Experimental Study on Improvement of Performance by Wave Form Cathode Channels in a PEM Fuel Cell
Abstract
:1. Introduction
2. Theoretical Background
2.1. Polarization Curve
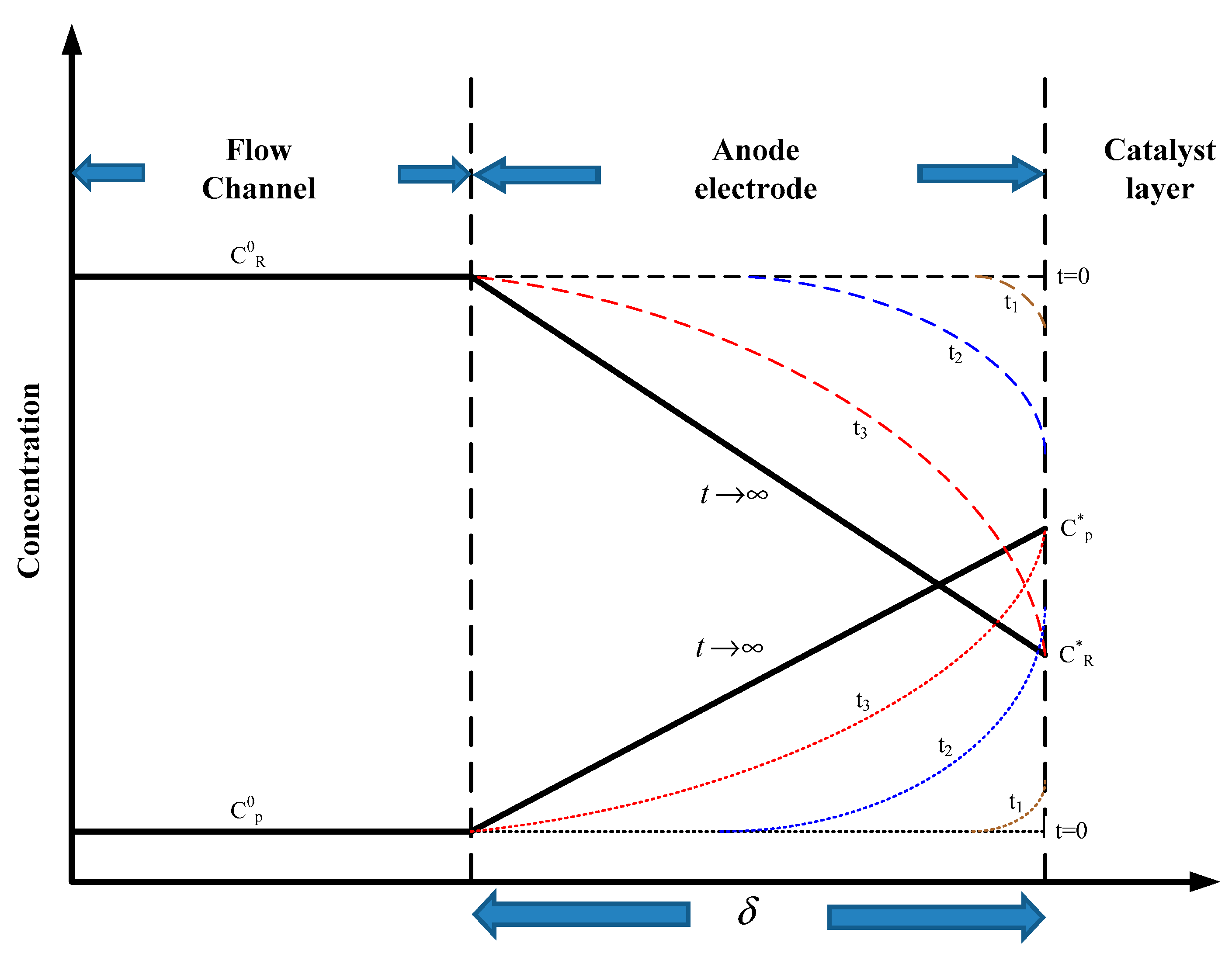
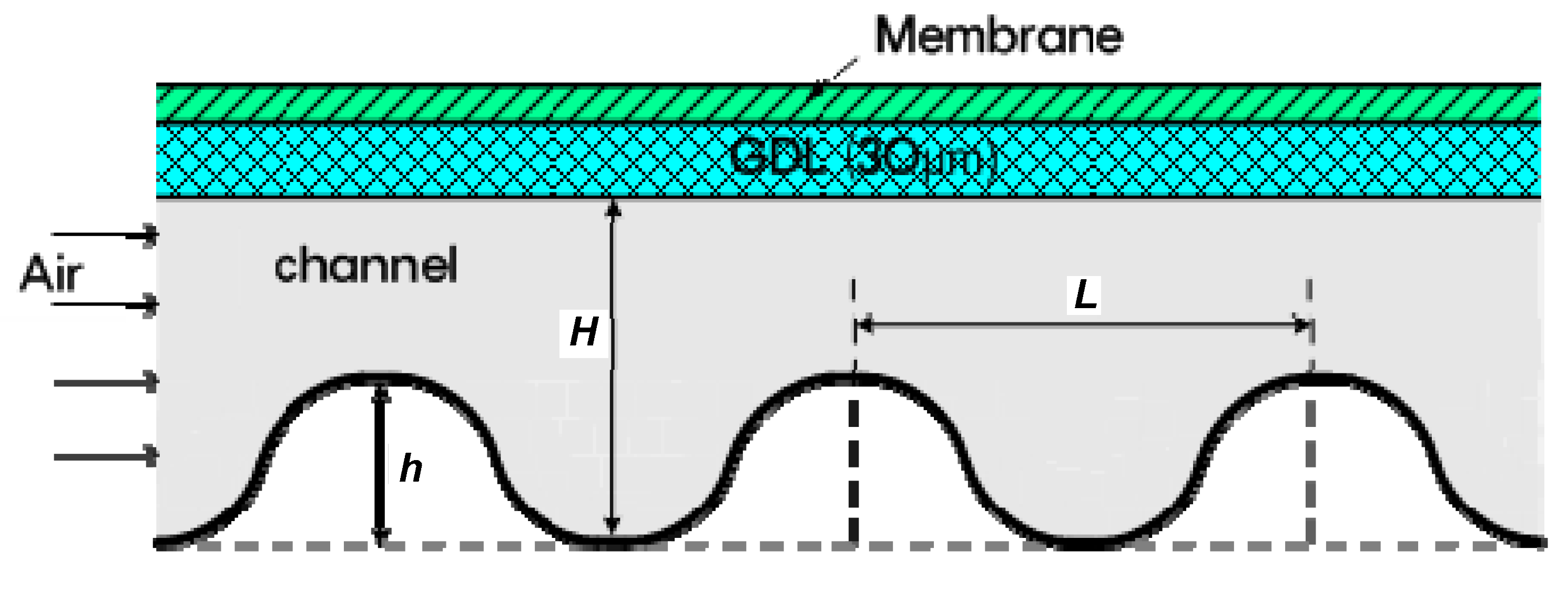
2.2. Factors for Displacement in the Wave Form Channels
3. Experimental Method
3.1. Experimental Apparatus
3.2. Experimental Conditions
4. Results and Discussion
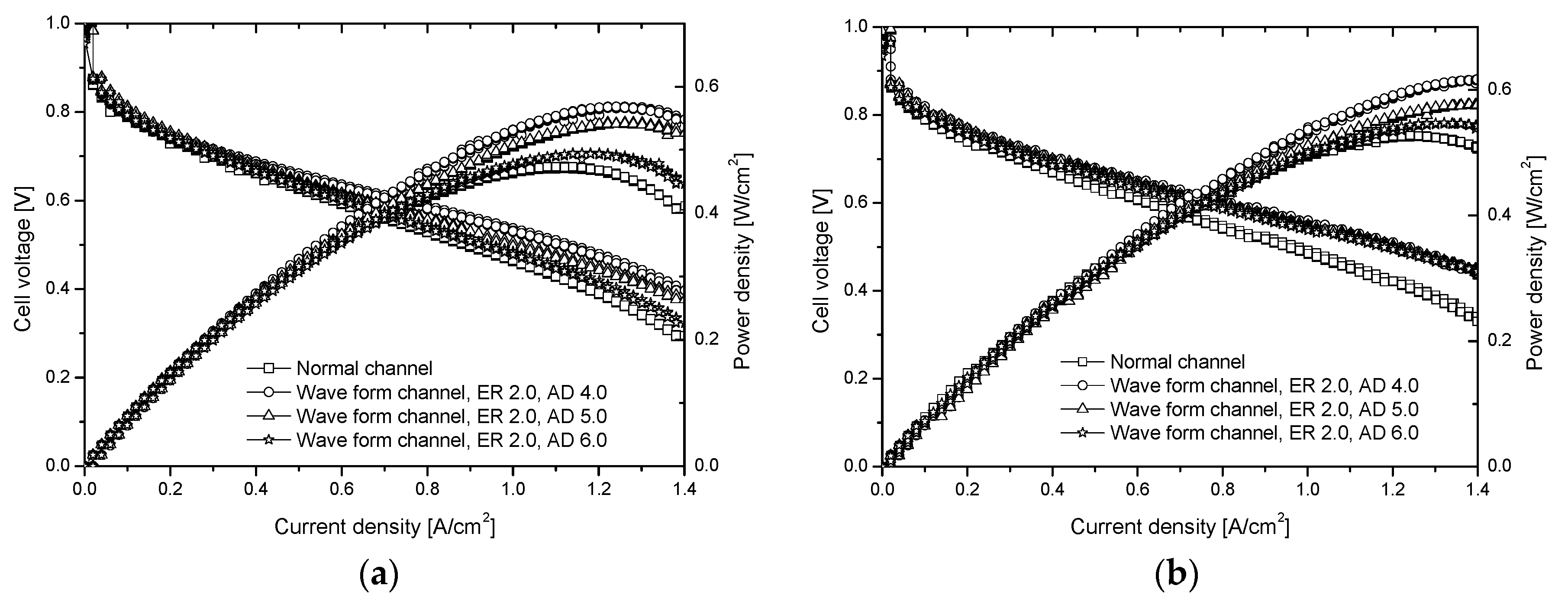
4.1. Fuel Cell Performance Variation According to the ER Variation
4.2. AD Variation and Fuel Cell Performance
5. Conclusions
- (1)
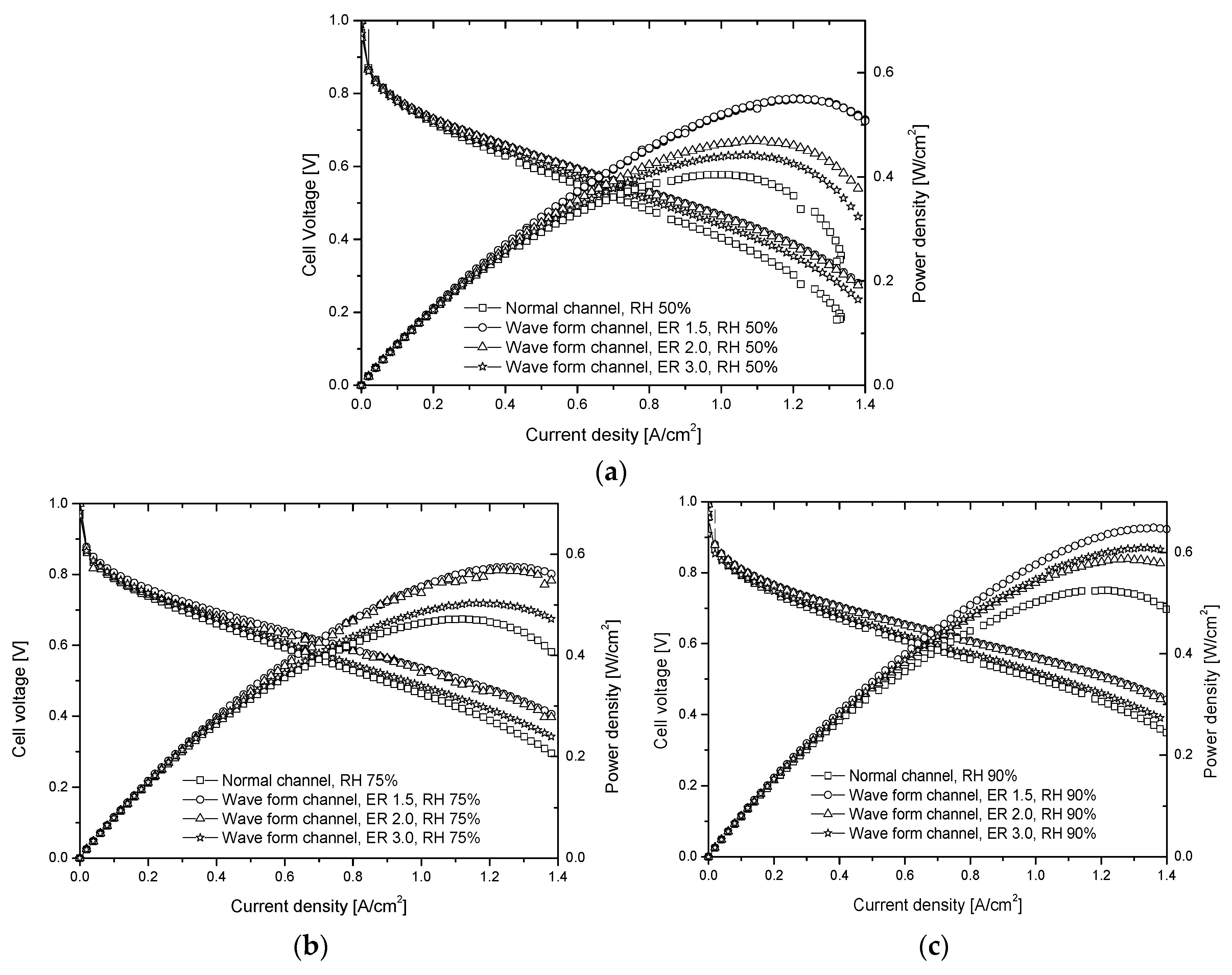
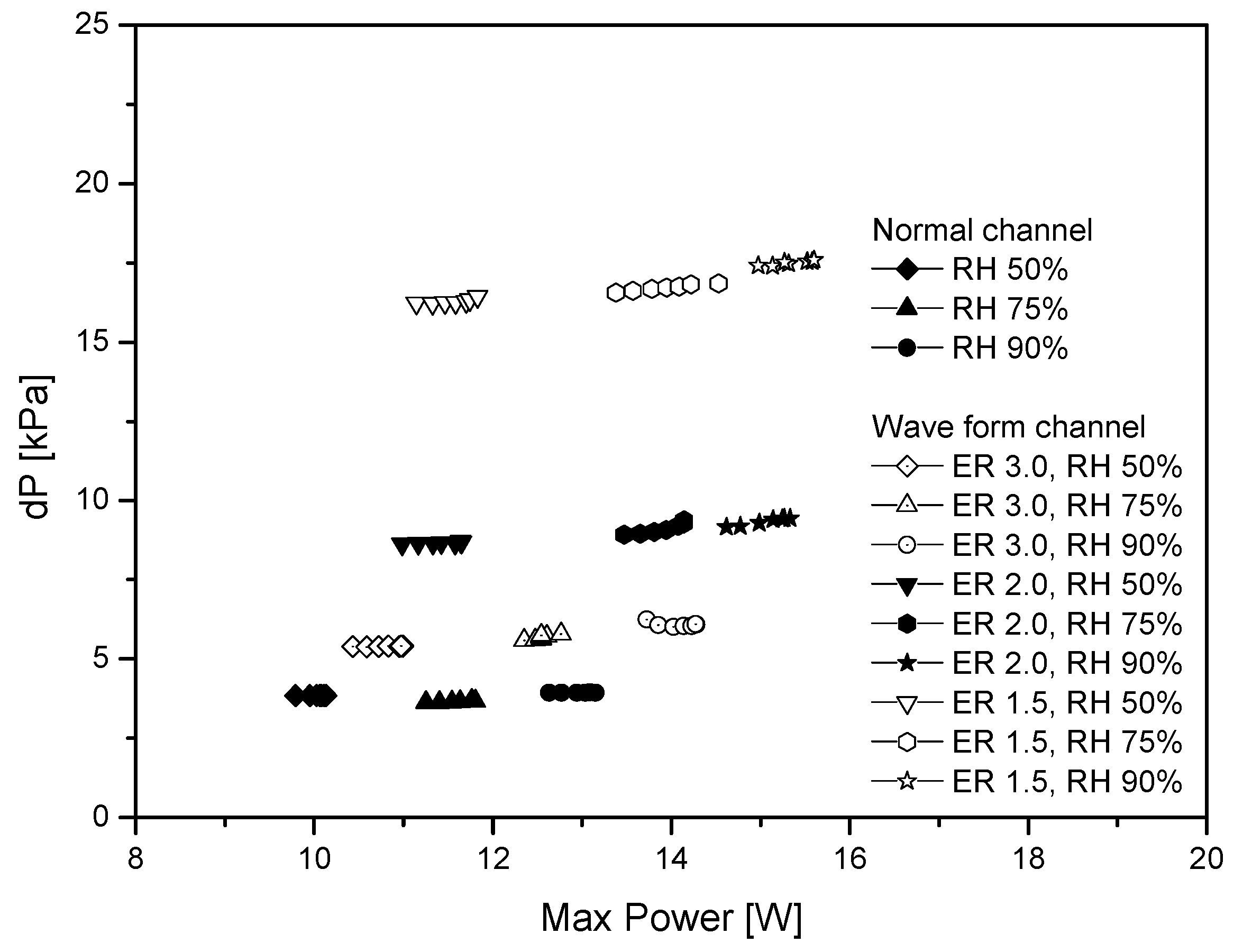
- As the ER decreases, the maximum power density increases further. The power density of the wave form channel (ER 1.5) is about 21% better than that of the normal channel. In a CD over 1.2, the power density of the wave form channel decreases slowly, unlike that of the normal channel. The pressure difference becomes larger as the ER becomes smaller. This can affect the stability and internal resistance of the fuel cell.
