A Wavelet-Based Optimization Method for Biofuel Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Waste Frying Peanuts Oil
- ✓
- Ultimate analysis (C, H, N contents): as specified by EN 15104:2011 [26].
- ✓
- Moisture content: as specified by EN 14774-1:2009 [27].
- ✓
- High and Low Heating Value (HHV and LHV): as specified by EN 14918:2009 [28].
- ✓
- Density: as specified by EN ISO 12185:1996 [29].
- ✓
- Viscosity: as specified by EN ISO 3104:2000 [30].
2.2. Transesterification Reaction
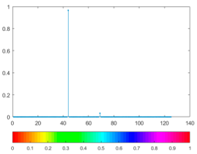
2.3. Wavelet Coefficients Space
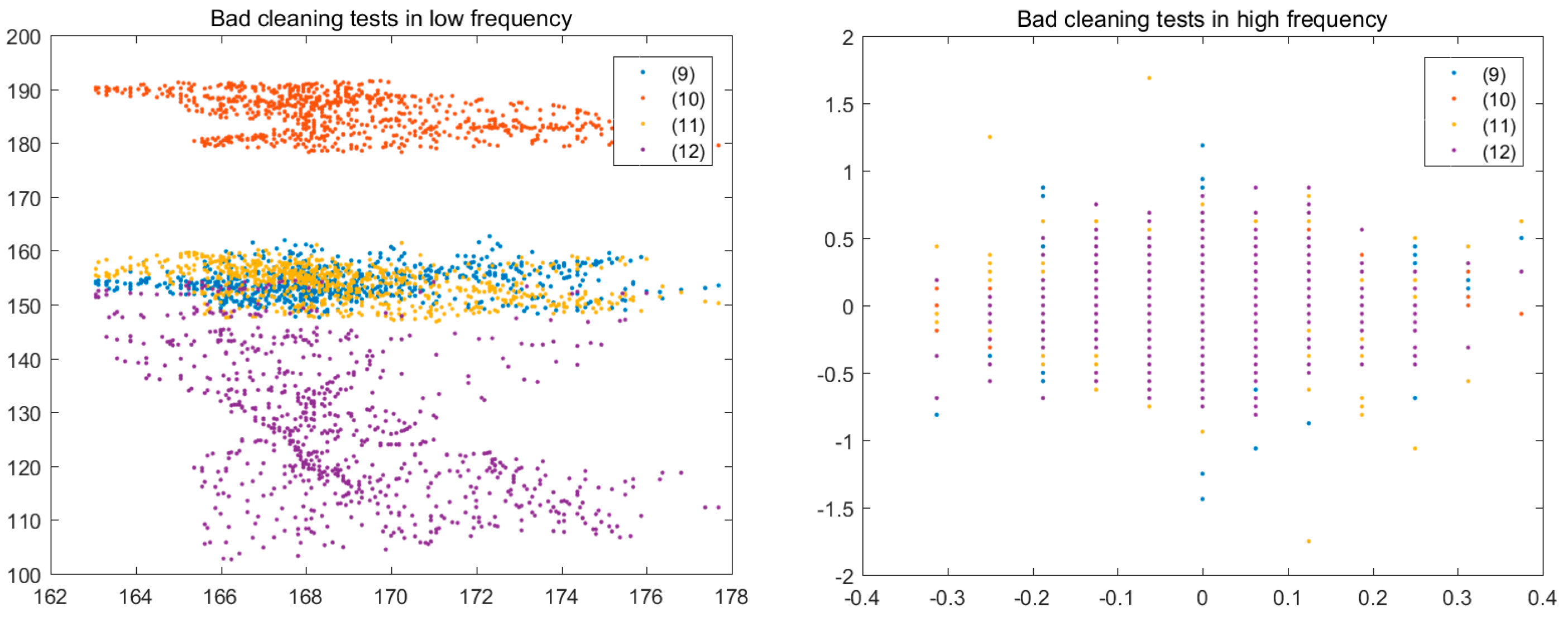
3. Results and Discussion
3.1. Analysis of Waste Peanut Frying Oil
3.2. Experimental Plan
3.3. Experimental Analysis
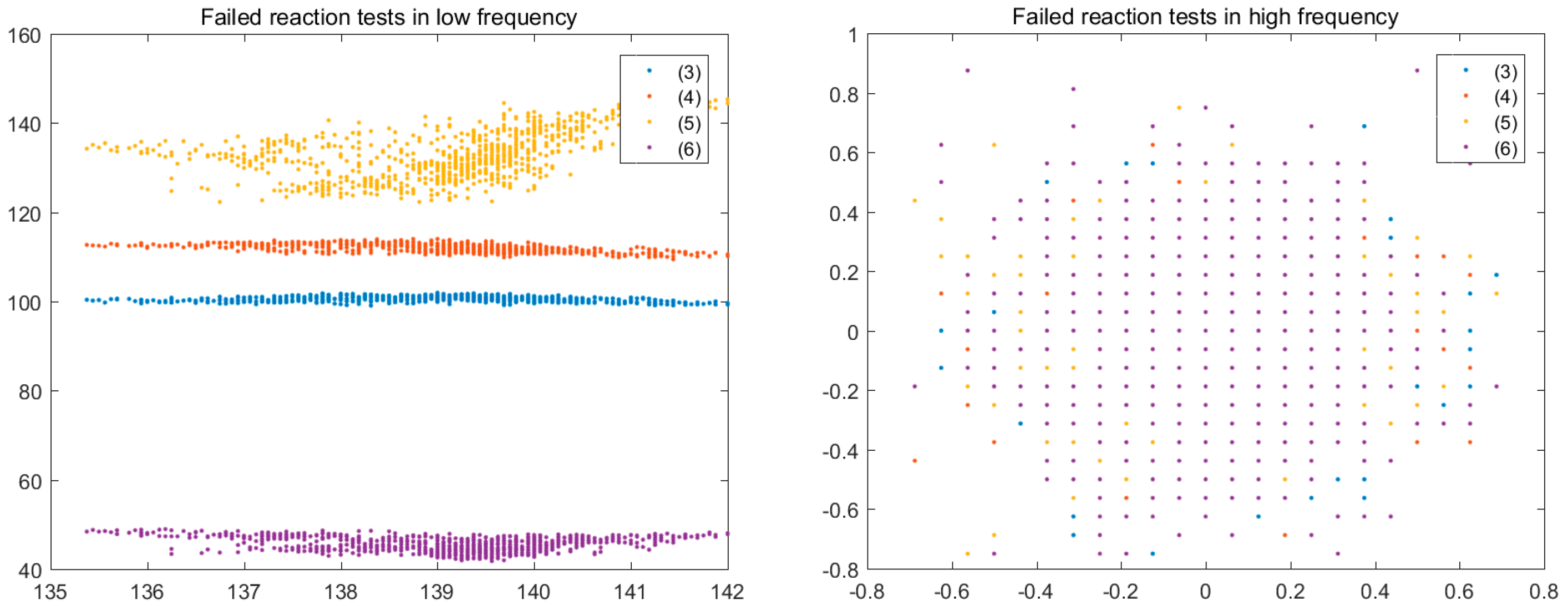
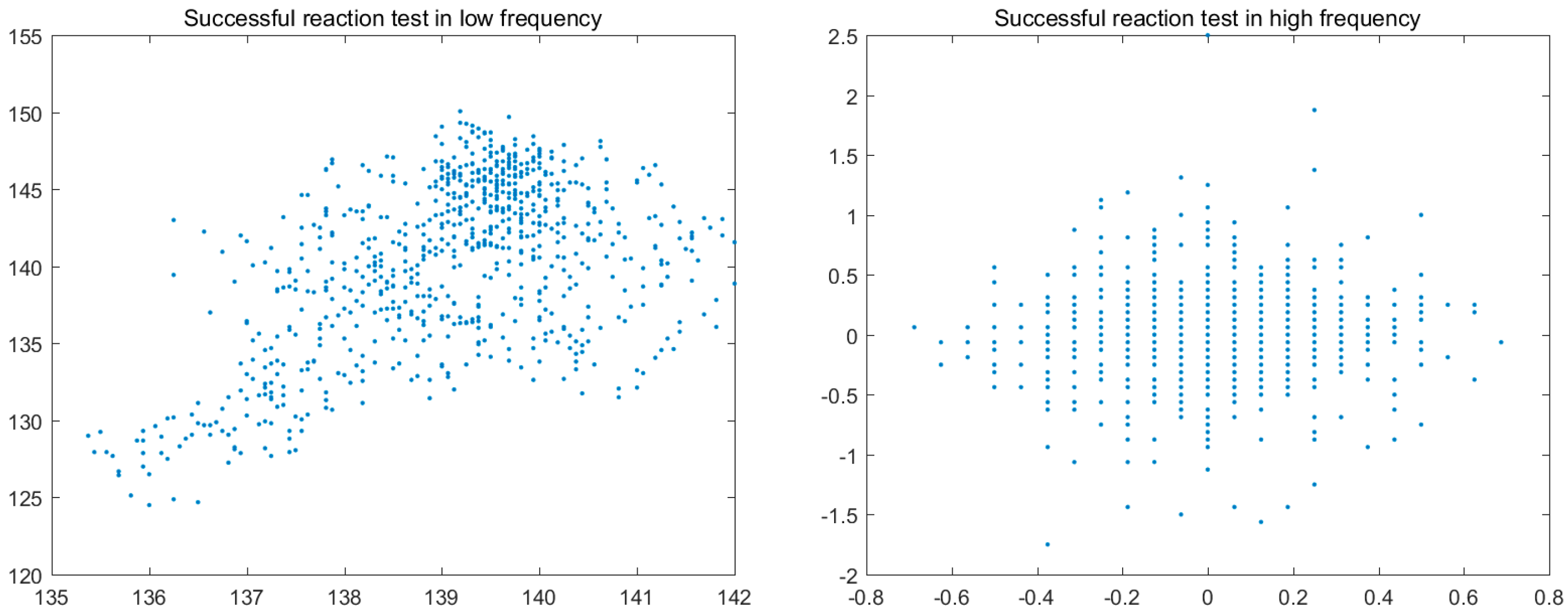
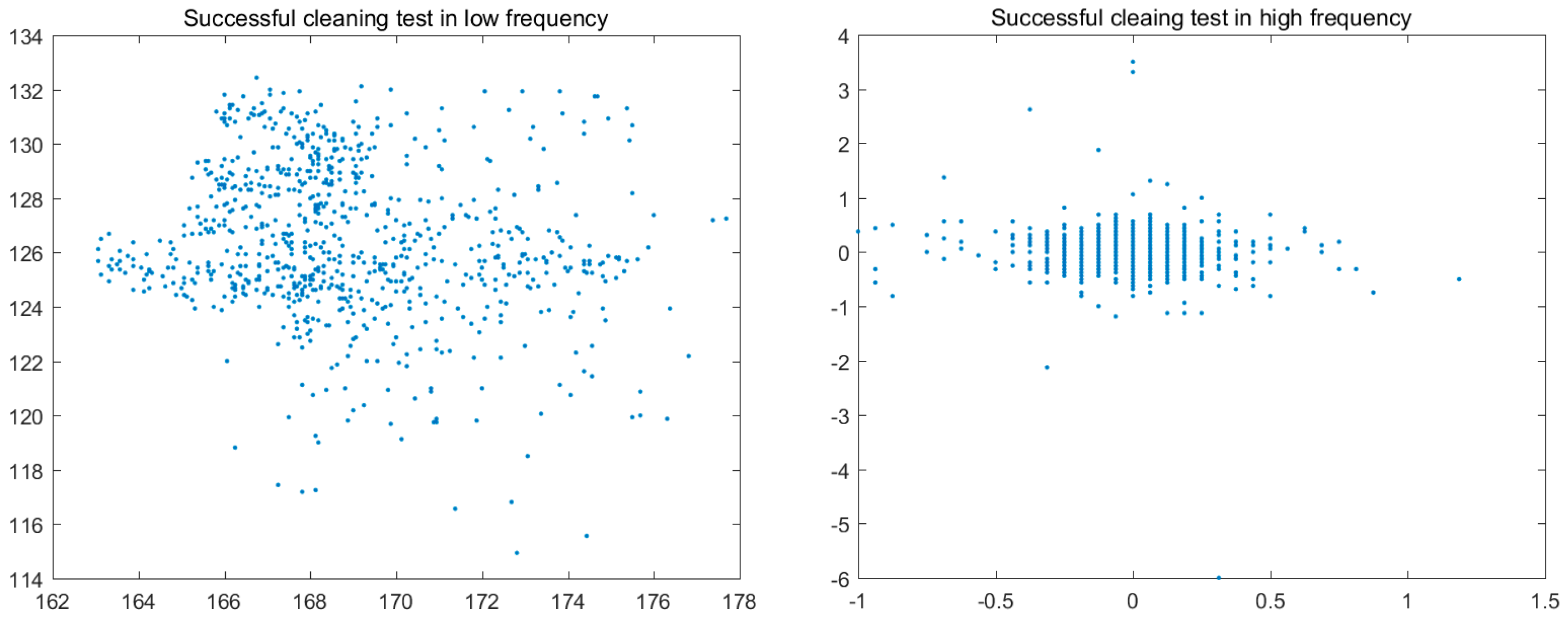
3.4. Colour Histogram
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carraretto, C.; Macor, A.; Mirandola, A.; Stoppato, A.; Tonon, S. Biodiesel as alternative fuel: Experimental analysis and energetic evaluations. Energy 2004, 29, 2195–2211. [Google Scholar] [CrossRef]
