Treatment of Oily Wastewater by the Optimization of Fe2O3 Calcination Temperatures in Innovative Bio-Electron-Fenton Microbial Fuel Cells
Abstract
:1. Introduction
2. Experimental Design
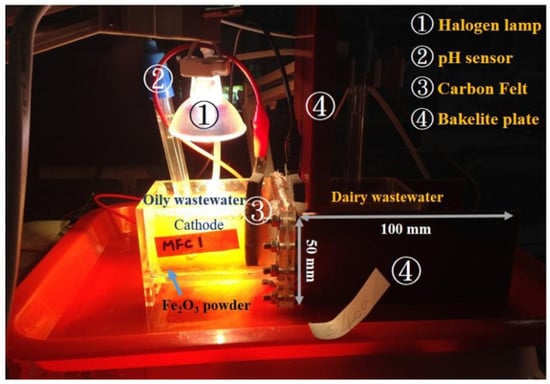
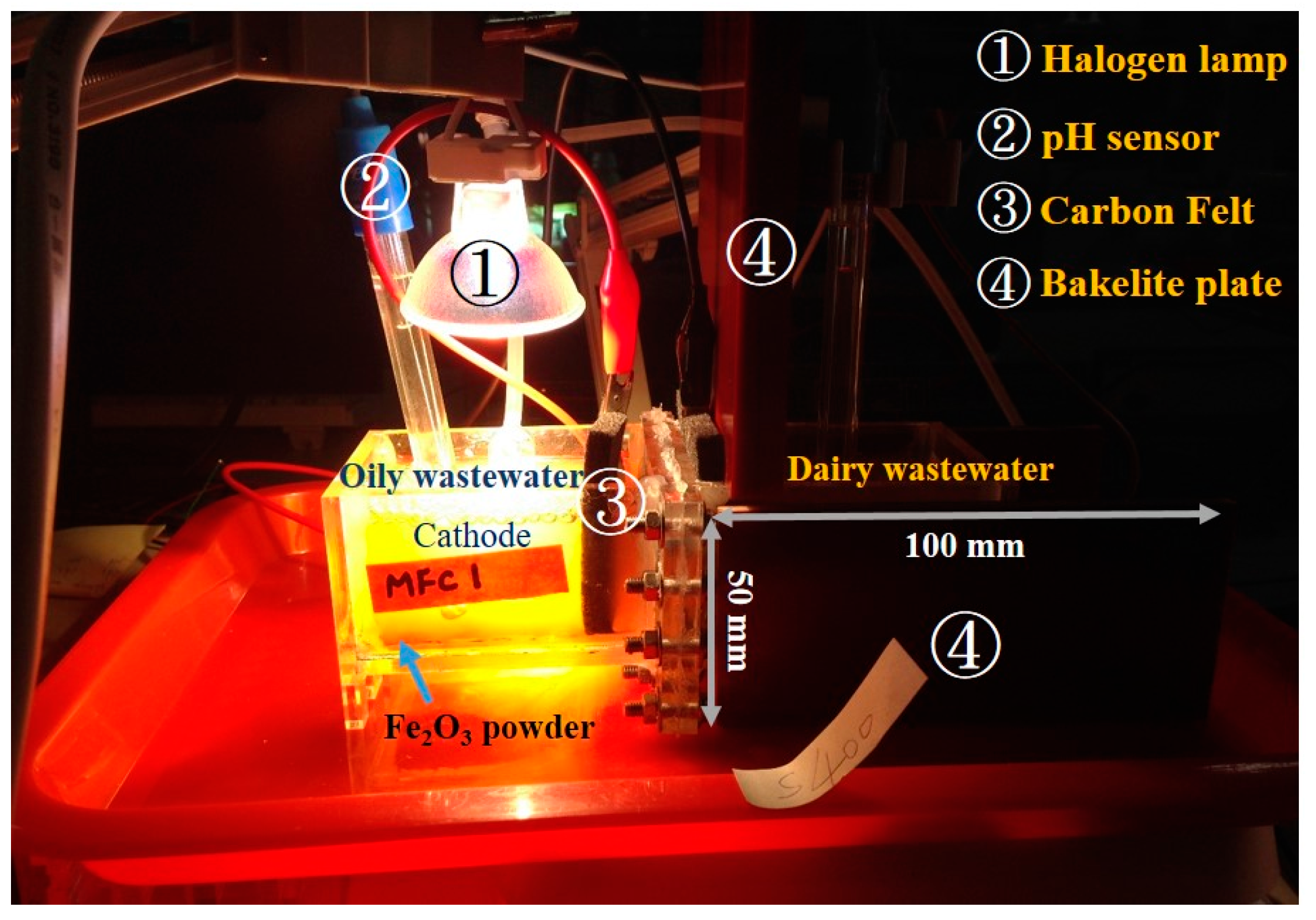
2.1. Reactor Construction and Operation
2.2. Anolyte/Catholyte and Fe2O3 Catalyst Preparation
2.3. Experimental Analysis
3. Results and Discussions
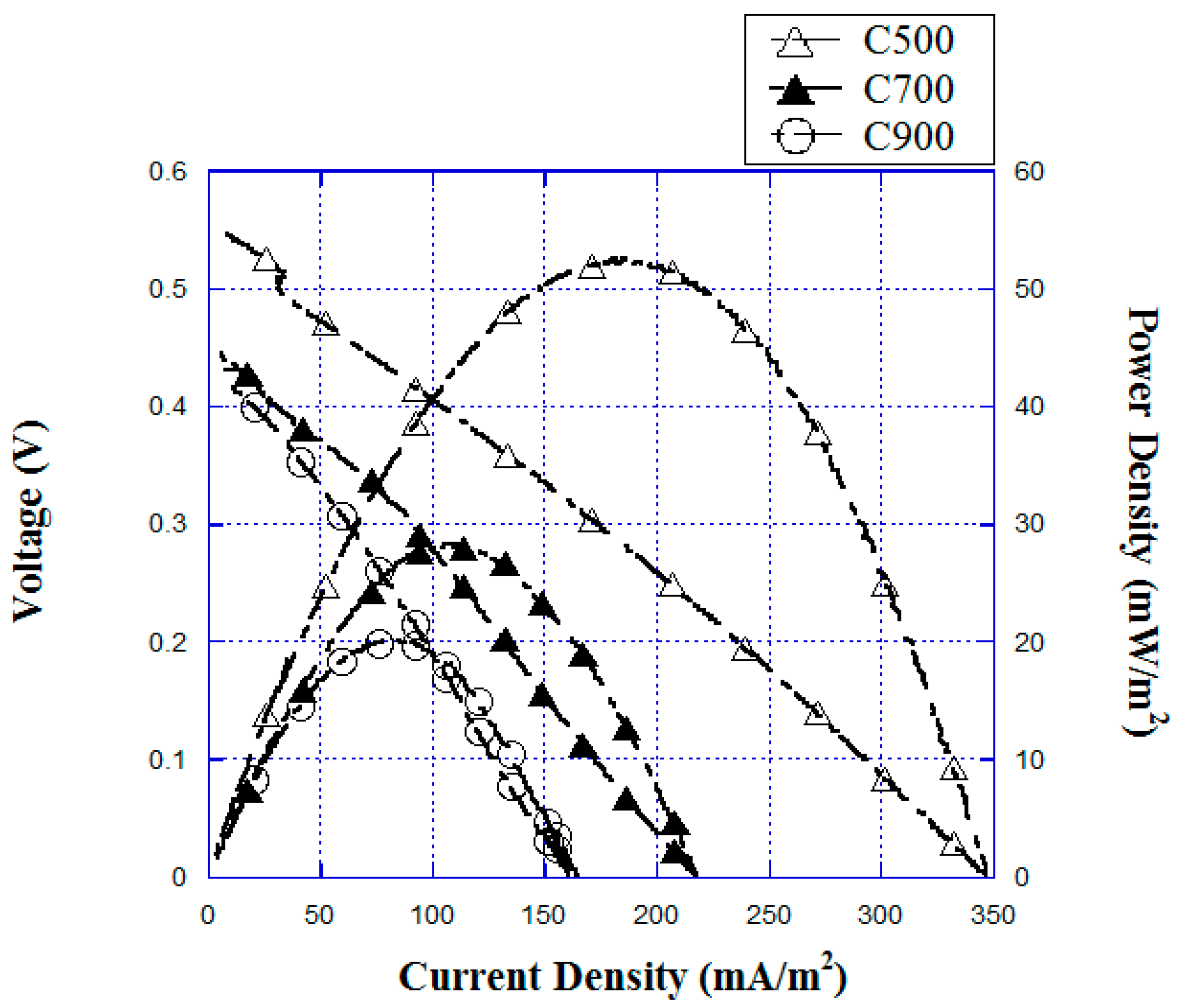
3.1. Performance of the Fe2O3-C500 °C/CF in Bio-E-Fenton MFCs Power Generation
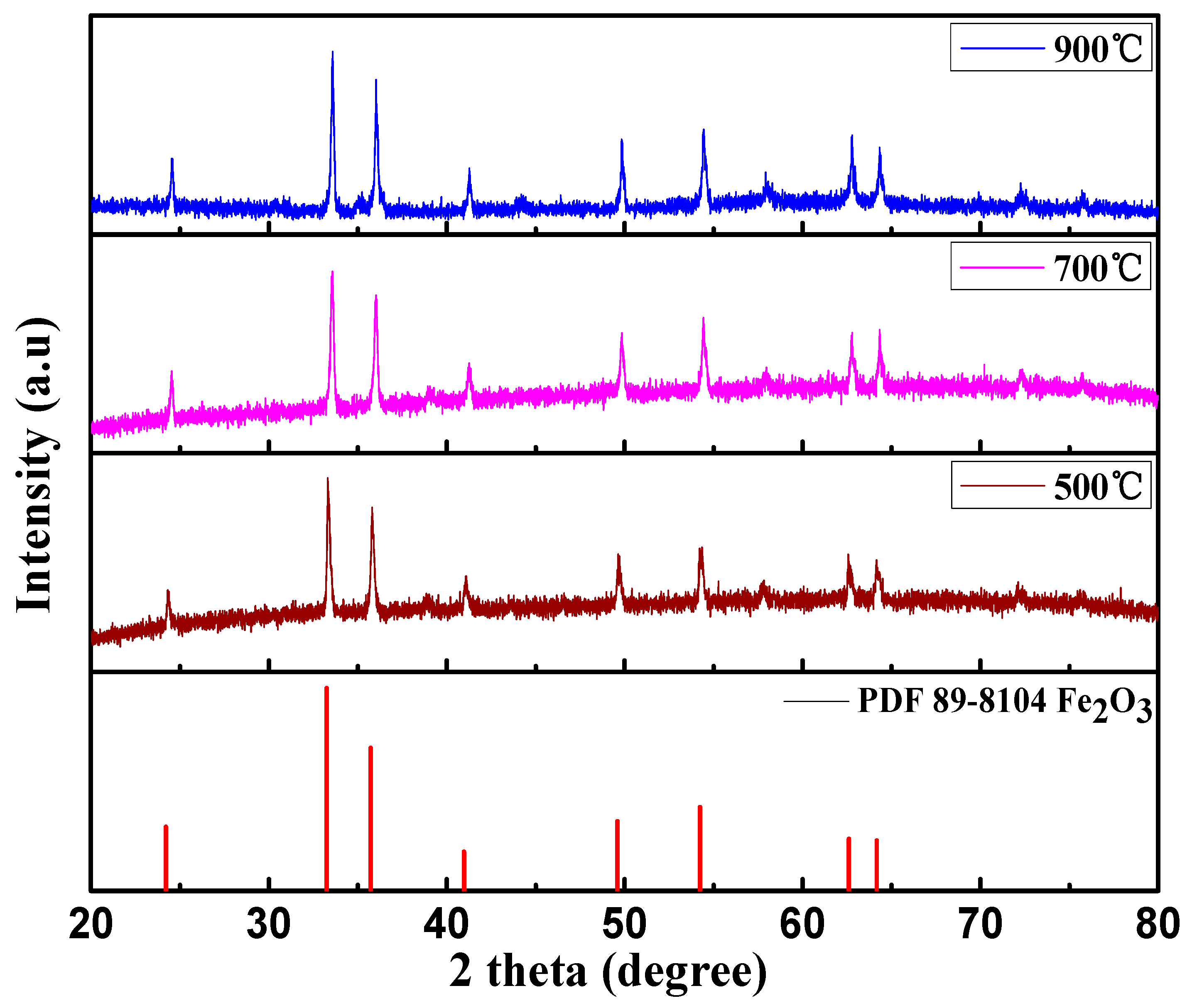
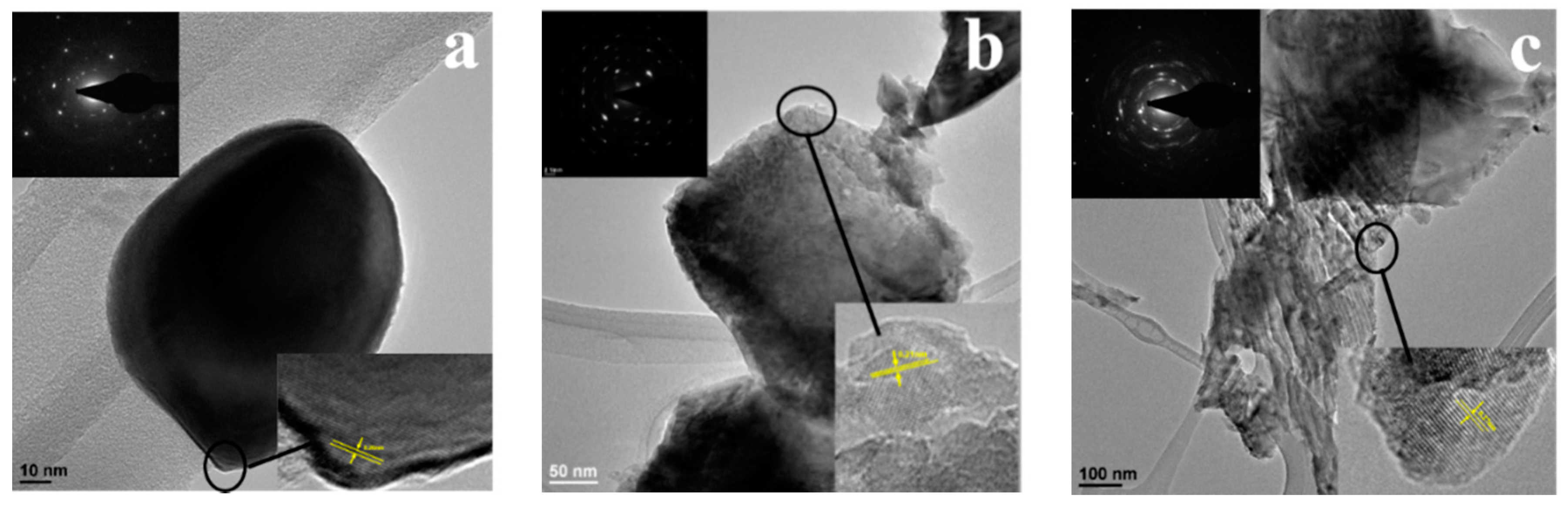
3.2. Characterizations of the Calcinated Fe2O3
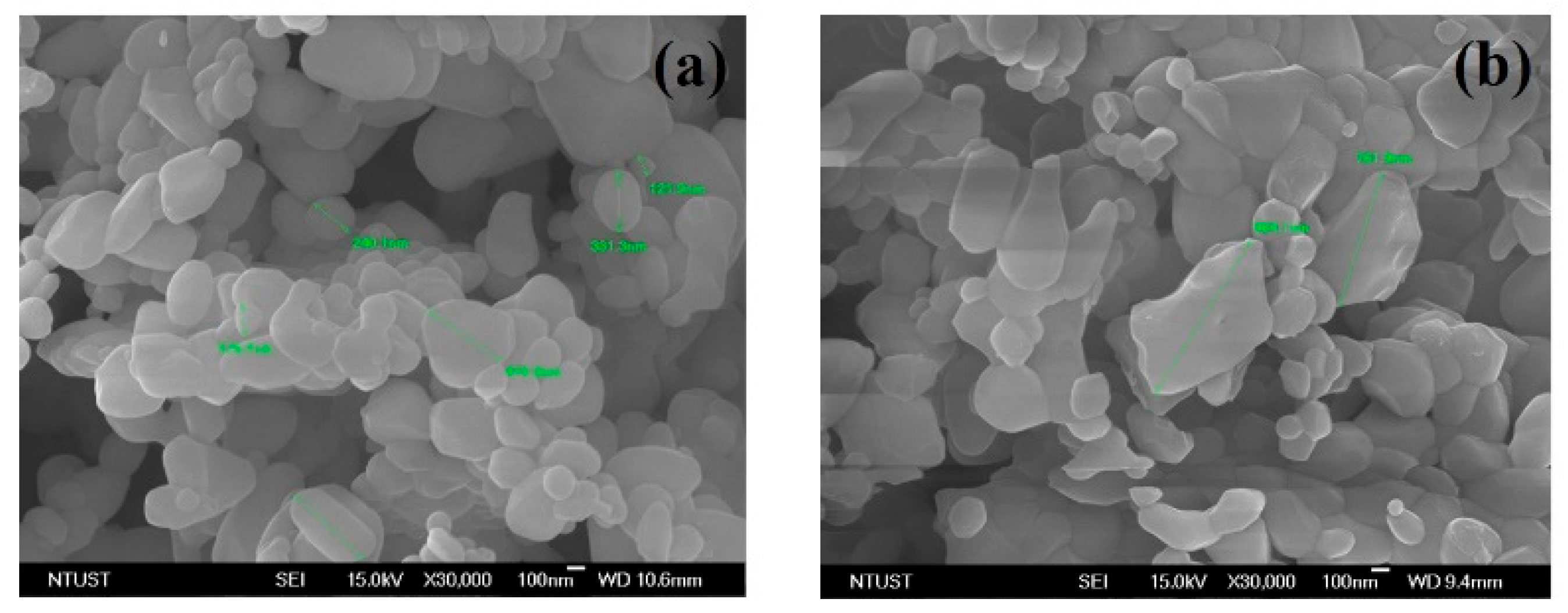
3.3. The Morphologies of the Fe2O3 at Different Calcination Temperatures
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khoufi, S.; Aloui, F.; Sayadi, S. Treatment of olive oil mill wastewater by combined process electro-Fenton reaction and anaerobic digestion. Water Res. 2006, 40, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E.; Casado, J. Aniline degradation by Electro-Fenton® and peroxi-coagulation processes using a flow reactor for wastewater treatment. Chemosphere 2002, 47, 241–248. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, X.; Xuan, X.; Jiang, C.; Cui, H.N. A novel electro-Fenton process for water treatment: Reaction-controlled pH adjustment and performance assessment. Environ. Sci. Technol. 2007, 41, 2937–2942. [Google Scholar] [CrossRef] [PubMed]
- Panizza, M.; Oturan, M.A. Degradation of Alizarin Red by electro-Fenton process using a graphite-felt cathode. Electrochim. Acta 2011, 56, 7084–7087. [Google Scholar] [CrossRef]
