Investigation on the Potential of High Efficiency for Internal Combustion Engines
Abstract
:1. Introduction
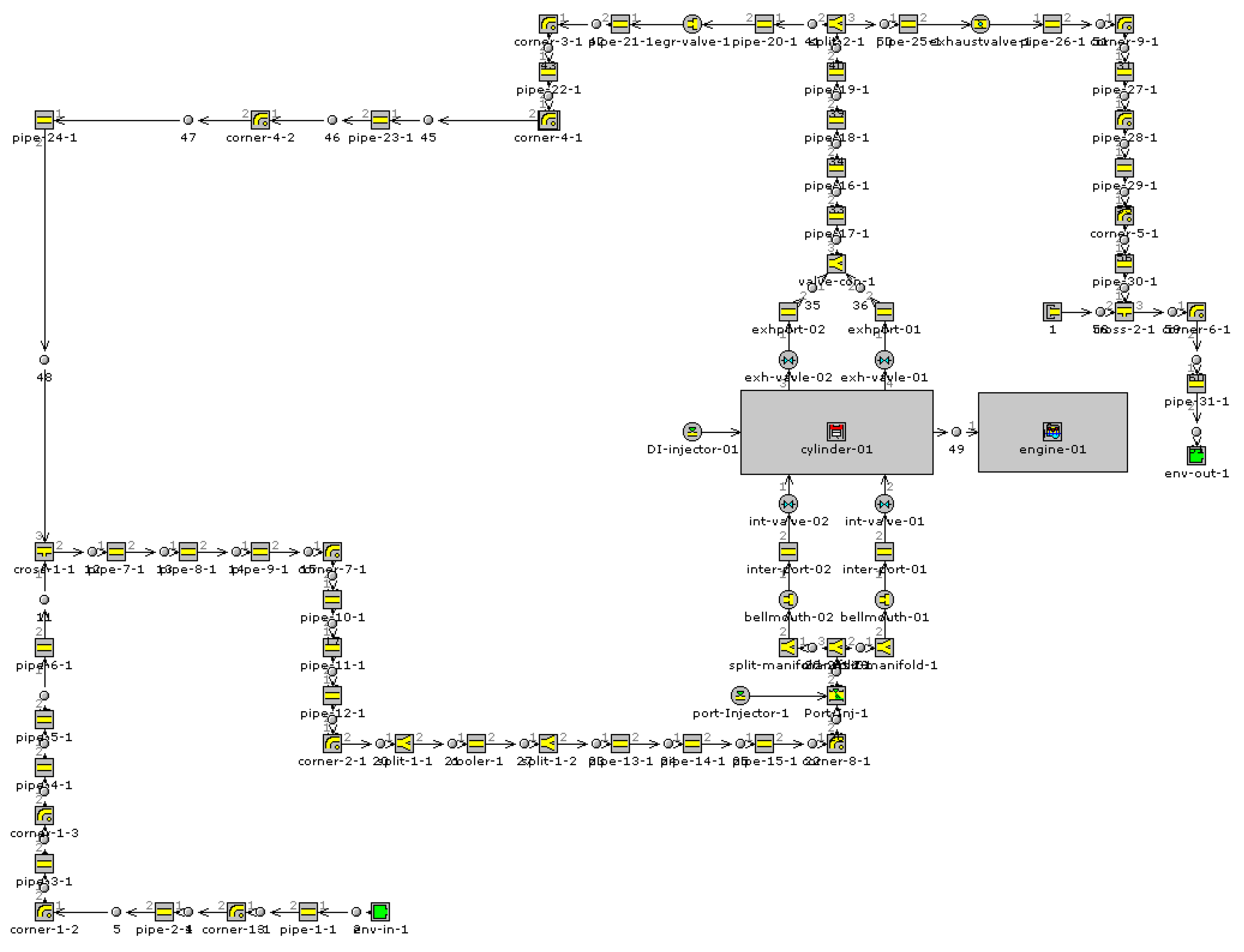
2. System Description and Thermodynamic Model
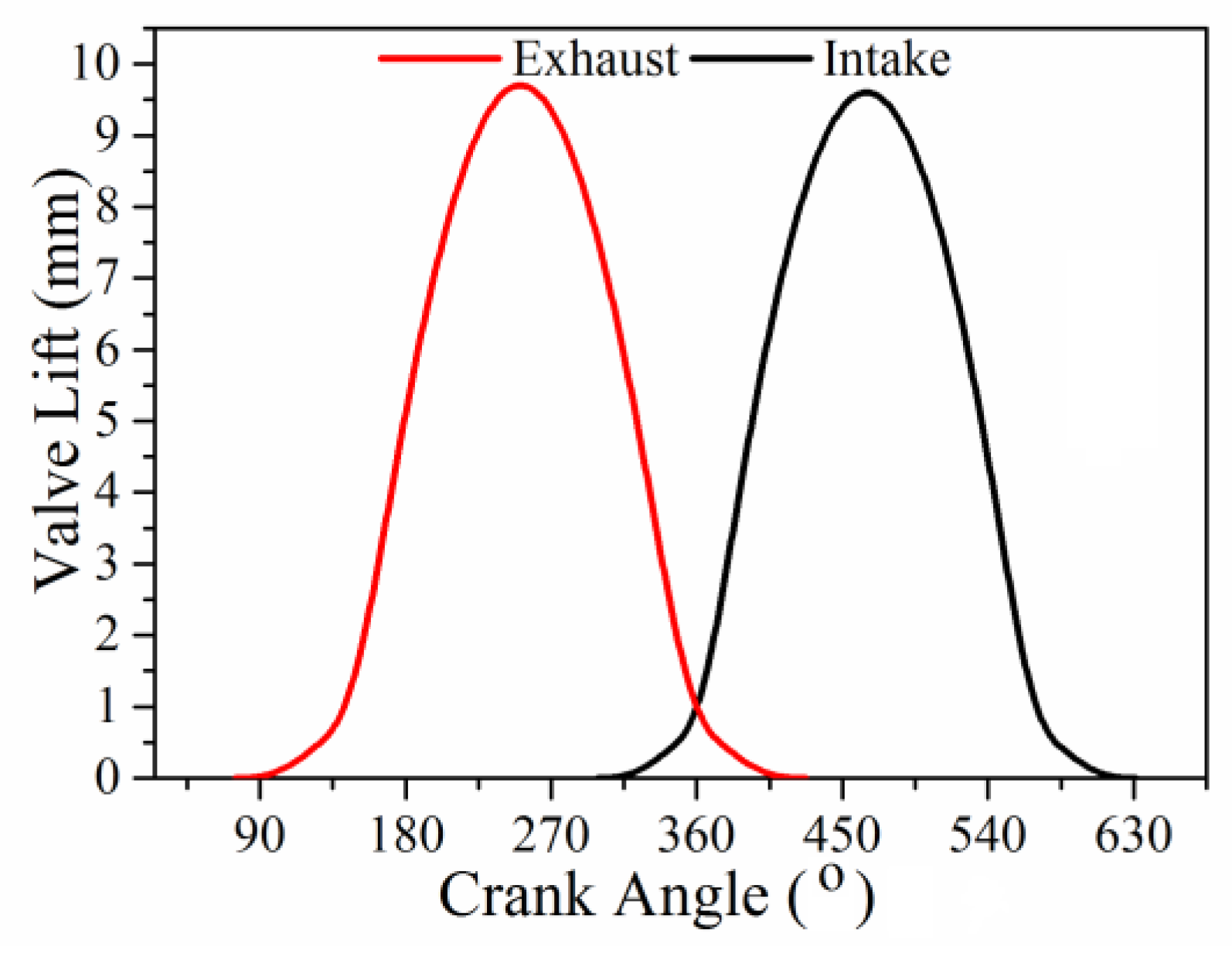
3. Engine and Operating Conditions
4. Results and discussion
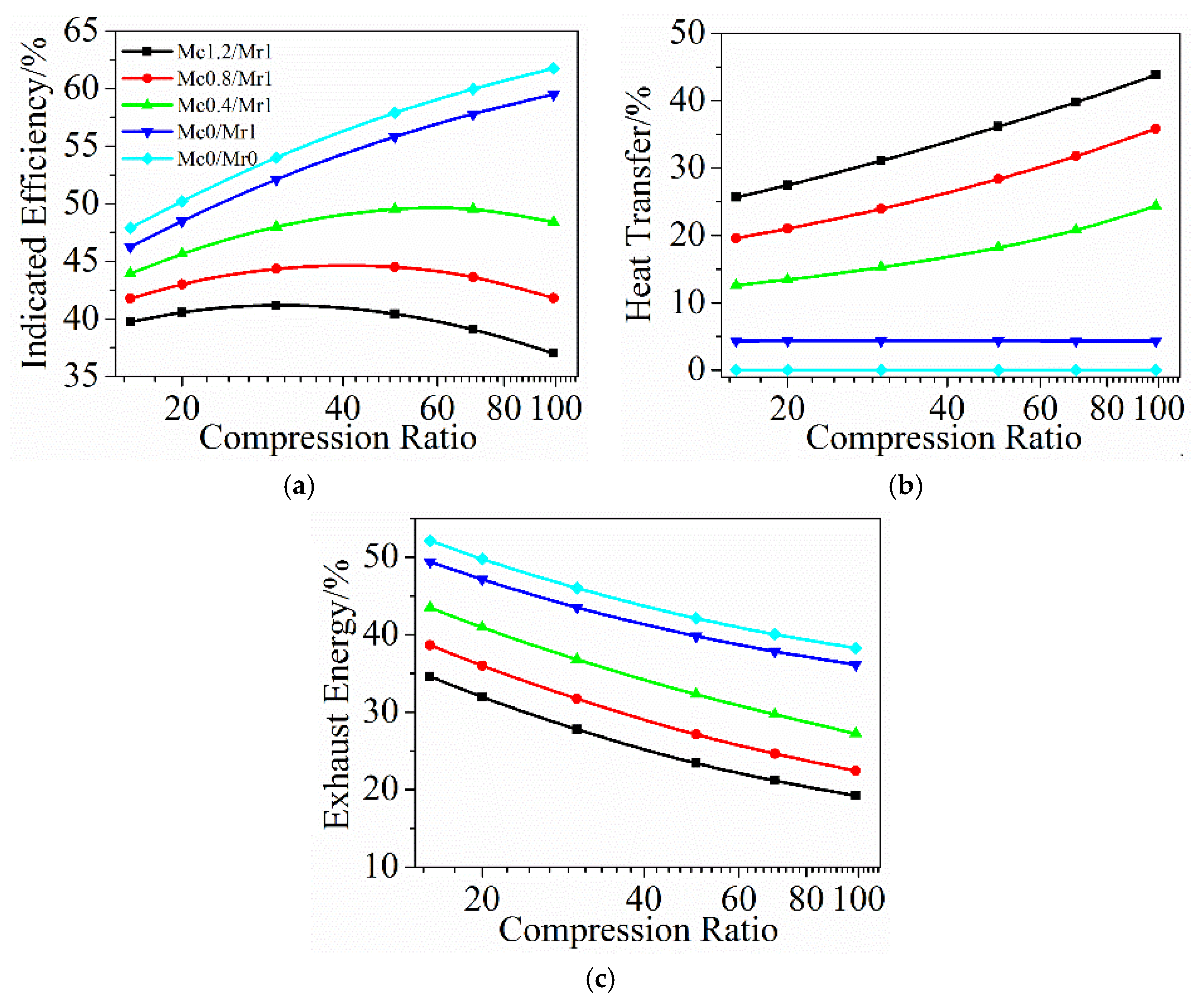
4.1. The Effects of Heat Transfer Coefficient and Compression Ratio
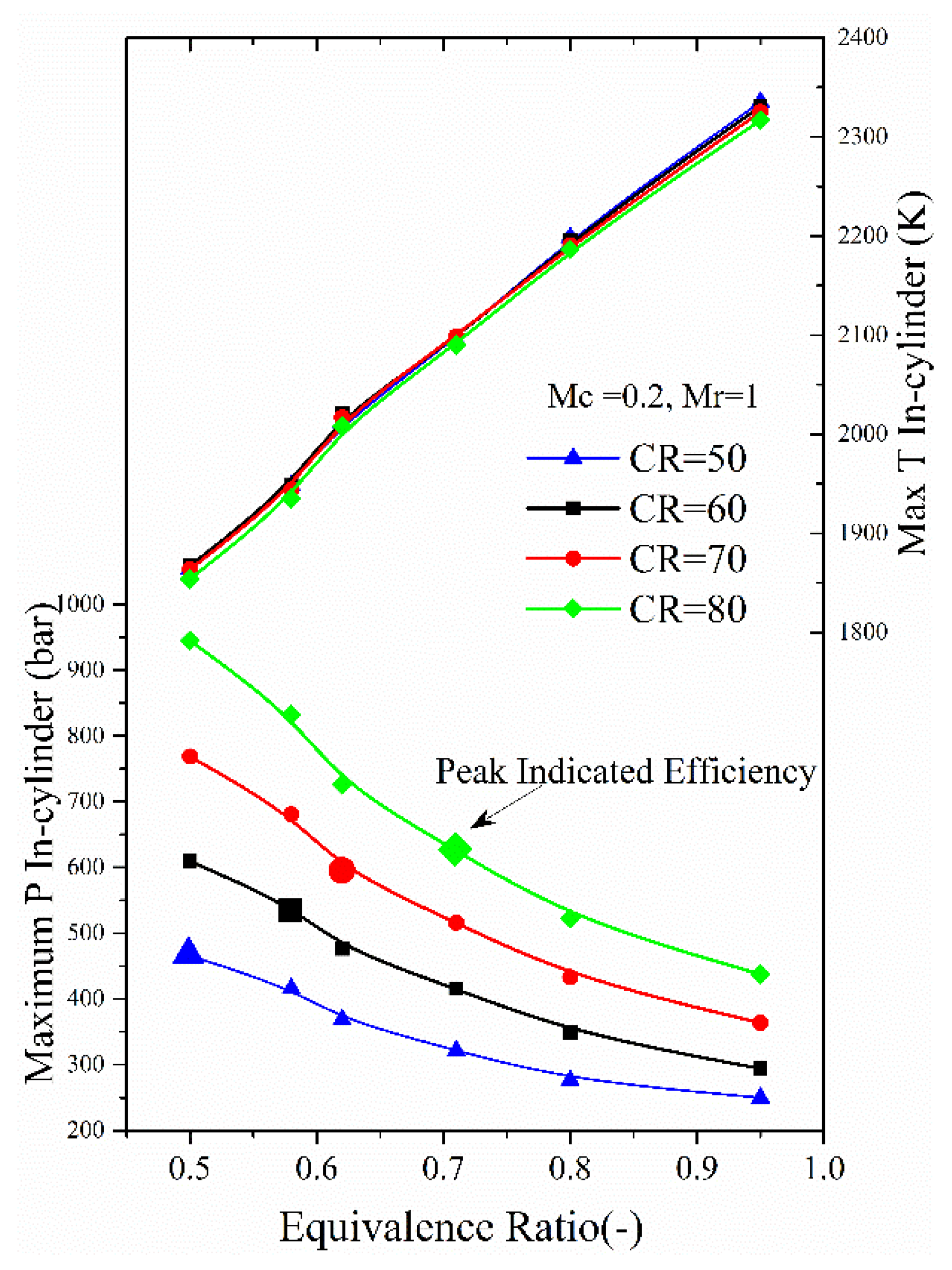
4.2. The Effects of Equivalence Ratios
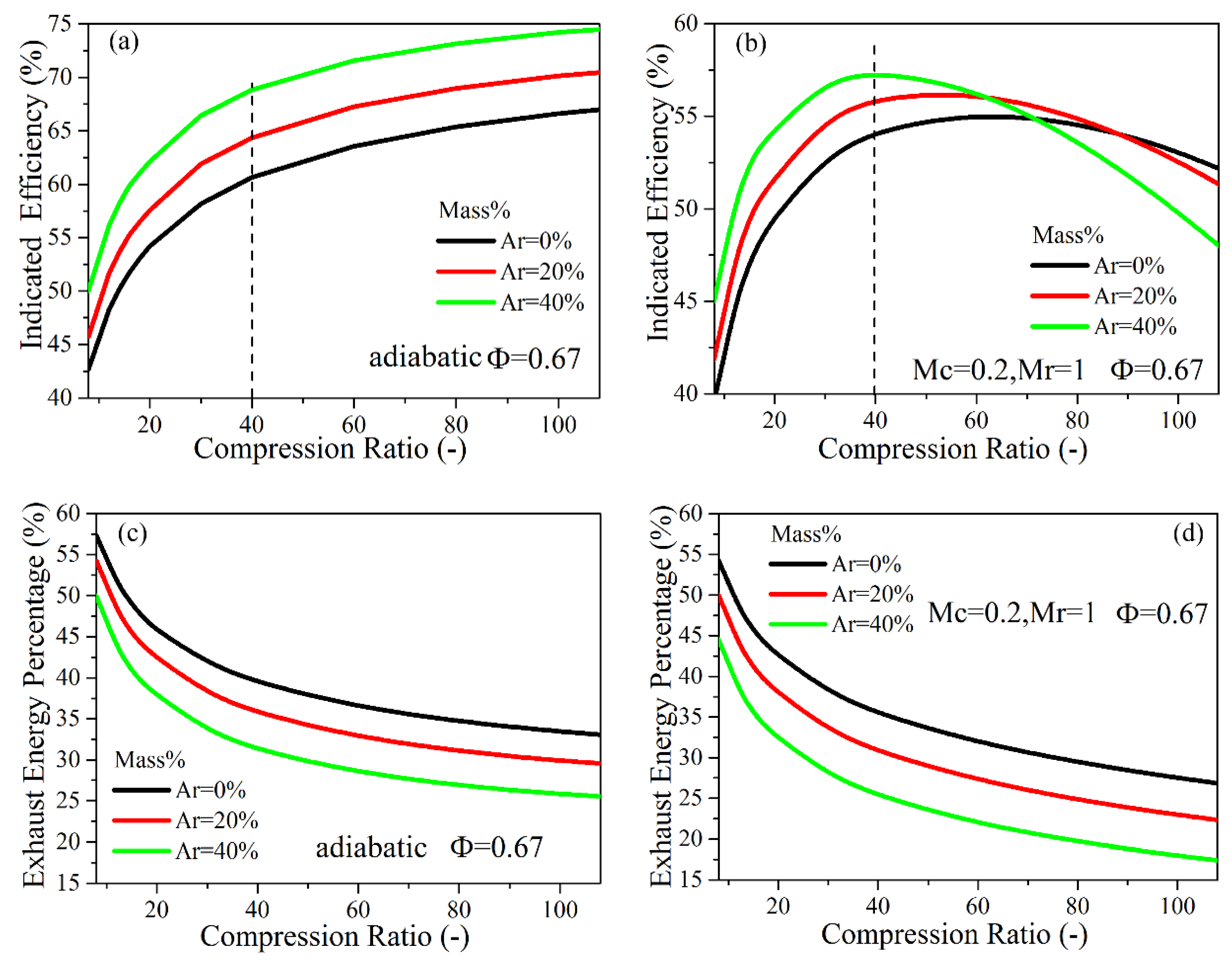
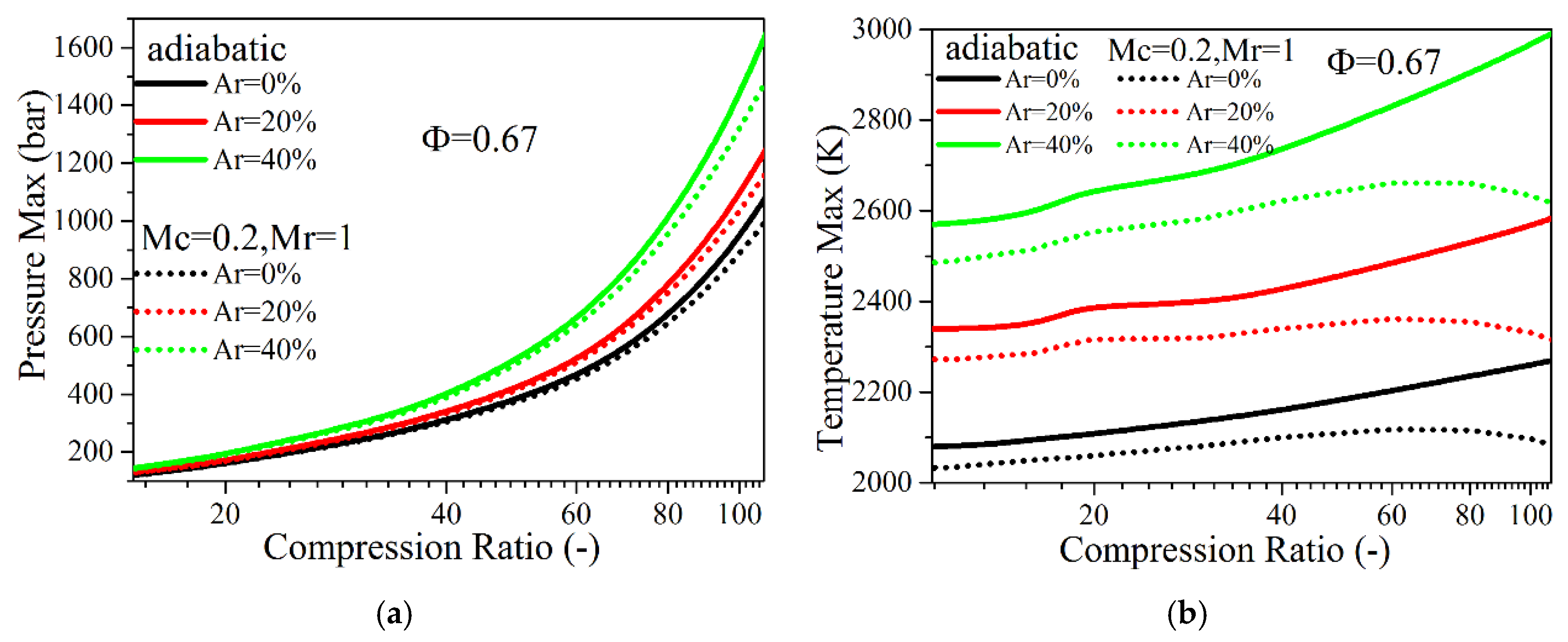
4.3. The Effects of Intake Charge Composition
4.3.1. Intake N2 Replacement by Argon
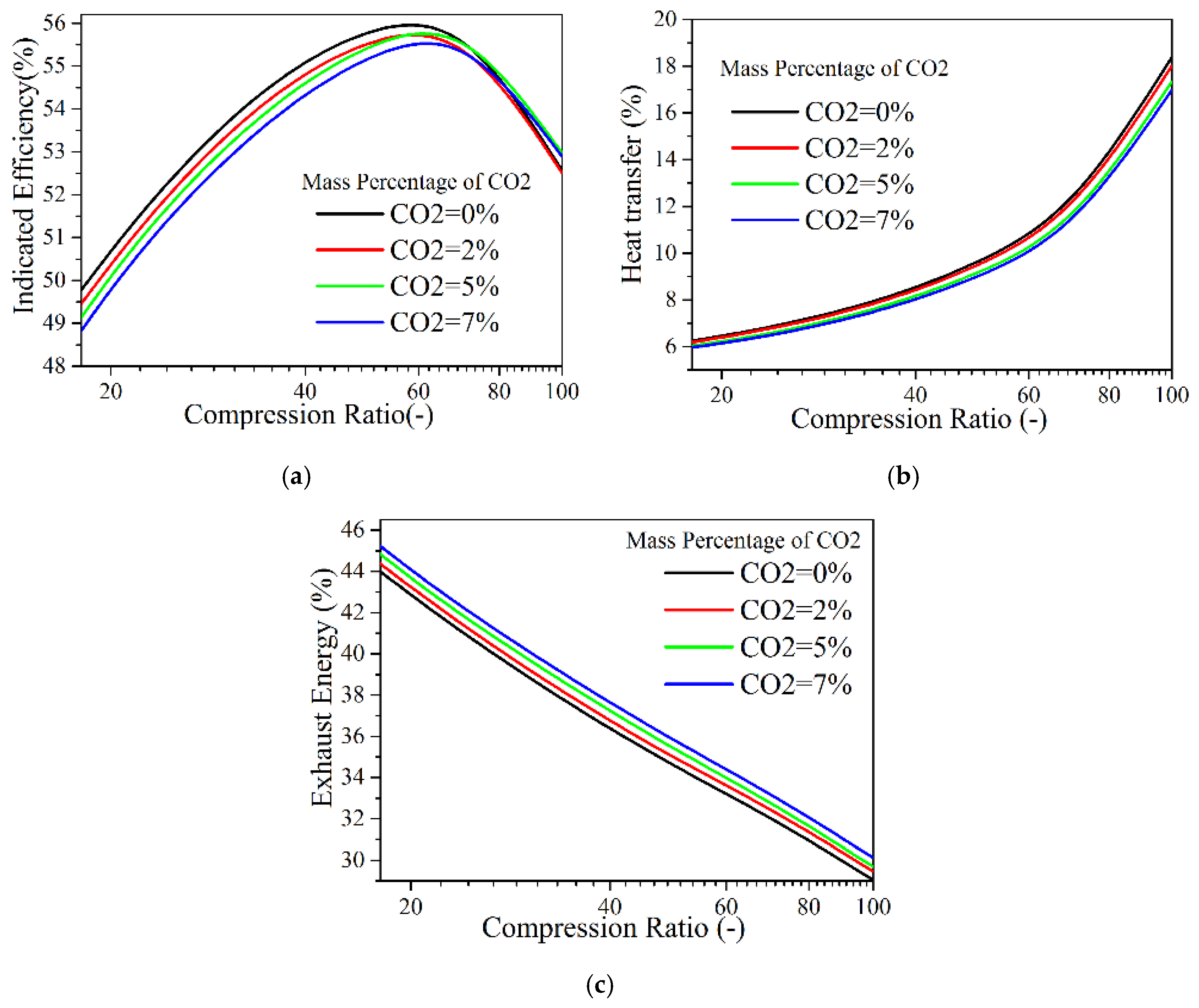
4.3.2. Intake N2 Replacement by CO2
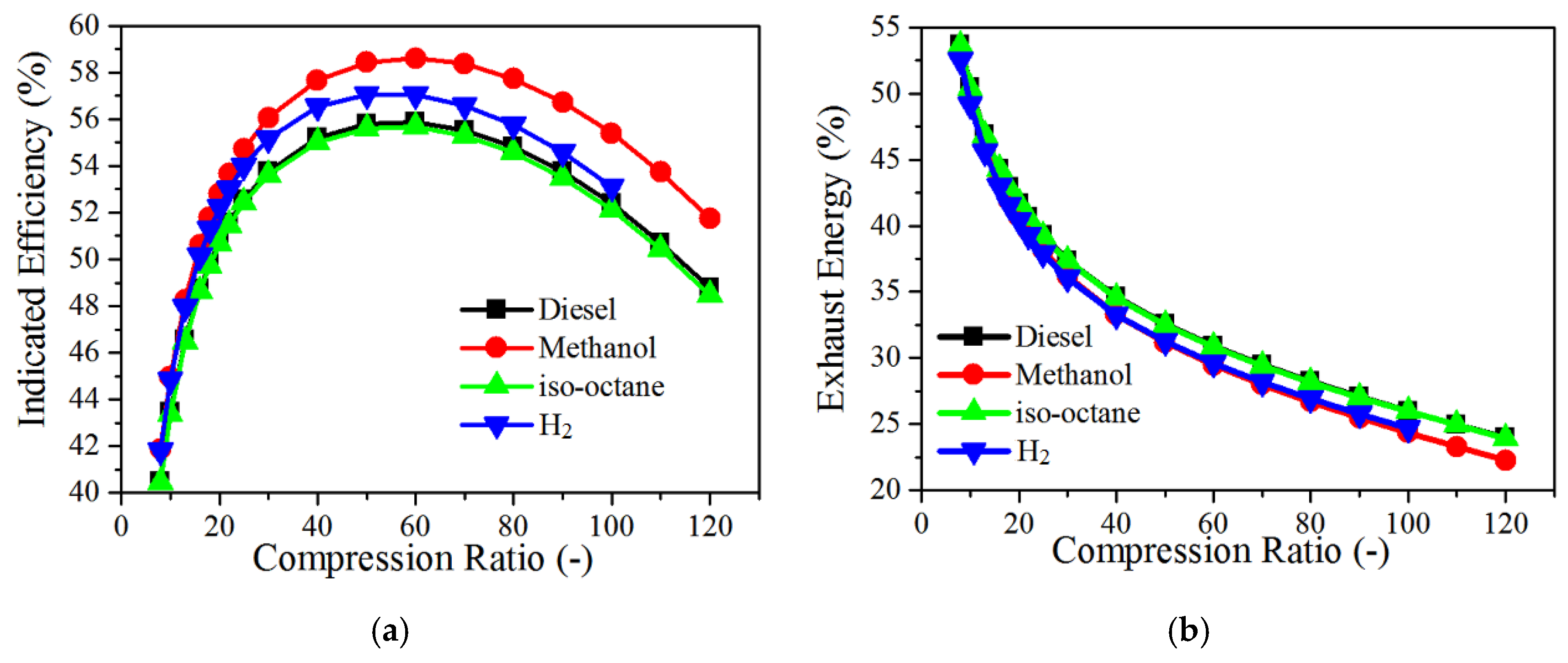
4.4. The Effects of Fuels Properties
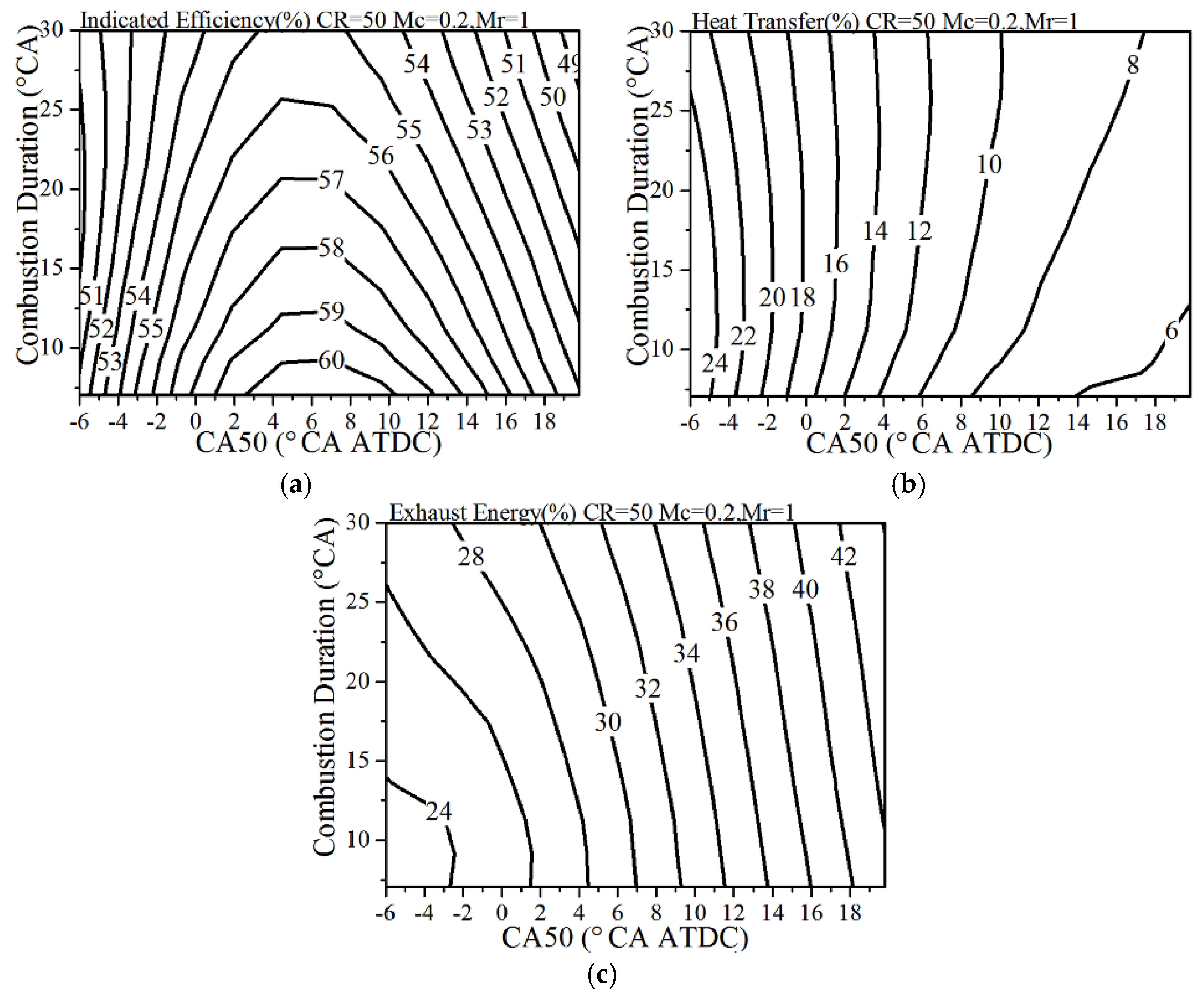
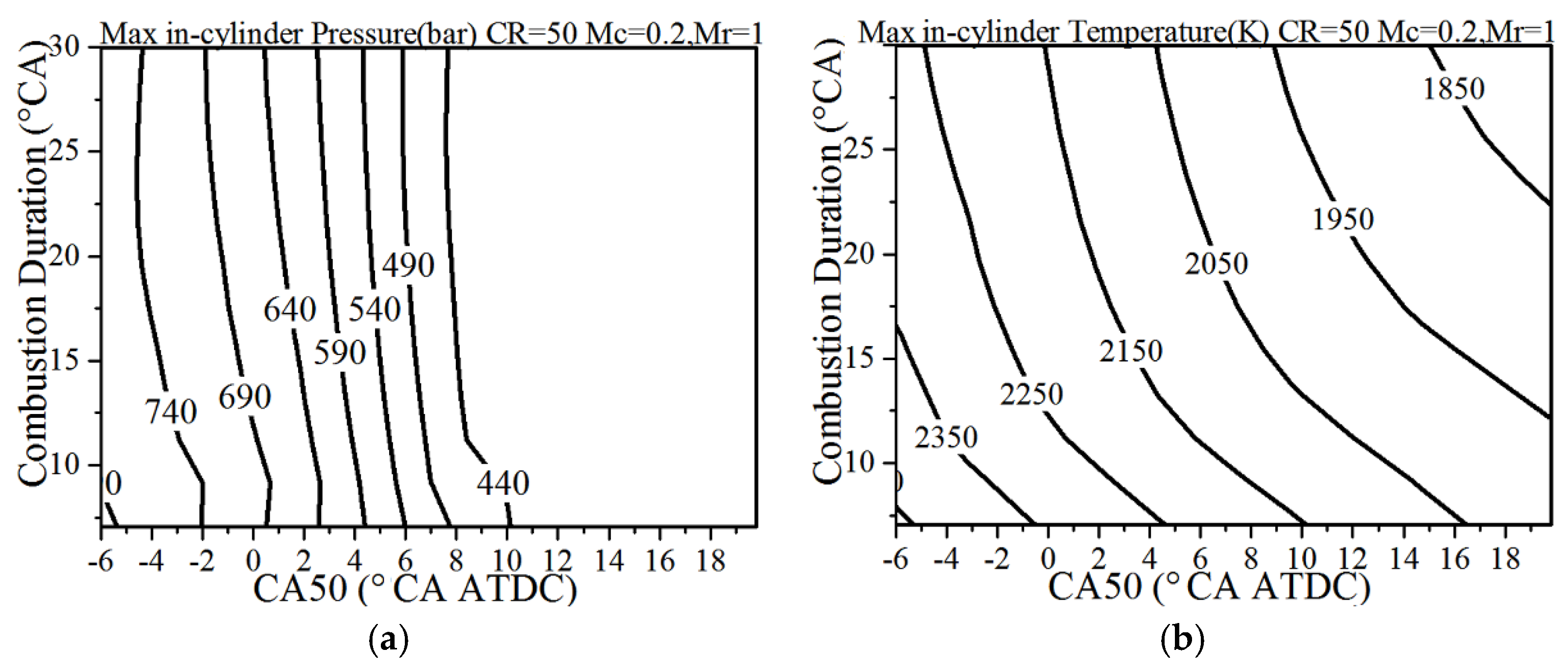
4.5. The Effects of Combustion Phasing
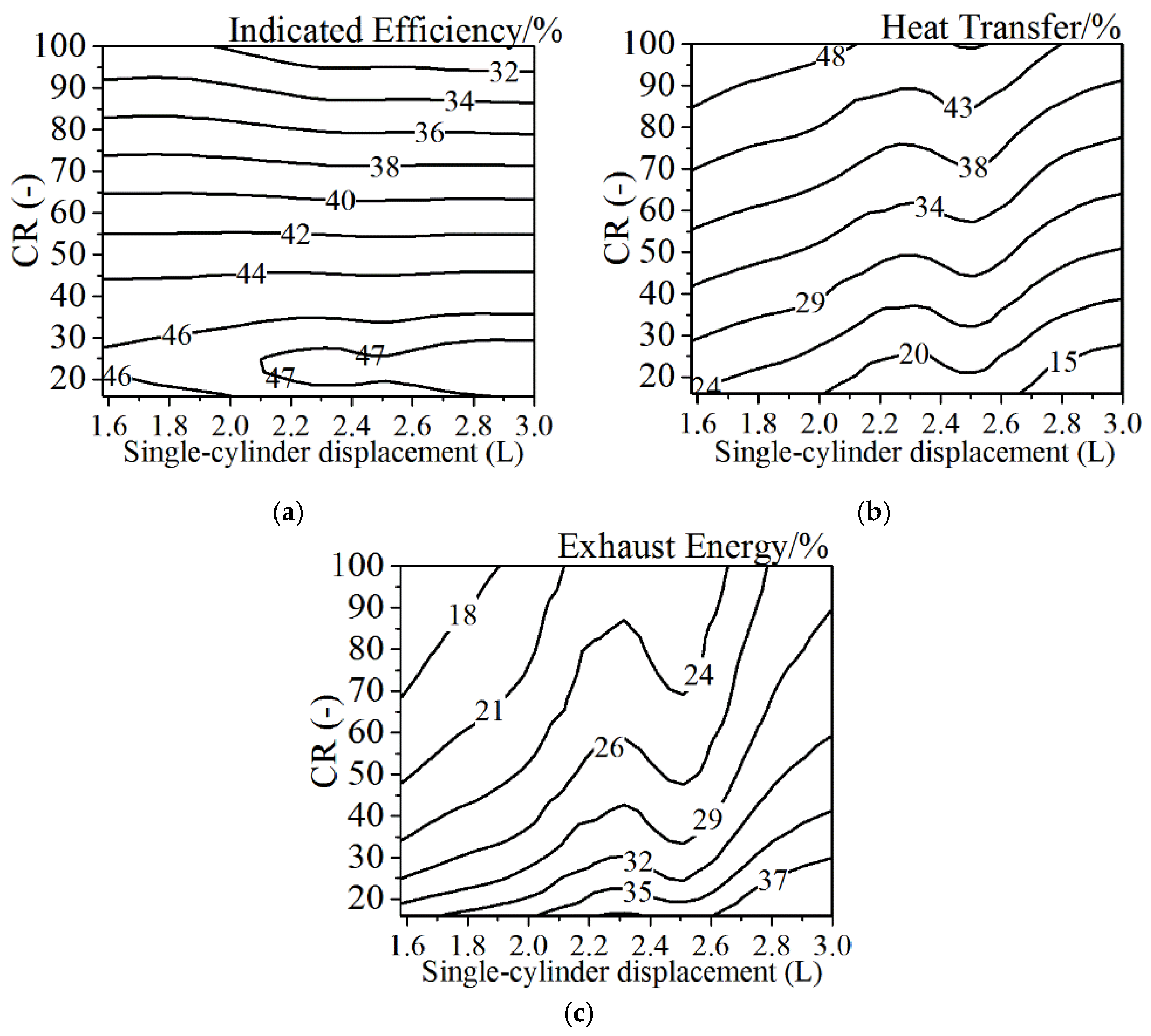