- Freitas, H.F.S.; Olivo, J.E.; Andrade, C.M.G. Optimization of Bioethanol in Silico Production Process in a Fed-Batch Bioreactor Using Non-Linear Model Predictive Control and Evolutionary Computation Techniques. Energies 2017, 10, 1763. [Google Scholar] [CrossRef]
- Gnanaprakasam, A.; Sivakumar, V.M.; Surendhar, A.; Thirumarimurugan, M.; Kannadasan, T. Recent Strategy of Biodiesel Production from Waste Cooking Oil and Process Influencing Parameters: A Review. J. Energy 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- El-Dalatony, M.M.; Salama, E.-S.; Kurade, M.B.; Hassan, S.H.A.; Oh, S.-E.; Kim, S.; Jeon, B.-H. Utilization of Microalgal Biofractions for Bioethanol, Higher Alcohols, and Biodiesel Production: A Review. Energies 2017, 10, 2110. [Google Scholar] [CrossRef]
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl. Acad. Sci. USA 2006, 103, 11206–11210. [Google Scholar] [CrossRef] [PubMed]
- Tamošiūnas, A.; Chouchène, A.; Valatkevičius, P.; Gimžauskaitė, D.; Aikas, M.; Uscila, R.; Ghorbel, M.; Jeguirim, M. The Potential of Thermal Plasma Gasification of Olive Pomace Charcoal. Energies 2017, 10, 710. [Google Scholar] [CrossRef]
