Simultaneous Production of Transformer Insulating Oil and Value-Added Glycerol Carbonates from Soybean Oil by Lipase-Catalyzed Transesterification in Dimethyl Carbonate
Abstract
:1. Introduction
2. Results and Discussion
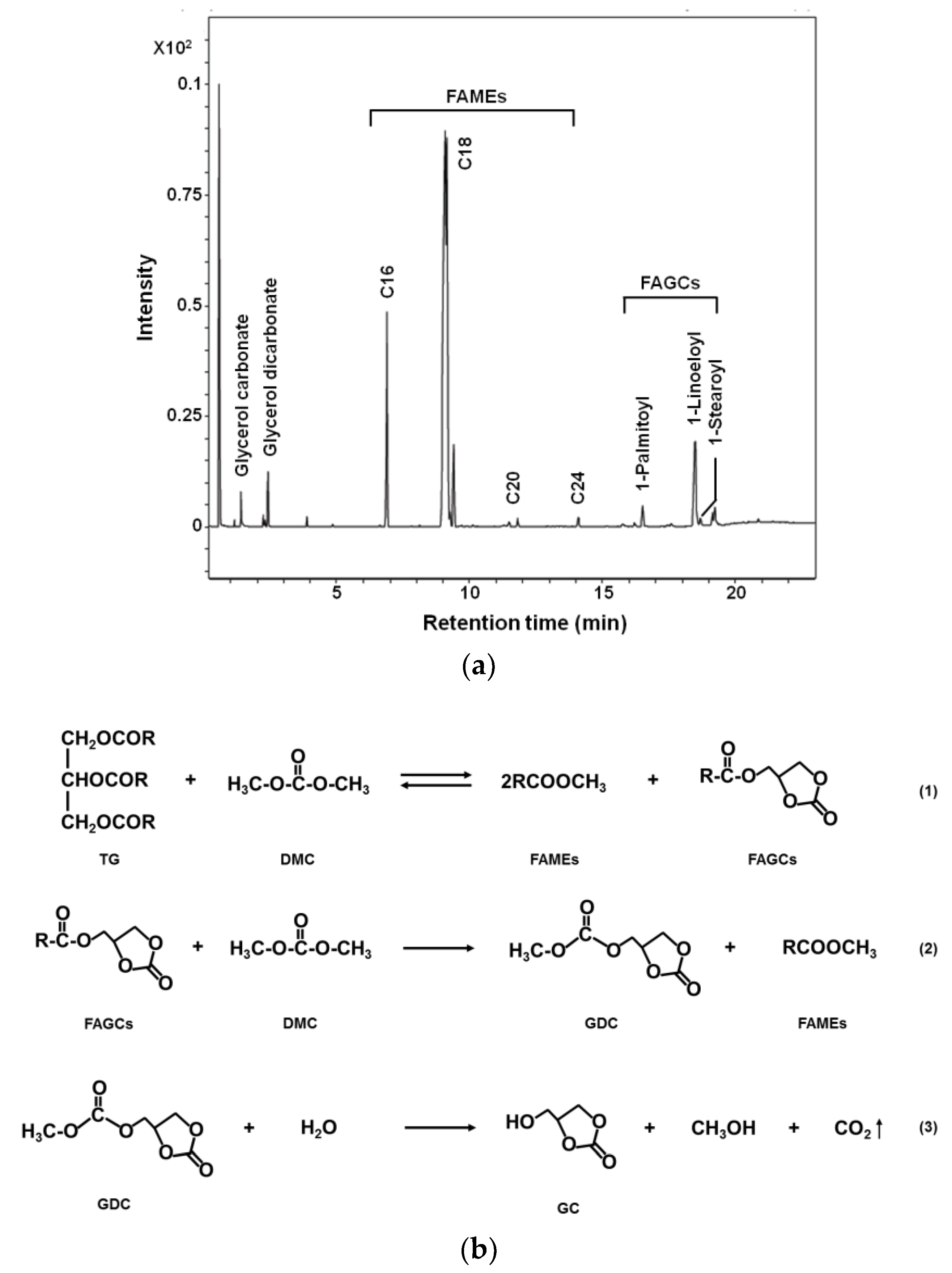