4.6. The Effects of Single-Cylinder Displacement
5. Conclusions
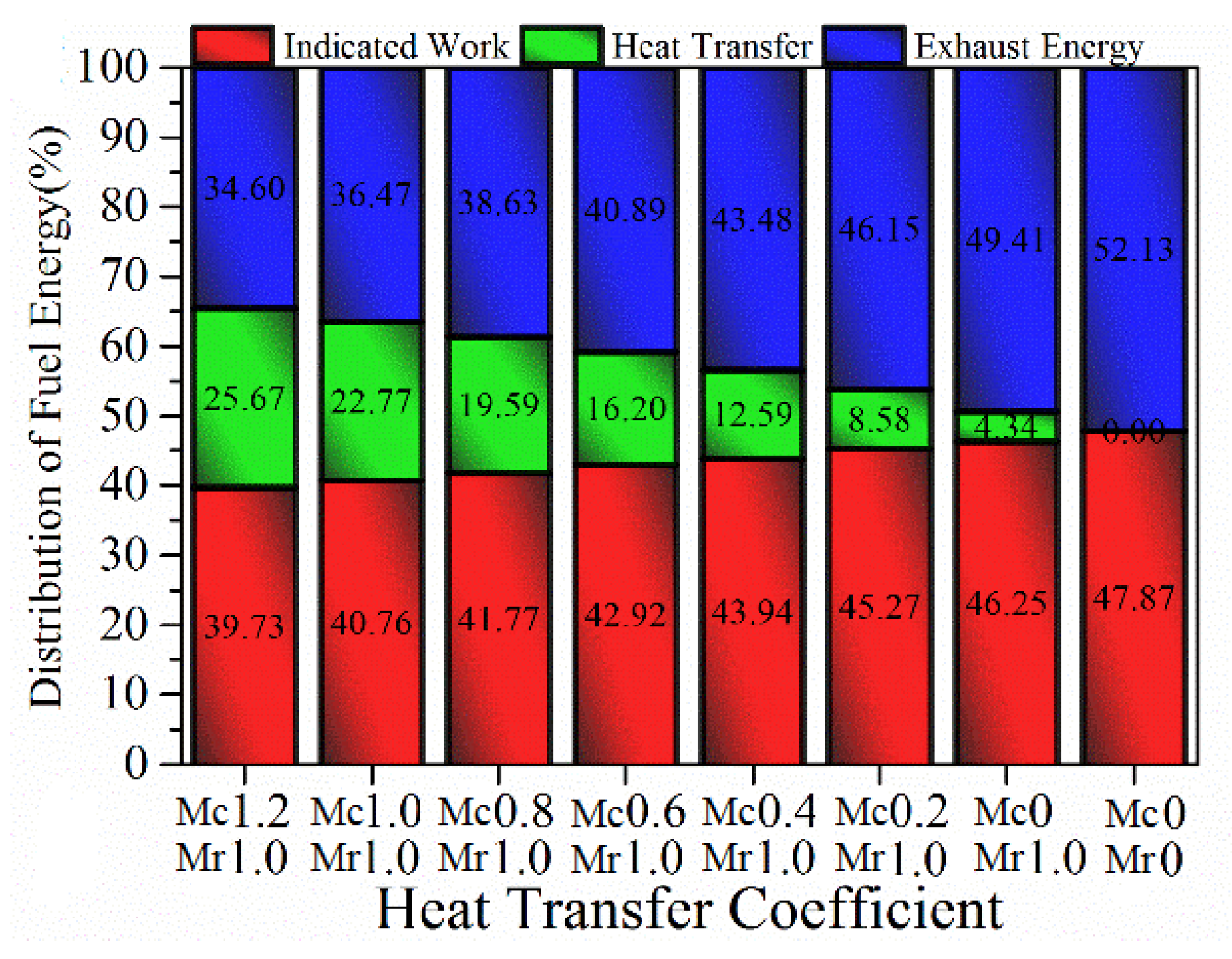
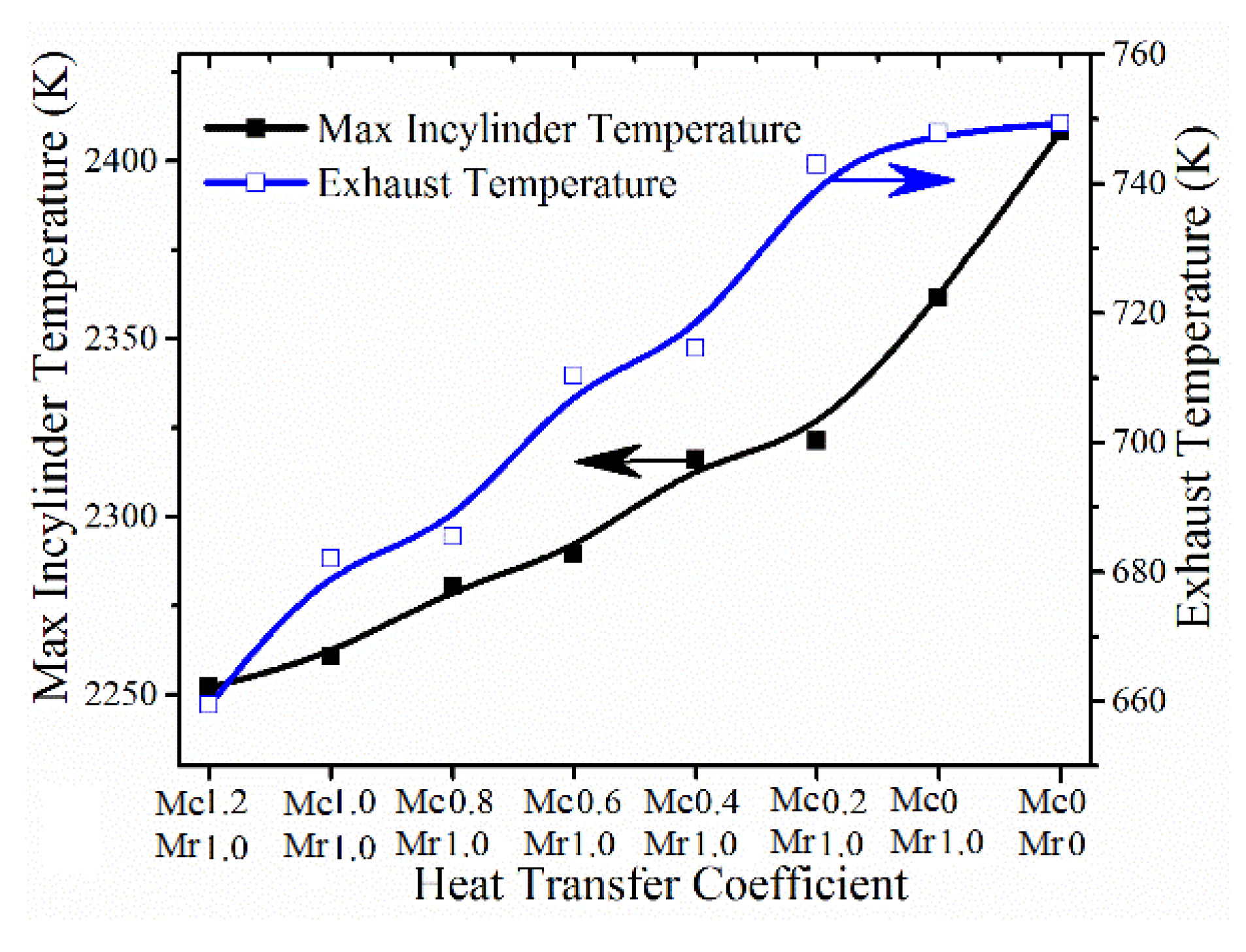
- At the normal compression ratio, with the decrease of heat transfer coefficient, heat transfer losses are reduced. However, the majority of saved energies are lost in the exhaust instead of useful work. If the compression ratio continues to increase, indicated efficiency does not always increase. Instead, the heat transfer coefficient determines optimal compression ratio. As the heat transfer coefficient decreases, the corresponding optimal compression ratio increases to obtain higher indicated efficiency. For the normal heat transfer coefficient (Mc = 1.2, Mr = 1), the peak indicated efficiency is achieved at a compression ratio of 30, which means that the indicated efficiency cannot be improved even if the compression ratio is increased to ultra-high compression ratio without changing the heat transfer coefficient.