- Borges, M.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 12, 2839–2849. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Encinar, J.M. Biodiesel from Used Frying Oil. Variables Affecting the Yields and Characteristics of the Biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Carlini, M.; Castellucci, S.; Cocchi, S.; Manzo, A. Waste wood biomass arising from pruning of urban green in Viterbo town: Energy characterization and potential uses. In Computational Science and Its Applications—ICCSA 2013; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 7972. [Google Scholar]
- Marucci, A.; Carlini, M.; Castellucci, S.; Cappuccini, A. Energy efficiency of a greenhouse for the conservation of forestry biodiversity. Math. Probl. Eng. 2013, 2013, 768658. [Google Scholar] [CrossRef]
- Mosconi, E.M.; Carlini, M.; Castellucci, S.; Allegrini, E.; Mizzelli, L.; di Trifiletti, M.A. Economical assessment of large-scale photovoltaic plants: An Italian case study. In Computational Science and Its Applications—ICCSA 2013; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 7972. [Google Scholar]
- Kulkarni, M.G.; Dalai, A.K. Waste Cooking OilAn Economical Source for Biodiesel: A Review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Supple, B. The Effect of Steam Treating Waste Cooking Oil on the Yield of Methyl Ester. J. Am. Oil Chem. Soc. 2002, 79, 175–178. [Google Scholar] [CrossRef]