- (2)
- From the performance curve of the fuel cell according to the relative humidity change (RH 50, 75, and 90%), the cell voltage, power density, and CD gradually increase at high relative humidity. At ER 1.5 and 2.0, performance is measured similarly, and the power density and cell voltage instability seen on the steady channel disappear. The performance of the fuel cell can be increased by activating the catalyst reactants, because high CD changes the flow in the channel.
- (3)
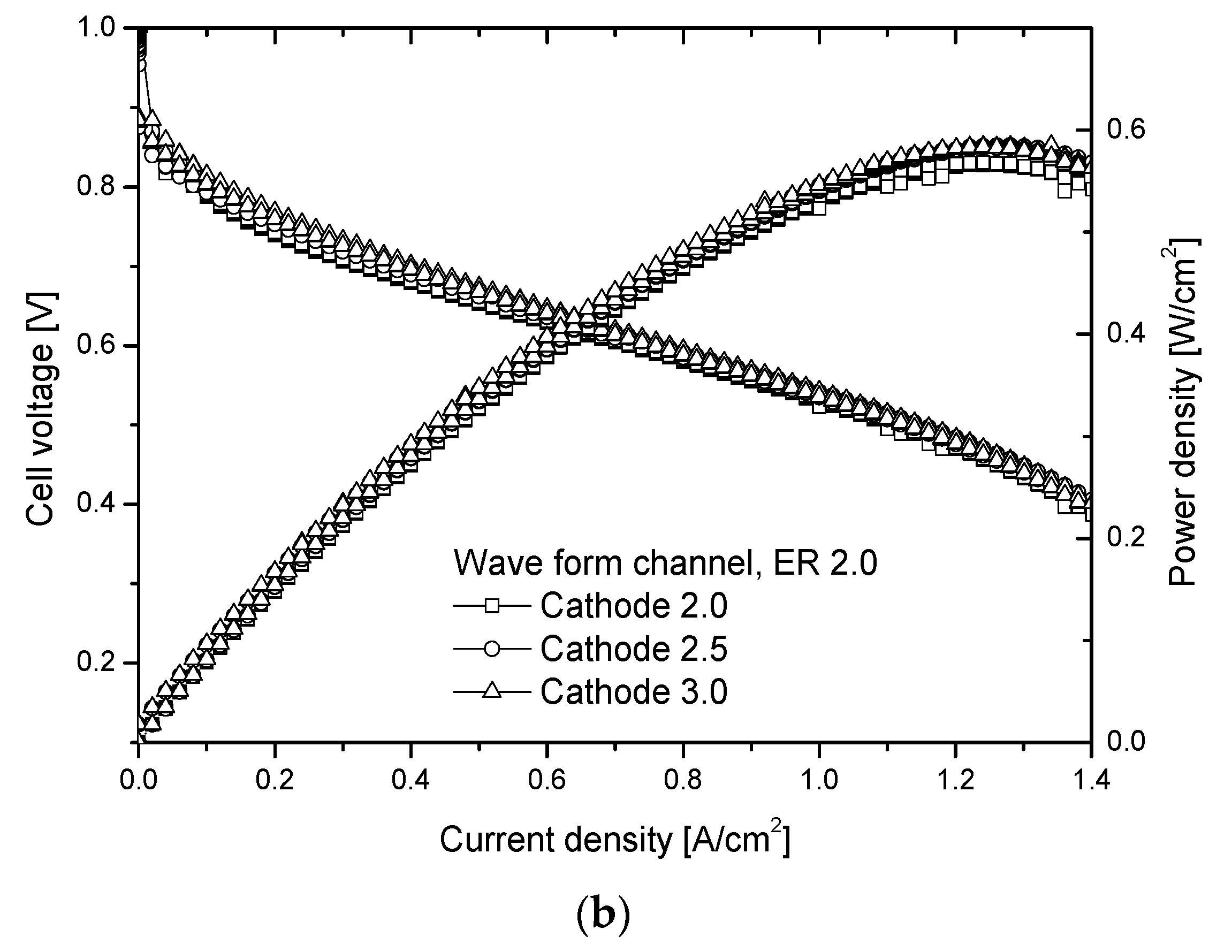
- From the fuel cell performance as a function of cathode stoichiometry, the maximum power density appears near CD 1.1 in the case of the normal channel, and near CD 1.3 in the case of the wave form channel (ER 2.0). Furthermore, the maximum power density of the wave form channel is about 20% better than that of the normal one.
- (4)