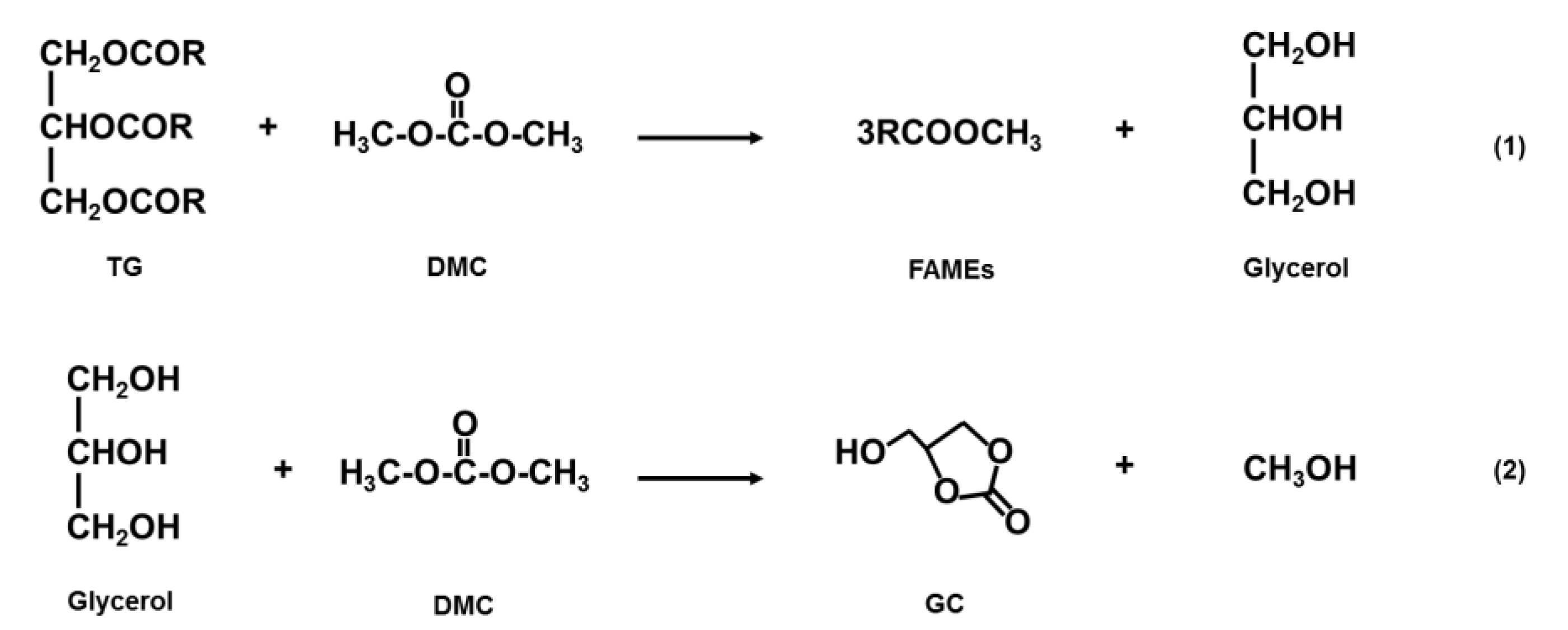
2.1. Basic Reaction Pathway of DMC-Based Transesterificaton
2.2. Biosynthesis of Transformer