- A lean combustion can decrease the heat transfer losses and exhaust energy, and the peak in-cylinder temperature is also suppressed effectively. For different equivalence ratios, the corresponding compression ratio to achieve peak indicated efficiency is varied. As the compression ratio is from 20 to 40, the lean mixtures will be favorable for higher indicated efficiency.
- If argon is used to replace N2, at the compression ratio of 20–40, a higher indicated thermal efficiency will be achieved with increasing intake argon percentage in either adiabatic or low heat transfer conditions. At the ultra-high compression ratio (40–100) with a low heat-transfer condition and the increase of the intake argon ratio, the indicated efficiency does not show a positive effect, and the maximum cylinder pressure increased more significantly. If CO2 is used to replace N2, the indicated efficiency decreases slightly with the increase of the intake CO2 ratio when the compression ratio is lower than 60, and the temperature in the cylinder with higher intake CO2 ratio decreases significantly at the compression ratio of 20–100.
- Methanol shows an excellent ability in decreasing the peak in-cylinder temperature, and the peak indicated efficiency is relatively higher than other tested fuels at the compression ratio of 10–120.
- A sharp and intensified combustion phasing is beneficial for the improvement of the indicated efficiency—there exists an optimal CA50 for the given heat transfer coefficient. The single-cylinder displacement has a much stronger effect on the heat transfer losses and exhaust energy compared to the effect on the indicated efficiency. Under the normal heat transfer condition, the larger single-cylinder displacement combined with the lower compression ratio is better to improve the thermal efficiency.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ribeiro, S.K.; Figueroa, M.J.; Creutzig, F.; Dubeux, C.; Hupe, J.; Kobayashi, S. Energy end-use: Transport. FEBS Lett. 2012, 565, 101–105. [Google Scholar]