- Wang, A.; Qu, J.; Ru, J.; Liu, H.; Ge, J. Mineralization of an azo dye Acid Red 14 by electro-Fenton’s reagent using an activated carbon fiber cathode. Dyes Pigment. 2005, 65, 227–233. [Google Scholar] [CrossRef]
- Luo, M.; Yuan, S.; Tong, M.; Liao, P.; Xie, W.; Xu, X. An integrated catalyst of Pd supported on magnetic Fe3O4 nanoparticles: Simultaneous production of H2O2 and Fe2+ for efficient electro-Fenton degradation of organic contaminants. Water Res. 2014, 48, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Nidheesh, P.; Gandhimathi, R.; Velmathi, S.; Sanjini, N. Magnetite as a heterogeneous electro Fenton catalyst for the removal of Rhodamine B from aqueous solution. RSC Adv. 2014, 4, 5698–5708. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; El-Desoky, H.S.; Zidan, N.M. Electro-Fenton oxidation of Sunset Yellow FCF azo-dye in aqueous solutions. Desalination 2011, 274, 22–30. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Ai, Z.; Mei, T.; Liu, J.; Li, J.; Jia, F.; Zhang, L.; Qiu, J. Fe@Fe2O3 core–shell nanowires as an iron reagent. 3. Their combination with CNTs as an effective oxygen-fed gas diffusion electrode in a neutral electro-Fenton system. J. Phys. Chem. C 2007, 111, 14799–14803. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhou, S.; Li, Y.; Liu, T.; Huang, D. In situ Fenton-enhanced cathodic reaction for sustainable increased electricity generation in microbial fuel cells. J. Power Sources 2010, 195, 1379–1382. [Google Scholar] [CrossRef]