- Fox, N.; Stachowiak, G. Vegetable oil-based lubricants—A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Savuto, E.; di Carlo, A.; Bocci, E.; D’Orazio, A.; Villarini, M.; Carlini, M.; Foscolo, P.U. Development of a CFD model for the simulation of tar and methane steam reforming through a ceramic catalytic filter. Int. J. Hydrogen Energy 2015, 40, 7991–8004. [Google Scholar] [CrossRef]
- Paul, S.; Mittal, G.S. Dynamics of fat/oil degradation during frying based on optical properties. J. Food Eng. 1996, 30, 389–403. [Google Scholar] [CrossRef]
- Grams, J.; Ruppert, A.M. Development of Heterogeneous Catalysts for Thermo-Chemical Conversion of Lignocellulosic Biomass. Energies 2017, 10, 545. [Google Scholar] [CrossRef]
- Semwal, S.; Arora, A.K.; Badoni, R.P.; Tuli, D.K. Biodiesel production using heterogeneous catalysts. Bioresour. Technol. 2011, 102, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, A. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Nohb, B.-S.; Bae, S.-Y.; Kima, K. Characterization of fatty acids composition in vegetable oils by gas chromatography and chemometrics. Anal. Chim. Acta 1998, 358, 163–175. [Google Scholar] [CrossRef]