Insulating Oil Using Dimethyl Carbonate as an Acyl Acceptor
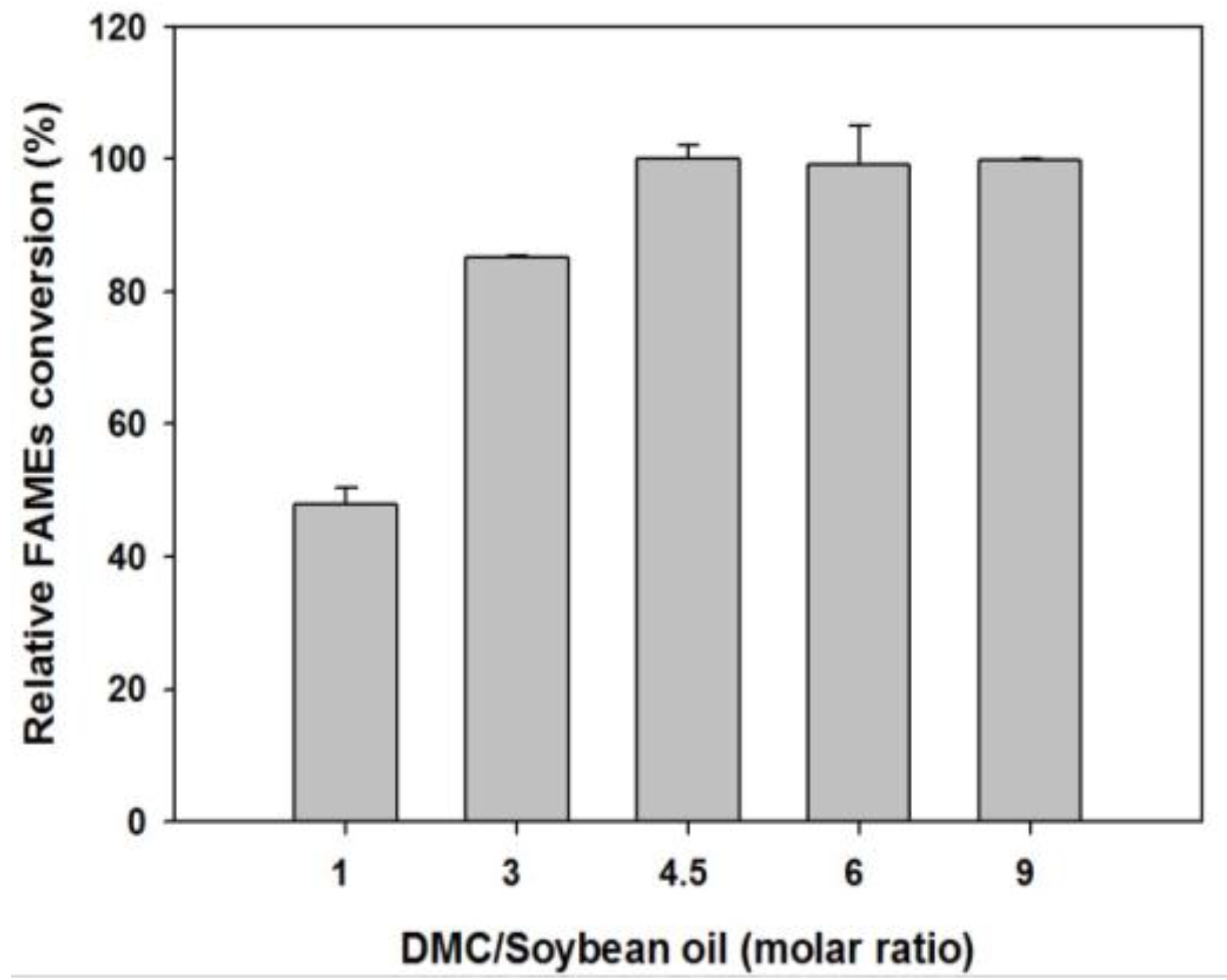
2.2.1. Effect of DMC-to-Soybean Oil Molar Ratio