- Li, Y.; Lu, A.; Ding, H.; Wang, X.; Wang, C.; Zeng, C.; Yan, Y. Microbial fuel cells using natural pyrrhotite as the cathodic heterogeneous Fenton catalyst towards the degradation of biorefractory organics in landfill leachate. Electrochem. Commun. 2010, 12, 944–947. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; Li, Y.; Gao, J.; Yu, C.-P. Enhancement of emerging contaminants removal using Fenton reaction driven by H2O2-producing microbial fuel cells. Chem. Eng. J. 2017, 307, 679–686. [Google Scholar] [CrossRef]
- Patil, M.R.; Shrivastava, V.S. Adsorption removal of carcinogenic acid violet19 dye from aqueous solution by polyaniline-Fe2O3 magnetic nano-composite. J. Mater. Environ. Sci 2015, 6, 11–21. [Google Scholar]
- Li, J.; Liu, X.; Pan, L.; Qin, W.; Chen, T.; Sun, Z. MoS2–reduced graphene oxide composites synthesized via a microwave-assisted method for visible-light photocatalytic degradation of methylene blue. RSC Adv. 2014, 4, 9647–9651. [Google Scholar] [CrossRef]
- Xu, N.; Zeng, Y.; Li, J.; Zhang, Y.; Sun, W. Removal of 17β-estrodial in a bio-electro-Fenton system: Contribution of oxidation and generation of hydroxyl radicals with the Fenton reaction and carbon felt cathode. RSC Adv. 2015, 5, 56832–56840. [Google Scholar] [CrossRef]
- Wang, C.-T.; Huang, R.-Y.; Lee, Y.-C.; Zhang, C.-D. Electrode material of carbon nanotube/polyaniline carbon paper applied in microbial fuel cells. J. Clean Energy Technol. 2013, 1, 206–210. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wang, C.-T.; Huang, H.-C.; Chang, S.-T.; Liao, F.-Y. High stability pyrolyzed vitamin B12 as a non-precious metal catalyst of oxygen reduction reaction in microbial fuel cells. RSC Adv. 2013, 3, 15375–15381. [Google Scholar] [CrossRef]