- Lobo, B.B.; da Costa, A.E.; Gouvêa, C.A.K.; Andreazza, J.K.; Al-Rubaie, K.S. Optimization for producing biodiesel from ethanol and waste frying oil with a high concentration of ester. Rev. Fac. Ing. Univ. Antioquia 2016. [Google Scholar] [CrossRef]
- BSI. Liquid Petroleum Products-Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods; UNI EN 14214:2014; BSI: London, UK, 2013. [Google Scholar]
- Ouanji, F.; Khachani, M.; Boualag, M.; Kacimi, M.; Ziyad, M. Large-scale biodiesel production from Moroccan used frying oil. Int. J. Hydrogen Energy 2016, 41, 21022–21029. [Google Scholar] [CrossRef]
- Carlini, M.; Castellucci, S.; Cocchi, S. A Pilot-Scale Study of Waste Vegetable Oil Transesterification with Alkaline and Acidic Catalysts. Energy Procedia 2014, 45, 198–206. [Google Scholar] [CrossRef]
- Determination of Total Content of Carbon, Hydrogen and Nitrogen Instrumental Methods; UNI EN 15104:2011; Italian National Unification authority UNI: Milan, Italy, 2011.
- Determination of Moisture Content—Oven Dry Method; UNI EN 14774-1; Italian National Unification authority UNI: Milan, Italy, 2010.
- Determination of Caloric Value; UNI EN 14918:2010; Italian National Unification authority UNI: Milan, Italy, 2010.