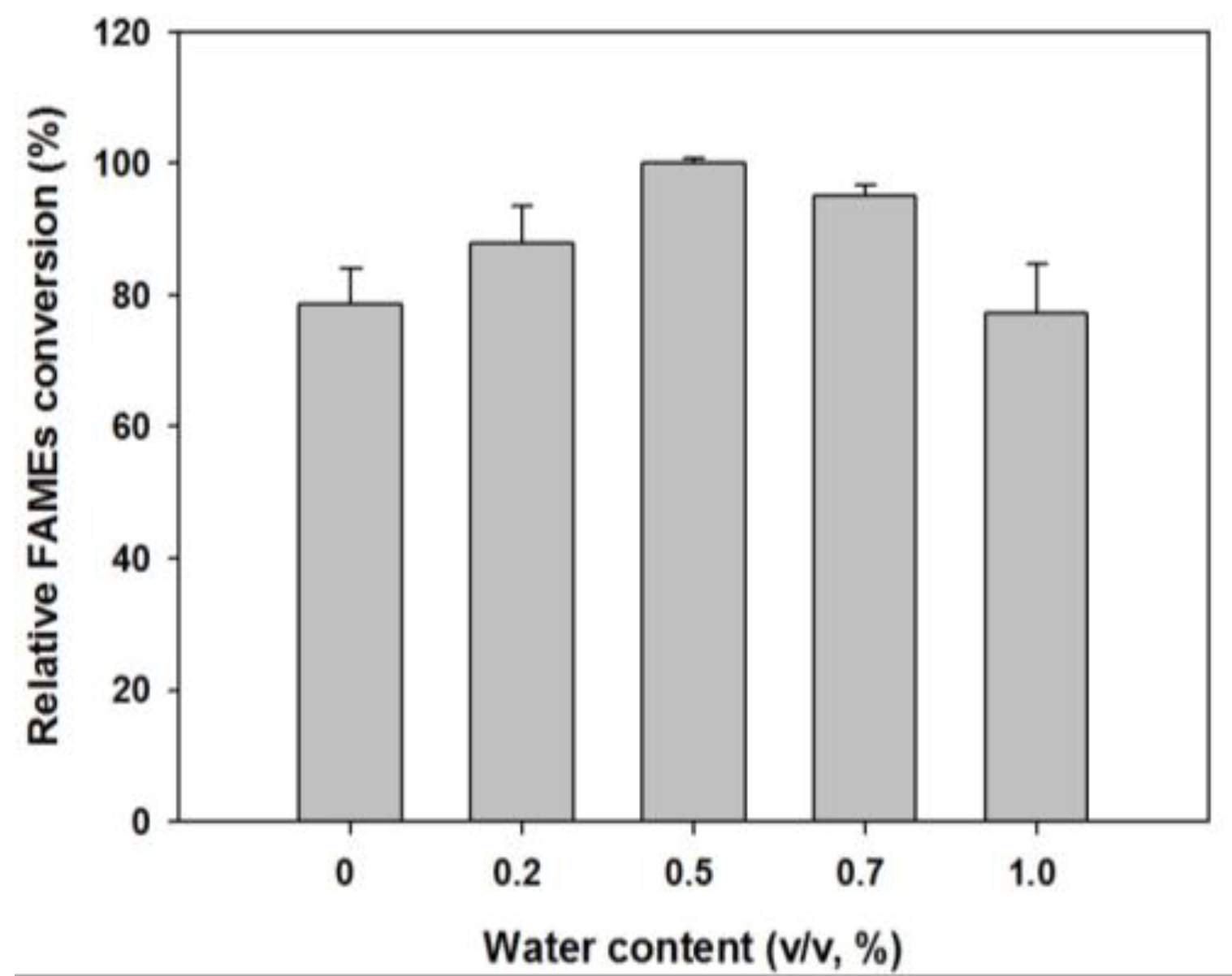
2.2.2. Effect of Water Addition
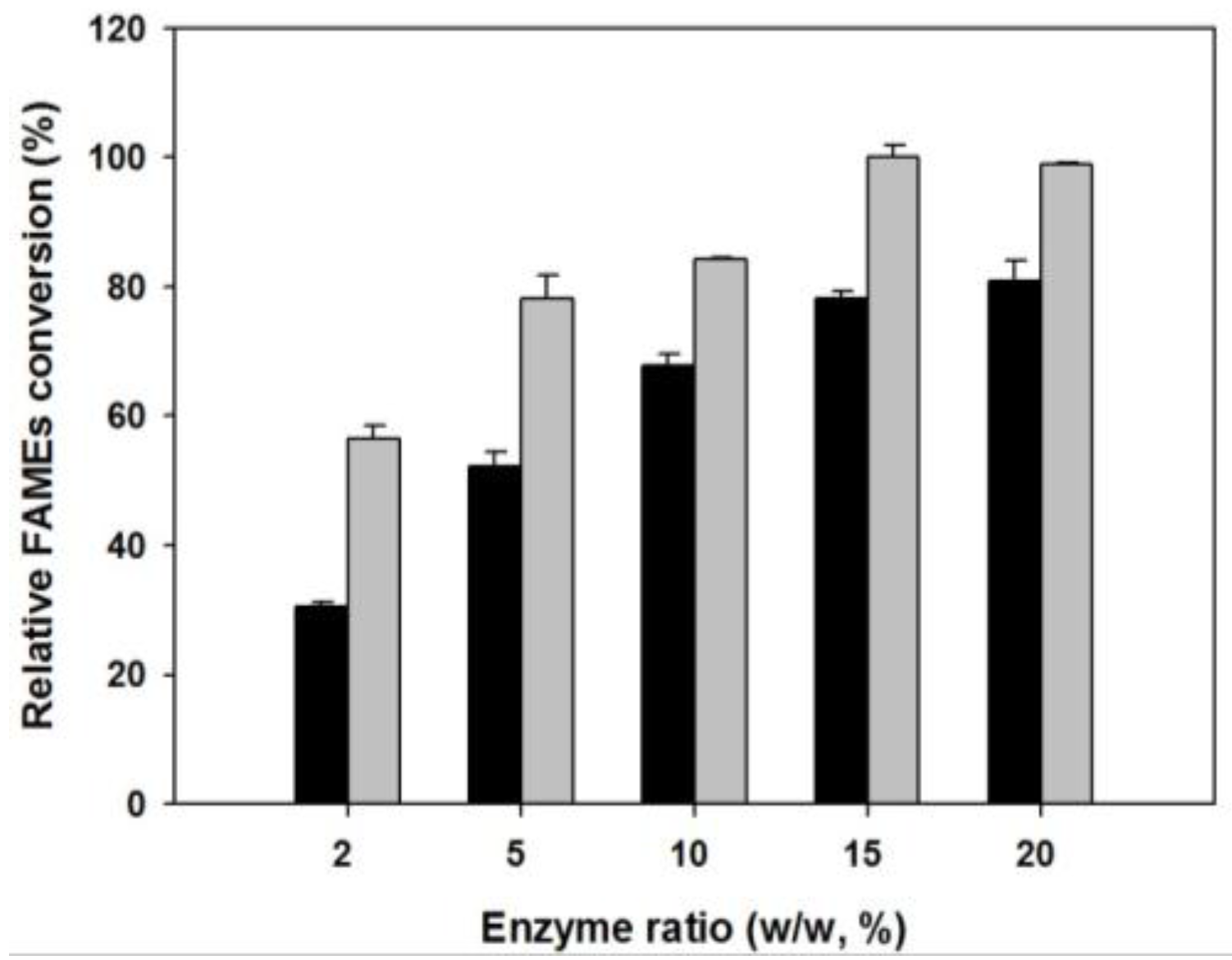