- Xu, N.; Zhou, S.; Yuan, Y.; Qin, H.; Zheng, Y.; Shu, C. Coupling of anodic biooxidation and cathodic bioelectro-Fenton for enhanced swine wastewater treatment. Bioresour. Technol. 2011, 102, 7777–7783. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H.J.H. LXXIII.—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Anotai, J.; Chen, C.-M.; Bellotindos, L.M.; Lu, M.-C. Treatment of TFT-LCD wastewater containing ethanolamine by fluidized-bed Fenton technology. Bioresour. Technol. 2012, 113, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ni, J. Simultaneous processes of electricity generation and p-nitrophenol degradation in a microbial fuel cell. Electrochem. Commun. 2009, 11, 274–277. [Google Scholar] [CrossRef]
- Fay, S.; Kroll, U.; Bucher, C.; Vallat-Sauvain, E.; Shah, A. Low pressure chemical vapour deposition of ZnO layers for thin-film solar cells: Temperature-induced morphological changes. Sol. Energy Mater. Sol. Cells 2005, 86, 385–397. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48, 2858–2860. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Cao, F.; Li, X.; Li, G.; Xie, Y.; Jimmy, C.Y.; Shu, Q.; Fan, Q.; Chen, J. Hydrothermal synthesis and characterization of novel PbWO4 microspheres with hierarchical nanostructures and enhanced photocatalytic performance in dye degradation. Chem. Eng. J. 2013, 219, 86–95. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, F.; Pan, K.; Tian, G.; Jiang, B.; Ren, Z.; Tian, C.; Fu, H. Well-Ordered Large-Pore Mesoporous Anatase TiO2 with Remarkably High Thermal Stability and Improved Crystallinity: Preparation, Characterization, and Photocatalytic Performance. Adv. Funct. Mater. 2011, 21, 1922–1930. [Google Scholar] [CrossRef]