- ISO. Crude Petroleum and Petroleum Products—Determination of Density—Oscillating U-tube Method; ISO 12185:1996; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- ISO. Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity; ISO 3104:2000; ISO: Geneva, Switzerland, 2000. [Google Scholar]
- Noureddini, H.; Zhu, D. Kinetics of transesterification of soybean oil. J. Am. Oil Chem. Soc. 1997, 74, 1457–1463. [Google Scholar] [CrossRef]
- Ouanji, F.; Nachid, M.; Kacimi, M.; Liotta, L.F.; Puleo, F.; Ziyad, M. Small scale biodiesel synthesis from waste frying oil and crude methanol in Morocco. Chin. J. Chem. Eng. 2016, 24, 1178–1185. [Google Scholar] [CrossRef]
- Daubechies, I. Ten Lectures on Wavelets; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1992. [Google Scholar]
- Chui, C.K. An Introduction to Wavelets; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Mallat, S.G. A theory for multiresolution signal decomposition: The wavelet representation. IEEE Trans. Pattern Anal. Mach. Intell. 1989, 11, 674–693. [Google Scholar] [CrossRef]
- Rioul, O.; Martin, V. Wavelets and signal processing. IEEE Signal Process. Mag. 1991, 8, 14–38. [Google Scholar] [CrossRef]
- Knothe, G.; van Gerpen, J.; Krahl, J. The Biodiesel Handbook; AOCS Press: Champaign, IL, USA, 2005. [Google Scholar]
- Leung, D.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]












| Properties | Unit | Value |
|---|---|---|
| Density | kg/m3 | 937.5 ± 0.01 |
| Viscosity | mm2/s | 36.6 ± 0.04 |
| Moisture | % | 3 ± 0.03 |
| C | % | 54.893 ± 0.05 |
| H | % | 8.708 ± 0.02 |
| N | % | 0.238 ± 0.03 |
| Hs | MJ/kg | 41.42 ± 0.02 |
| Hi | MJ/kg | 39.62 ± 0.01 |
| Test | Catalyst [%] | Temperature [°C] | Product Yield [%] |