- As AD decreases, the maximum power density increases further. The power density of AD 4 (at ER 2.0) is about 20% better than that of the normal channel. As the relative humidity increases, the performance of the fuel cell is improved. From this, the optimal shape of the wave form channel if found to have ER 2.0 and AD 4.0. In the time-wise fuel cell performance variation on the normal channel and waveform channel, the wave form channel exhibits less voltage and performance degradation than the normal channel. In a high CD, the performance curve of the normal channel and wave form channel is more stable.
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| F | Force, N |
| h | Channel amplitude, mm |
| H | Channel height, mm |
| T | Temperature, °C |
| RH | Relative humidity, % |
| Q | Flow rate, kg/s |
| T | Time, s |
| P | Operating pressure, kPa |
| W | Work |
| u | Velocity component, m/s |
| R | Radius, m |
| V | Voltage, V |
| CD | Current density, A/cm2 |
| GDL | Gas diffusion layer |
| MEA | Membrane electrode assembly |
| Greek symbols | |
| λ | Stoichiometry |
| ρ | Density, kg/m3 |
| б | Surface tension, N/m |
| Subscripts | |
| a | Air |
| g | Gas |
| act | Activation |
| sat | Saturated |
| c | Cell |
| gen | Generating force |
| adh | Adhesion |
| r | Radiation |
| in | Inlet |
| out | Outlet |
| h | Hydrostatic |
| fore | Force contact angle |
References
- Escribano, S.; Blachot, J.F.; Ethève, J. Characterization of PEMFCs gas diffusion layers properties. J. Power Sources 2006, 156, 8–13. [Google Scholar] [CrossRef]
- Lin, J.H.; Chen, W.H.; Su, Y.J.; Ko, T.H. Effect of gas diffusion layer compression on the performance in a proton exchange membrane fuel cell. Fuel 2008, 87, 2420–2424. [Google Scholar] [CrossRef]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer electrolyte fuel cell model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Natarajan, D.; Nguyen, T.V. A two-dimensional, two-phase, multicomponent, transient model for the cathode of a proton exchange membrane fuel cell using conventional gas distributors. J. Electrochem. Soc. 2001, 148, A1324–A1335. [Google Scholar] [CrossRef]