- Xiong, J.; Cheng, G.; Li, G.; Qin, F.; Chen, R. Well-crystallized square-like 2D BiOCl nanoplates: Mannitol-assisted hydrothermal synthesis and improved visible-light-driven photocatalytic performance. RSC Adv. 2011, 1, 1542–1553. [Google Scholar] [CrossRef]
- Feng, C.-H.; Li, F.-B.; Mai, H.-J.; Li, X.-Z. Bio-electro-Fenton process driven by microbial fuel cell for wastewater treatment. Environ. Sci. Technol. 2010, 44, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Oonnittan, A.; Sillanpaa, M.E.T. Water treatment by electro-Fenton process. Curr. Org. Chem. 2012, 16, 2060–2072. [Google Scholar] [CrossRef]
- Kim, H.J.; Hyun, M.S.; Chang, I.S.; Kim, B.H. A microbial fuel cell type lactate biosensor using a metal-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 1999, 9, 365–367. [Google Scholar]
- Diels, A.M.; Callewaert, L.; Wuytack, E.Y.; Masschalck, B.; Michiels, C.W. Inactivation of Escherichia coli by high-pressure homogenisation is influenced by fluid viscosity but not by water activity and product composition. Int. J. Food Microbiol. 2005, 101, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Zou, X.G. Treatment in Water Bodies Pollution by Ecological Floating Bed Technology. China Water Kesources 2005, 5, 022. [Google Scholar]
- Toyoda, M.; Inagaki, M. Heavy oil sorption using exfoliated graphite: New application of exfoliated graphite to protect heavy oil pollution. Carbon 2000, 38, 199–210. [Google Scholar] [CrossRef]
- Blumer, M. Oil pollution of the ocean. In Oil on the Sea; Springer: Berlin, Germany, 1969; pp. 5–13. [Google Scholar]