|---|---|---|---|
| 1 | 0.25 | 70 | 47.57 ± 0.09 |
| 2 | 0.50 | 70 | 78.26 ± 0.05 |
| 3 | 0.75 | 70 | 44.25 ± 0.06 |
| 4 | 0.25 | 80 | 53.27 ± 0.07 |
| 5 | 0.50 | 80 | 52.85 ± 0.05 |
| 6 | 0.75 | 80 | 37.06 ± 0.04 |
| 7 | 0.25 | 90 | 52.31 ± 0.03 |
| 8 | 0.50 | 90 | 49.33 ± 0.02 |
| 9 | 0.75 | 90 | 2.25 ± 0.06 |
| 10 | 0.25 | 110 | 41.64 ± 0.01 |
| 11 | 0.50 | 110 | 21.97 ± 0.05 |
| 12 | 0.75 | 110 | - |
| Chemical Reaction | Colour Histogram | Chemical Reaction | Colour Histogram |
|---|---|---|---|
 (1) |  |  (2) | 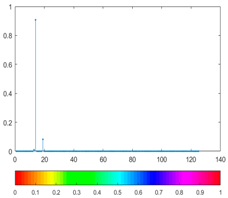 |
| Chemical Reaction | Colour Histogram | Chemical Reaction | Colour Histogram |
|---|---|---|---|
 (3) |  |  (4) | 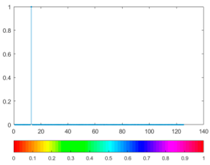 |
 (5) |  |  (6) |  |
| Chemical Reaction | Colour Histogram | Chemical Reaction | Colour Histogram |
|---|---|---|---|
 (7) |  |  (8) | 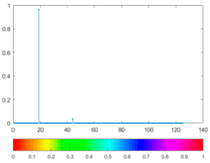 |
 (9) | 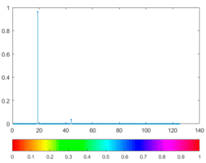 |  (10) | 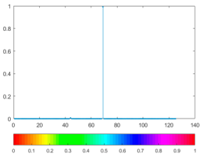 |
 (11) |  |  (12) |  |
| Chemical Reaction | Colour Histogram |
|---|---|
 (13) | 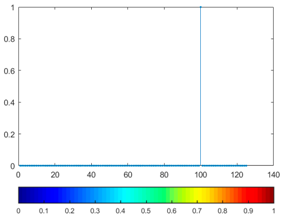 |
| Figure | Chemical Reaction | Scalogram: Percentage of Energy for Each Wavelet Coefficient | Percentage of Energy for Each Wavelet Coefficient |
|---|---|---|---|
| (1) |  | 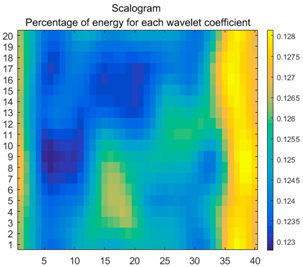 |  |
| (2) |  |  | 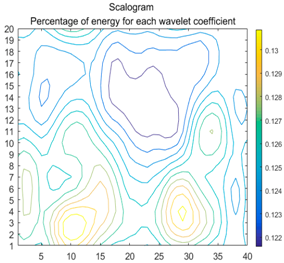 |
| Figure | Chemical Reaction | Scalogram: Percentage of Energy for Each Wavelet Coefficient | Percentage of Energy for Each Wavelet Coefficient |
|---|---|---|---|
| (3) |  |  |  |
| (4) |  | 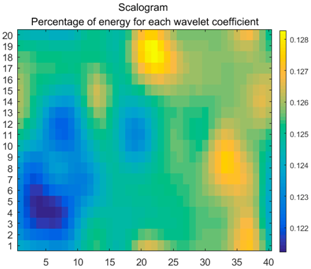 | 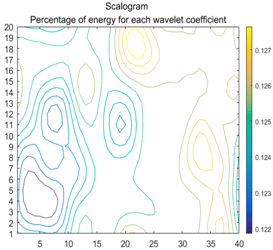 |