- Yoon, Y.G.; Lee, W.Y.; Park, G.G.; Yang, T.H.; Kim, C.S. Effects of channel configurations of flow field plates on the performance of a PEMFC. Electrochim. Acta 2004, 50, 709–712. [Google Scholar] [CrossRef]
- Kuo, J.K.; Chen, C.K. The effects of buoyancy on the performance of a PEM fuel cell with a wave-like gas flow channel design by numerical investigation. Int. J. Heat Mass Transf. 2007, 21–22, 4166–4179. [Google Scholar] [CrossRef]
- Kuo, J.K.; Yen, T.S.; Chen, C.K. Improvement of performance of gas flow channel in PEM fuel cells. Energy Convers. Manag. 2008, 49, 2776–2787. [Google Scholar] [CrossRef]
- Kuo, J.K.; Yen, T.H.; Chen, C.K. Three-dimensional numerical analysis of PEM fuel cells with straight and wave-like gas flow fields channels. J. Power Sources 2008, 177, 96–103. [Google Scholar] [CrossRef]
- Li, X.; Sabir, I. Review of bipolar plates in PEM fuel cells: Flow-field designs. Int. J. Hydrogen Energy 2005, 30, 359–371. [Google Scholar] [CrossRef]
- Su, A.; Chiu, Y.C.; Weng, F.B. The impact of flow field pattern on concentration and performance in PEMFC. Int. J. Energy Res. 2005, 29, 409–425. [Google Scholar] [CrossRef]
- Perng, S.W.; Wu, H.W. Effect of the prominent catalyst layer surface on reactant gas transport and cell performance at the cathodic side of a PEMFC. Appl. Energy 2010, 87, 1386–1399. [Google Scholar] [CrossRef]
- Liu, F.; Lu, G.; Wang, C.Y. Water transport coefficient distribution through the membrane in a polymer electrolyte fuel cell. J. Membr. Sci. 2007, 287, 126–131. [Google Scholar] [CrossRef]
- Wan, Z.M.; Huawei, H.W.; Shu, S.M.; Wang, Y.X.; Tang, H.L. A review on cold start of proton exchange membrane fuel cells. Energies 2014, 7, 3179–3203. [Google Scholar] [CrossRef]
- Pharoa, J.G. On the permeability of gas diffusion media used in PEM fuel cells. J. Power Sources 2005, 144, 77–82. [Google Scholar] [CrossRef]
- O’Hayre, R.; Cha, S.W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2006. [Google Scholar]