- Nelson-Smith, A. The problem of oil pollution of the sea. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1971; Volume 8, pp. 215–306. [Google Scholar]
- Molognoni, D.; Chiarolla, S.; Cecconet, D.; Callegari, A.; Capodaglio, A.G. Industrial wastewater treatment with a bioelectrochemical process: Assessment of depuration efficiency and energy production. Water Sci. Technol. 2018, 77, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Marashi, S.; Kariminia, H. Performance of a single chamber microbial fuel cell at different organic loads and pH values using purified terephthalic acid wastewater. J. Environ. Health Sci. Eng. 2015, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Yang, W.; Ye, Y.; LaBarge, N.; Logan, B.E. Performance of anaerobic fluidized membrane bioreactors using effluents of microbial fuel cells treating domestic wastewater. Bioresour. Technol. 2016, 208, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, Y.; Tao, H.; Zhou, S.; Zeng, Y. Bio-electro-Fenton system for enhanced estrogens degradation. Bioresour. Technol. 2013, 138, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, G.; Ghangrekar, M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 2009, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Adani, F.; Baido, D.; Calcaterra, E.; Genevini, P. The influence of biomass temperature on biostabilization–biodrying of municipal solid waste. Bioresour. Technol. 2002, 83, 173–179. [Google Scholar] [CrossRef]