| (5) |  | 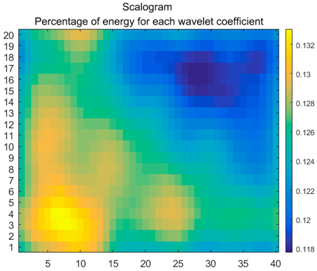 | 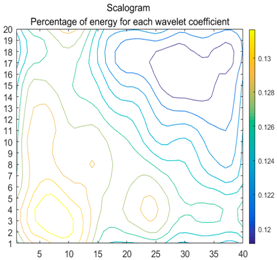 |
| (6) |  | 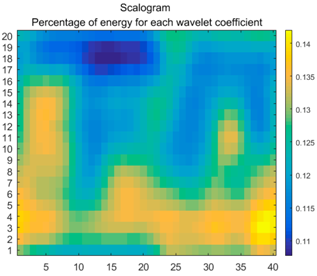 | 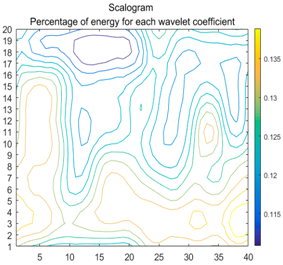 |
| Figure | Chemical Reaction | Scalogram: Percentage of Energy for Each Wavelet Coefficient | Percentage of Energy for Each Wavelet Coefficient |
|---|---|---|---|
| (7) |  | 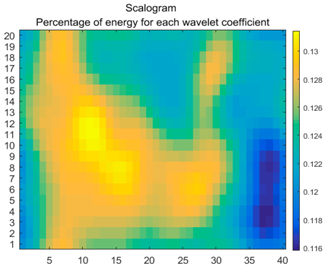 | 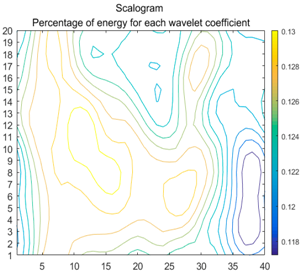 |
| (8) |  | 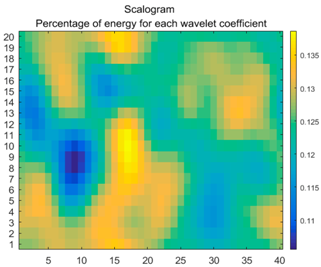 | 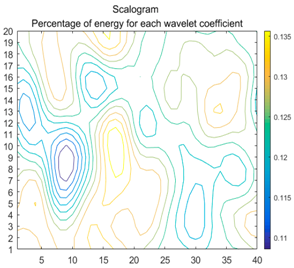 |
| (9) |  | 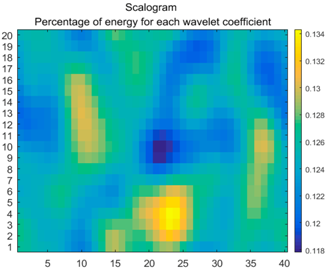 | 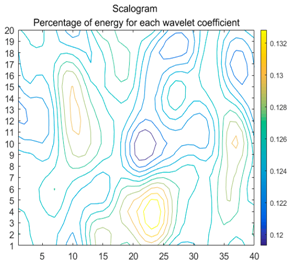 |
| (10) |  |  |  |
| (11) |  | 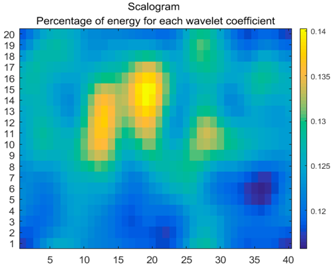 | 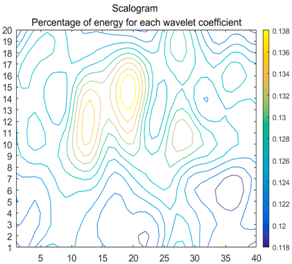 |
| (12) |  |  |  |
| Figure | Chemical Reaction | Scalogram: Percentage of Energy for Each Wavelet Coefficient | Percentage of Energy for Each Wavelet Coefficient |
|---|---|---|---|
| (13) |  | 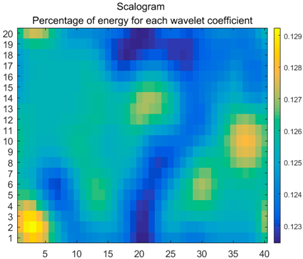 | 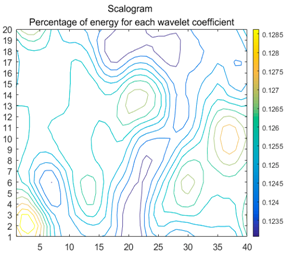 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlini, M.; Castellucci, S.; Sun, G.; Leng, J.; Cattani, C.; Cardarelli, A. A Wavelet-Based Optimization Method for Biofuel Production. Energies 2018, 11, 377. https://doi.org/10.3390/en11020377
Carlini M, Castellucci S, Sun G, Leng J, Cattani C, Cardarelli A. A Wavelet-Based Optimization Method for Biofuel Production. Energies. 2018; 11(2):377. https://doi.org/10.3390/en11020377
Chicago/Turabian StyleCarlini, Maurizio, Sonia Castellucci, Guomin Sun, Jinsong Leng, Carlo Cattani, and Alessandro Cardarelli. 2018. "A Wavelet-Based Optimization Method for Biofuel Production" Energies 11, no. 2: 377. https://doi.org/10.3390/en11020377