- Davis, S.C. Transportation Energy Data Book: Edition 23; National Technical Information Servic: Springfield, VA, USA, 2003. [Google Scholar]
- Song, H.; Quinton, K.; Peng, Z.; Zhao, H.; Ladommatos, N. Effects of oxygen content of fuels on combustion and emissions of diesel engines. Energies 2016, 9, 28. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A.; Faussone, G.C.; Karonis, D.; Sciubba, E. Alternative diesel from waste plastics. Energies 2017, 10, 1750. [Google Scholar] [CrossRef]
- Feng, L.; Du, B.; Tian, J.; Long, W.; Tang, B. Combustion performance and emission characteristics of a diesel engine using a water-emulsified heavy fuel oil and light diesel blend. Energies 2015, 8, 13628–13640. [Google Scholar] [CrossRef]
- Han, D.; Duan, Y.; Wang, C.; Lin, H.; Huang, Z.; Wooldridge, M.S. Experimental study of the two-stage injection process of fatty acid esters on a common rail injection system. Fuel 2016, 163, 214–222. [Google Scholar] [CrossRef]
- Han, D.; Zhai, J.; Duan, Y.; Ju, D.; Lin, H.; Huang, Z. Macroscopic and microscopic spray characteristics of fatty acid esters on a common rail injection system. Fuel 2017, 203, 370–379. [Google Scholar] [CrossRef]
- Han, D.; Li, K.; Duan, Y.; Lin, H.; Huang, Z. Numerical study on fuel physical effects on the split injection processes on a common rail injection system. Energy Convers. Manag. 2017, 134, 47–58. [Google Scholar] [CrossRef]
- Qasim, M.; Ansari, T.M.; Hussain, M.; Sciubba, E. Combustion, performance, and emission evaluation of a diesel engine with biodiesel like fuel blends derived from a mixture of pakistani waste canola and waste transformer oils. Energies 2017, 10, 1023. [Google Scholar] [CrossRef]
- Azami, M.H.; Savill, M. Pulse detonation assessment for alternative fuels. Energies 2017, 10, 369. [Google Scholar] [CrossRef]
- Papagiannakis, R.G.; Rakopoulos, D.C.; Rakopoulos, C.D. Theoretical study of the effects of spark timing on the performance and emissions of a light-duty spark ignited engine running under either gasoline or ethanol or butanol fuel operating modes. Energies 2017, 10, 1198. [Google Scholar] [CrossRef]
- Boretti, A.A. Energy recovery in passenger cars. J. Energy Resour. Technol. 2012, 134, 022203. [Google Scholar] [CrossRef]
- Ryan, L.B.; Cuenot, F.; Fulton, L. Technology Roadmap: Fuel Economy of Road Vehicles; Techinical Report; International Energy Agency (IEA): Paris, France, 2012. [Google Scholar]
- Ntziachristos, L.; Mellios, G.; Samaras, Z. What is the real-world CO2 reduction benefit of the 95 g/km passenger car average emission target to be reached by 2020? Procedia Soc. Behav. Sci. 2012, 48, 2048–2057. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (EPA); National Highway Traffic Safety Administration (NHTSA). Finalize Historic National Program to Reduce Greenhouse Gases and Improve Fuel Economy for Cars and Trucks; US Environmental Protection Agency Regulatory Announcement: Washington, DC, USA, 2011.