- Kuo, J.K.; Chen, C.K. Evaluating the enhanced performance of a novel wave-like form gas flow channel in the PEMFC using the field synergy principle. J. Power Sources 2006, 162, 1122–1129. [Google Scholar] [CrossRef]
- Miansari, M.; Sedighi, K.; Amidpour, M.; Alizadeh, E.; Miansari, M. Experimental and thermodynamic approach on proton exchange membrane fuel cell performance. J. Power Sources 2009, 190, 356–361. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, K.R.; Ahn, D.K.; Choi, Y.D. Experimental analysis of a dimensionless number in the cathode channels of a polymer electrolyte membrane fuel cell with different head losses. Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci. 2009, 224, 95–108. [Google Scholar] [CrossRef]
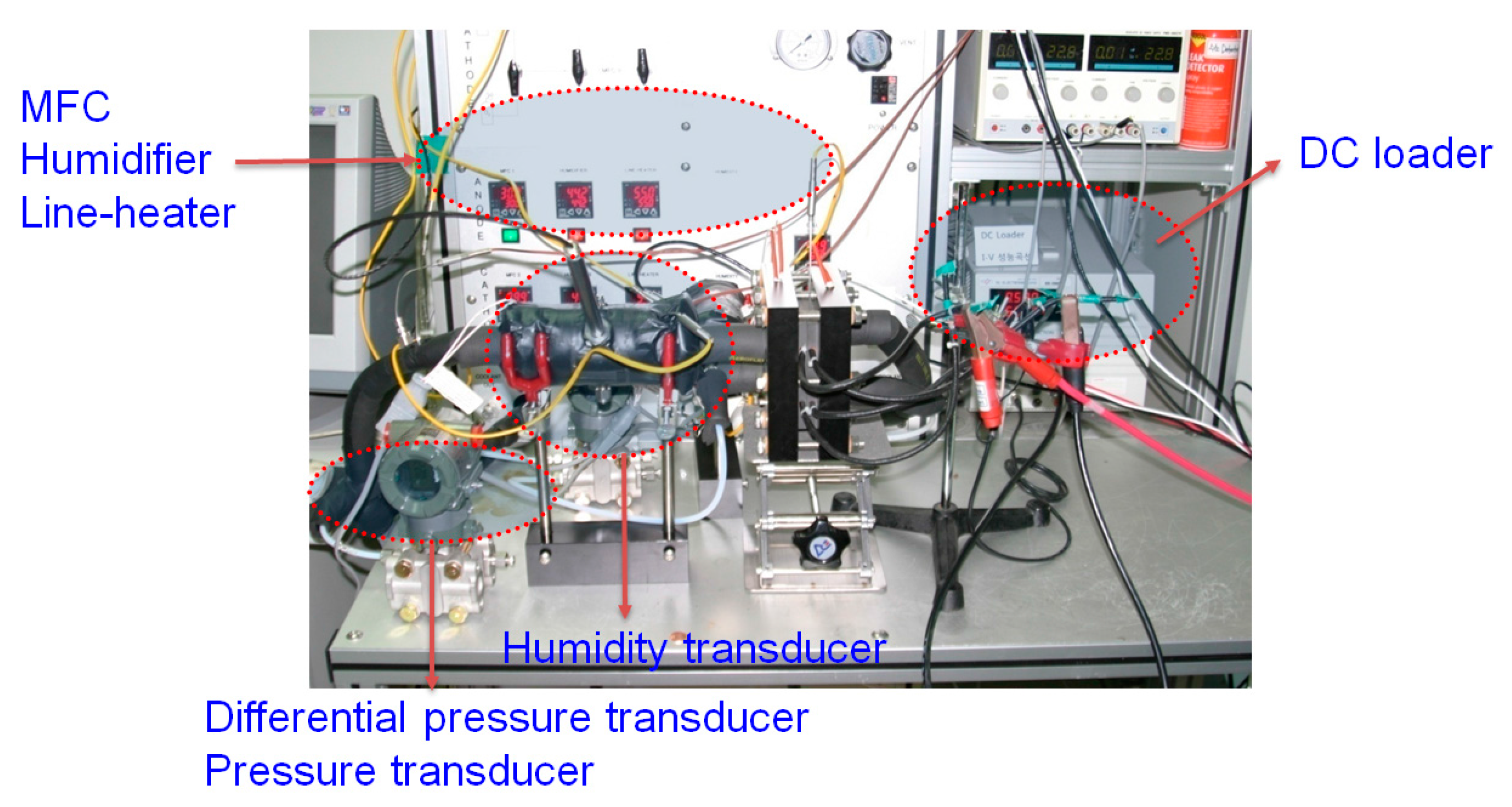
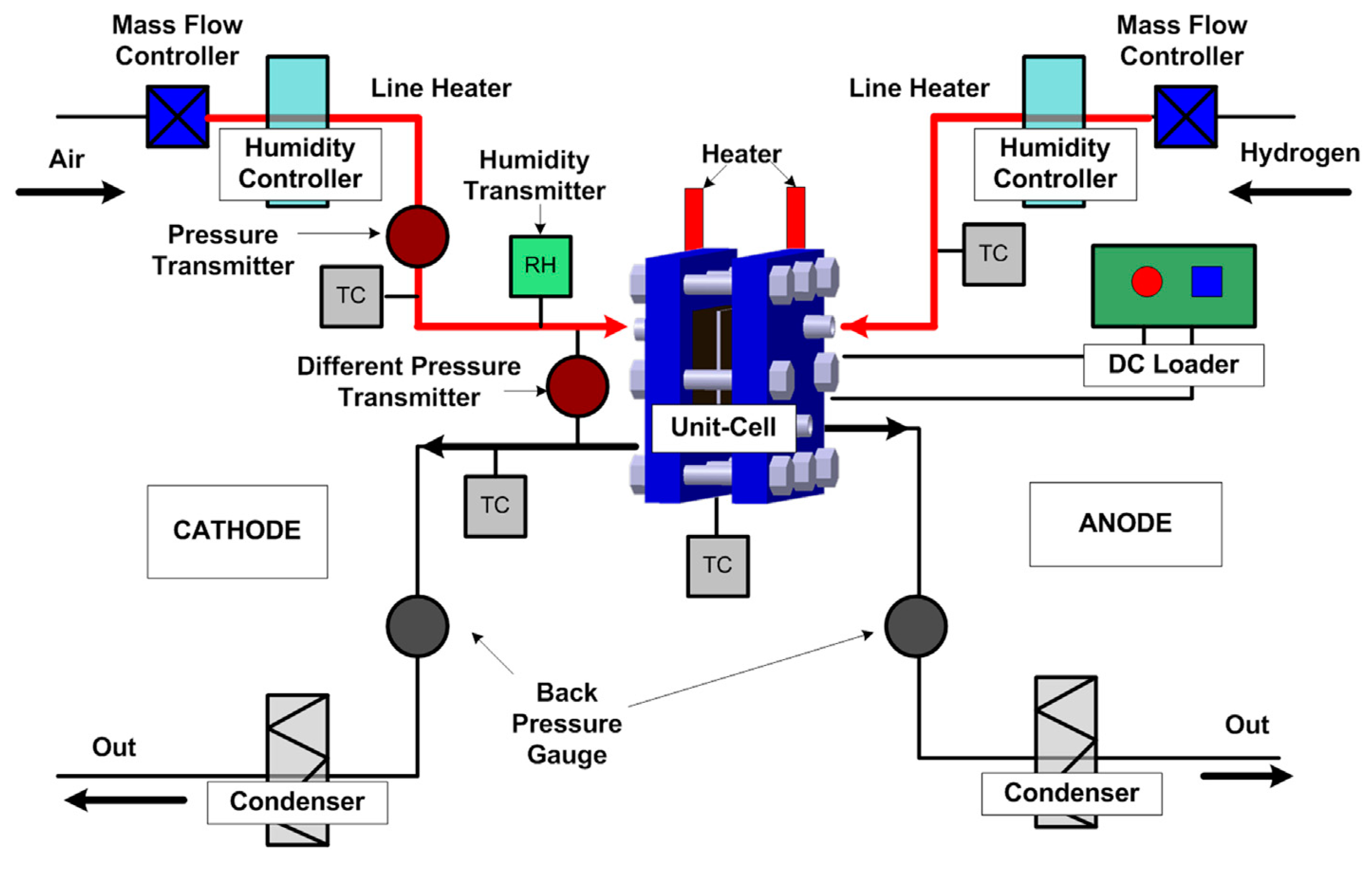


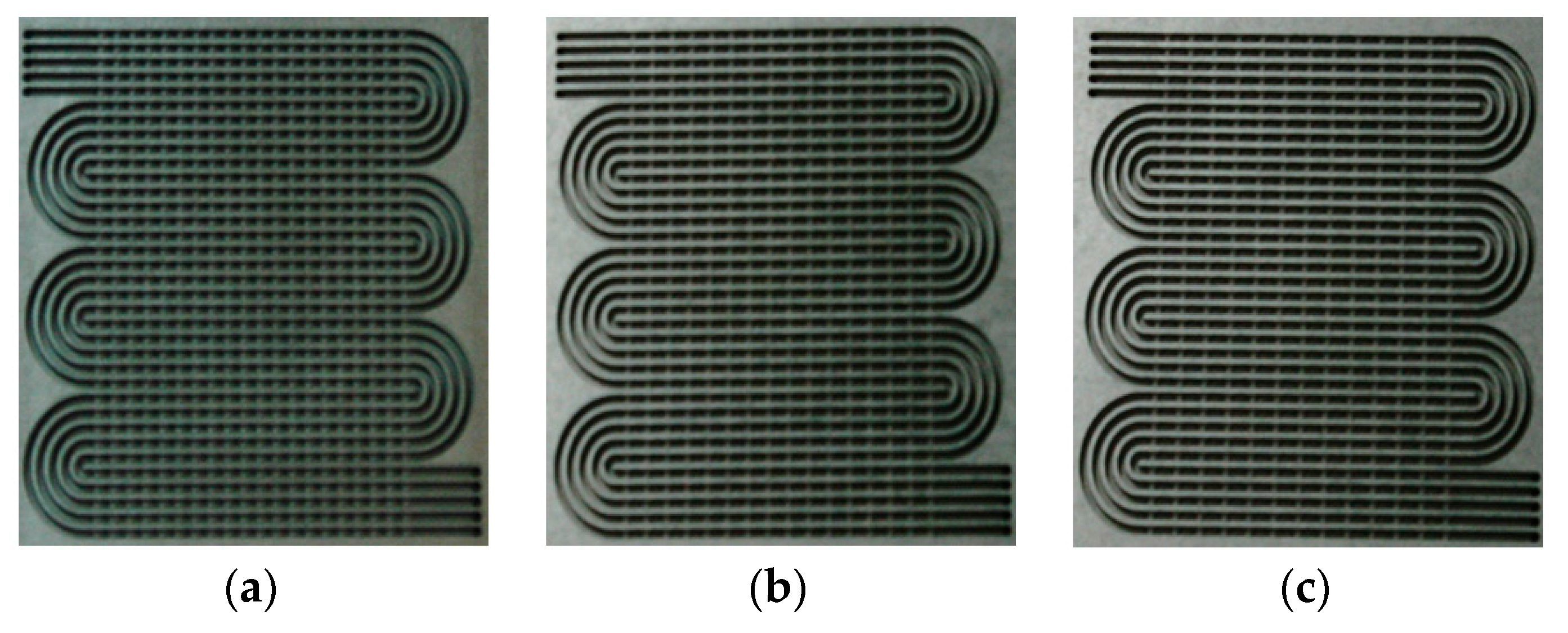





| Items | Location | Manufacturer | Model No. | Accuracy |
|---|---|---|---|---|
| Pressure gauge | Cathode inlet | YOKOGAWA | EJA530A | ±0.075% |
| Differential pressure gauge | Cathode | YOKOGAWA | EJA110A | ±0.075% |