- Liang, C.; Das, K.; McClendon, R. The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend. Bioresour. Technol. 2003, 86, 131–137. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zeng, L.; Cui, D.; Xiang, X.; Li, W. Polyaniline/mesoporous tungsten trioxide composite as anode electrocatalyst for high-performance microbial fuel cells. Biosens. Bioelectron. 2013, 41, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, Y.; Xiao, Y.; Wu, S.; Wu, Y.; Yang, Z.; Zhao, F. Analysis of oxygen reduction and microbial community of air-diffusion biocathode in microbial fuel cells. Bioresour. Technol. 2013, 144, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Smejkal, Q.; Linke, D.; Bentrup, U.; Pohl, M.-M.; Berndt, H.; Baerns, M.; Brückner, A. Combining accelerated activity tests and catalyst characterization: A time-saving way to study the deactivation of vinylacetate catalysts. Appl. Catal. A Gen. 2004, 268, 67–76. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Rezaei, F.; Richard, T.L.; Logan, B.E. Analysis of chitin particle size on maximum power generation, power longevity, and Coulombic efficiency in solid–substrate microbial fuel cells. J. Power Sources 2009, 192, 304–309. [Google Scholar] [CrossRef]
- Xiao, L.; Damien, J.; Luo, J.; Jang, H.D.; Huang, J.; He, Z. Crumpled graphene particles for microbial fuel cell electrodes. J. Power Sources 2012, 208, 187–192. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, S.; Schaller, R.; Jiao, J.; Chaplen, F.; Liu, H. Nanoparticle decorated anodes for enhanced current generation in microbial electrochemical cells. Biosens. Bioelectron. 2011, 26, 1908–1912. [Google Scholar] [CrossRef] [PubMed]


| Power Density (mW/m2) | COD Removal (%/h) | Ref. |
|---|---|---|
| 430 | 78/1080 | [13] |
| 625 | 88/48 | [14] |
| 52.5 | 99.3/1 | This study |
