 12 | 0.0025 | 3.85 × 10−6 | 0.00098 | 0.00016 |
| MFC-3 | 5 | 0.016 | 40.5 | 49.1 | 0.0018 | 11 | 1.7 × 10−6 | 13.5 | 0.0008 | 0.00085 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-M.; Wang, C.-T.; Yang, Y.-C. Effect of Wall Boundary Layer Thickness on Power Performance of a Recirculation Microbial Fuel Cell. Energies 2018, 11, 1003. https://doi.org/10.3390/en11041003
Chen Y-M, Wang C-T, Yang Y-C. Effect of Wall Boundary Layer Thickness on Power Performance of a Recirculation Microbial Fuel Cell. Energies. 2018; 11(4):1003. https://doi.org/10.3390/en11041003
Chicago/Turabian StyleChen, Yan-Ming, Chin-Tsan Wang, and Yung-Chin Yang. 2018. "Effect of Wall Boundary Layer Thickness on Power Performance of a Recirculation Microbial Fuel Cell" Energies 11, no. 4: 1003. https://doi.org/10.3390/en11041003