| Electronic load | - | E.L.P. Tek | ESL-300Z | ±0.1% |
| Mass flow controller | Anode and cathode | Bronkhorst High-Tech | EL-flow F-201C | 0.5% |
| Thermocouple | Test section | Omega Engineering Inc. | T-type | 0.5 °C or 0.4% |
| Humidity | Cathode inlet | VAISALA | HMT 337 | ±1.5% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, S.-J.; Wang, Z.H.; Son, J.; Kwak, D.-K.; Kwon, Y.-C. Experimental Study on Improvement of Performance by Wave Form Cathode Channels in a PEM Fuel Cell. Energies 2018, 11, 319. https://doi.org/10.3390/en11020319
Byun S-J, Wang ZH, Son J, Kwak D-K, Kwon Y-C. Experimental Study on Improvement of Performance by Wave Form Cathode Channels in a PEM Fuel Cell. Energies. 2018; 11(2):319. https://doi.org/10.3390/en11020319
Chicago/Turabian StyleByun, Sun-Joon, Zhen Huan Wang, Jun Son, Dong-Kurl Kwak, and Young-Chul Kwon. 2018. "Experimental Study on Improvement of Performance by Wave Form Cathode Channels in a PEM Fuel Cell" Energies 11, no. 2: 319. https://doi.org/10.3390/en11020319