| Material | Wavelength (nm) | COD Removal (%/min) | pH | Ref. |
|---|---|---|---|---|
| Fe@Fe2O3/CNT | 555 | 91.5/120 | 8 | [11] |
| PANI-Fe2O3 | 545 | 98.5/90 | 8 | [15] |
| MoS2-RGO | 450 | 99.0/60 | 7 | [16] |
| Fe2O3-C500 °C/CF | 410 | 99.3/60 | 3 | This study |
| Parameter | 500 °C | 700 °C | 900 °C | BF [30] |
|---|---|---|---|---|
| COD removal (%) @cathode | 99.3 | 83.2 | 75.2 | 74.0 |
| DO (ppm) @cathode | 4.13 | 2.82 | 3.59 | 2.44 |
| pHaverage | 2.1 | 1.8 | 2.4 | 3.0 |
| PDmax (mW/m2) | 52.5 | 28.1 | 20.1 | 26.0 |
| Temp. average/°C anode | 34.6 | 43.7 | 42.7 | 22.6 |
| Temp. average/°C cathode | 71.6 | 74.9 | 72.7 | 24.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-C.; Yan, W.-M.; Wang, C.-T.; Wang, C.-H.; Pai, Y.-H.; Wang, K.-C.; Chen, Y.-M.; Lan, T.-H.; Thangavel, S. Treatment of Oily Wastewater by the Optimization of Fe2O3 Calcination Temperatures in Innovative Bio-Electron-Fenton Microbial Fuel Cells. Energies 2018, 11, 565. https://doi.org/10.3390/en11030565
Wu J-C, Yan W-M, Wang C-T, Wang C-H, Pai Y-H, Wang K-C, Chen Y-M, Lan T-H, Thangavel S. Treatment of Oily Wastewater by the Optimization of Fe2O3 Calcination Temperatures in Innovative Bio-Electron-Fenton Microbial Fuel Cells. Energies. 2018; 11(3):565. https://doi.org/10.3390/en11030565
Chicago/Turabian StyleWu, Jung-Chen, Wei-Mon Yan, Chin-Tsan Wang, Chen-Hao Wang, Yi-Hao Pai, Kai-Chin Wang, Yan-Ming Chen, Tzu-Hsuan Lan, and Sangeetha Thangavel. 2018. "Treatment of Oily Wastewater by the Optimization of Fe2O3 Calcination Temperatures in Innovative Bio-Electron-Fenton Microbial Fuel Cells" Energies 11, no. 3: 565. https://doi.org/10.3390/en11030565