- Gillis, J.; Cooper, M. On the Road to 54 Mpg: A Progress Report on Achievability; Technical Report; Consumer Federation of America: Washington, DC, USA, 2013. [Google Scholar]
- DOE. Fuel Economy: Where the Energy Goes. 2012. Available online: www.fueleconomy.gov/feg/atv.shtml (accessed on 12 November 2017).
- Primus, R.J.; Hoag, K.L.; Flynn, P.F.; Brands, M.C. An Appraisal of Advanced Engine Concepts Using Second Law Analysis Techniques; Technical Report; International Energy Agency (IEA): Paris, France, 1984. [Google Scholar]
- Taymaz, I.; Cakir, K.; Gur, M.; Mimaroglu, A. Experimental investigation of heat losses in a ceramic coated diesel engine. Surf. Coat. Technol. 2003, 169, 168–170. [Google Scholar] [CrossRef]
- Taymaz, I. An experimental study of energy balance in low heat rejection diesel engine. Energy 2006, 31, 364–371. [Google Scholar] [CrossRef]
- Parlak, A. The effect of heat transfer on performance of the diesel cycle and exergy of the exhaust gas stream in a lhr diesel engine at the optimum injection timing. Energy Convers. Manag. 2005, 46, 167–179. [Google Scholar] [CrossRef]
- Parlak, A.; Yasar, H.; Eldogan, O. The effect of thermal barrier coating on a turbo-charged diesel engine performance and exergy potential of the exhaust gas. Energy Convers. Manag. 2005, 46, 489–499. [Google Scholar] [CrossRef]
- Heywood, J. Internal Combustion Engine Fundamentals; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Edson, M.H. The influence of compression ratio and dissociation on ideal otto cycle engine thermal efficiency. In Digital Calculations of Engine Cycles; Elsevier: Amsterdam, The Netherlands, 1964; pp. 49–64. Available online: https://www.sciencedirect.com/science/article/pii/B9780080111049500077 (accessed on 12 November 2017).
- Edwards, C.F.; Miller, S.L.; Teh, K.Y. Thermodynamic requirements for maximum internal combustion engine cycle efficiency. Part 2: Work extraction and reactant preparation strategies. Int. J. Engine Res. 2008, 9, 467–481. [Google Scholar]
- Wang, H.; Liu, S.; He, J. Performance analysis and parametric optimum criteria of a quantum otto heat engine with heat transfer effects. Appl. Therm. Eng. 2009, 29, 706–711. [Google Scholar] [CrossRef]
- Payri, F.; Olmeda, P.; Martín, J.; Carreño, R. Experimental analysis of the global energy balance in a di diesel engine. Appl. Therm. Eng. 2015, 89, 545–557. [Google Scholar] [CrossRef]
- Abedin, M.J.; Masjuki, H.H.; Kalam, M.A.; Sanjid, A.; Rahman, S.M.A.; Masum, B.M. Energy balance of internal combustion engines using alternative fuels. Renew. Sustain. Energy Rev. 2013, 26, 20–33. [Google Scholar] [CrossRef]
- Svrcek, M.N.; Miller, S.L.; Edwards, C.F. Diesel spray behavior at compression ratios up to 100:1. At. Sprays 2010, 20, 453–465. [Google Scholar] [CrossRef]
- Teh, K.Y.; Miller, S.L.; Edwards, C.F. Thermodynamic requirements for maximum internal combustion engine cycle efficiency. Part 1: Optimal combustion strategy. Int. J. Engine Res. 2008, 9, 449–465. [Google Scholar] [CrossRef]
- Eilts, P.; Stoeber-Schmidt, C.P. Investigation of Extreme Mean Effective and Maximum Cylinder Pressures in a Passenger Car Diesel Engine; Technical Report; International Energy Agency (IEA): Paris, France, 2013. [Google Scholar]
- Laumann, E.A.; Reynolds, R.K. Hydrogen-Fueled Engine. U.S. Patent 4,112,875, 12 September 1978. [Google Scholar]
- Kuroki, R.; Kato, A.; Kamiyama, E.; Sawada, D. Study of High Efficiency Zero-Emission Argon Circulated Hydrogen Engine; Technical Report; International Energy Agency (IEA): Paris, France, 2010. [Google Scholar]




| Parameters | Value |
|---|---|
| Engine Type | Single cylinder 4-stroke, turbocharged, water coolant |
| Displacement | 1.08 L |
| Bore × stroke | 105 × 125 mm |
| Connecting road length | 210 mm |
| Number of valve | 4 |
| Original compression ratio | 16:1 |
| Wrist-pin-to-crank offset | 0 mm |
| TDC (Top Dead Center) clearance height | 1.2 mm |
| Fuel injection system | Common rail |
| Fuel Species | Lower Heating Value (kJ/kg) | Latent Heat of Vaporization (kJ/kg) | Stoichiometric Air/Fuel Ratio | Mass Flow Rate of Fuel (mg/cycle) |
|---|---|---|---|---|
| Diesel | 42,800 | 301 | 14.3 | 80 |
| Methanol | 21,110 | 1109 | 6.47 | 162.2 |
| Isooctane | 44,344 | 510 | 15.2 | 78 |
| Hydrogen | 119,940 | - | 34.3 | 23.94 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Ma, J.; Tong, L.; Ma, G.; Zheng, Z.; Yao, M. Investigation on the Potential of High Efficiency for Internal Combustion Engines. Energies 2018, 11, 513. https://doi.org/10.3390/en11030513
Liu H, Ma J, Tong L, Ma G, Zheng Z, Yao M. Investigation on the Potential of High Efficiency for Internal Combustion Engines. Energies. 2018; 11(3):513. https://doi.org/10.3390/en11030513
Chicago/Turabian StyleLiu, Haifeng, Junsheng Ma, Laihui Tong, Guixiang Ma, Zunqing Zheng, and Mingfa Yao. 2018. "Investigation on the Potential of High Efficiency for Internal Combustion Engines" Energies 11, no. 3: 513. https://doi.org/10.3390/en11030513