2.2.3. Effect of Enzyme-to-Soybean Oil Ratio
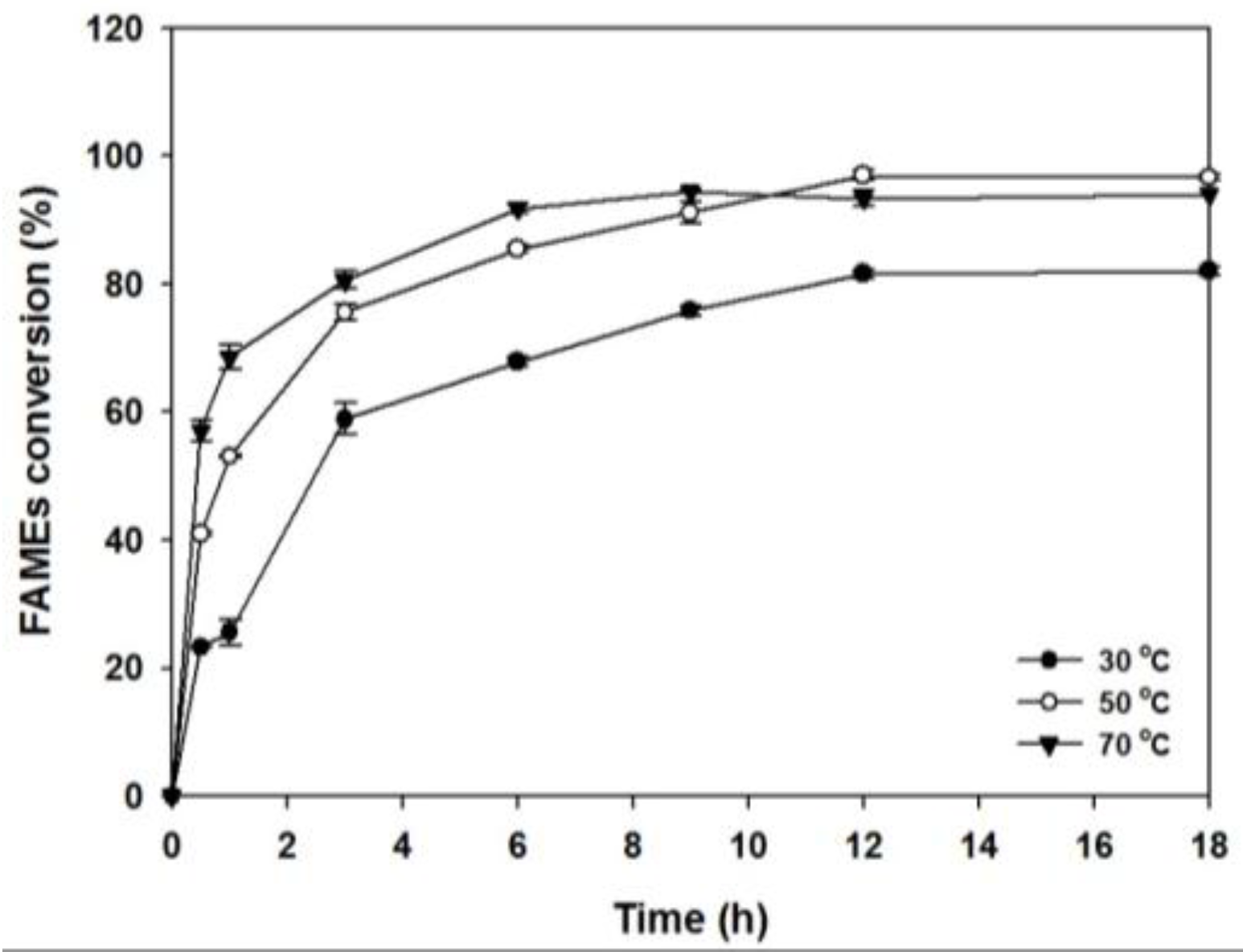
2.2.4. Effect of Reaction Temperature and Time
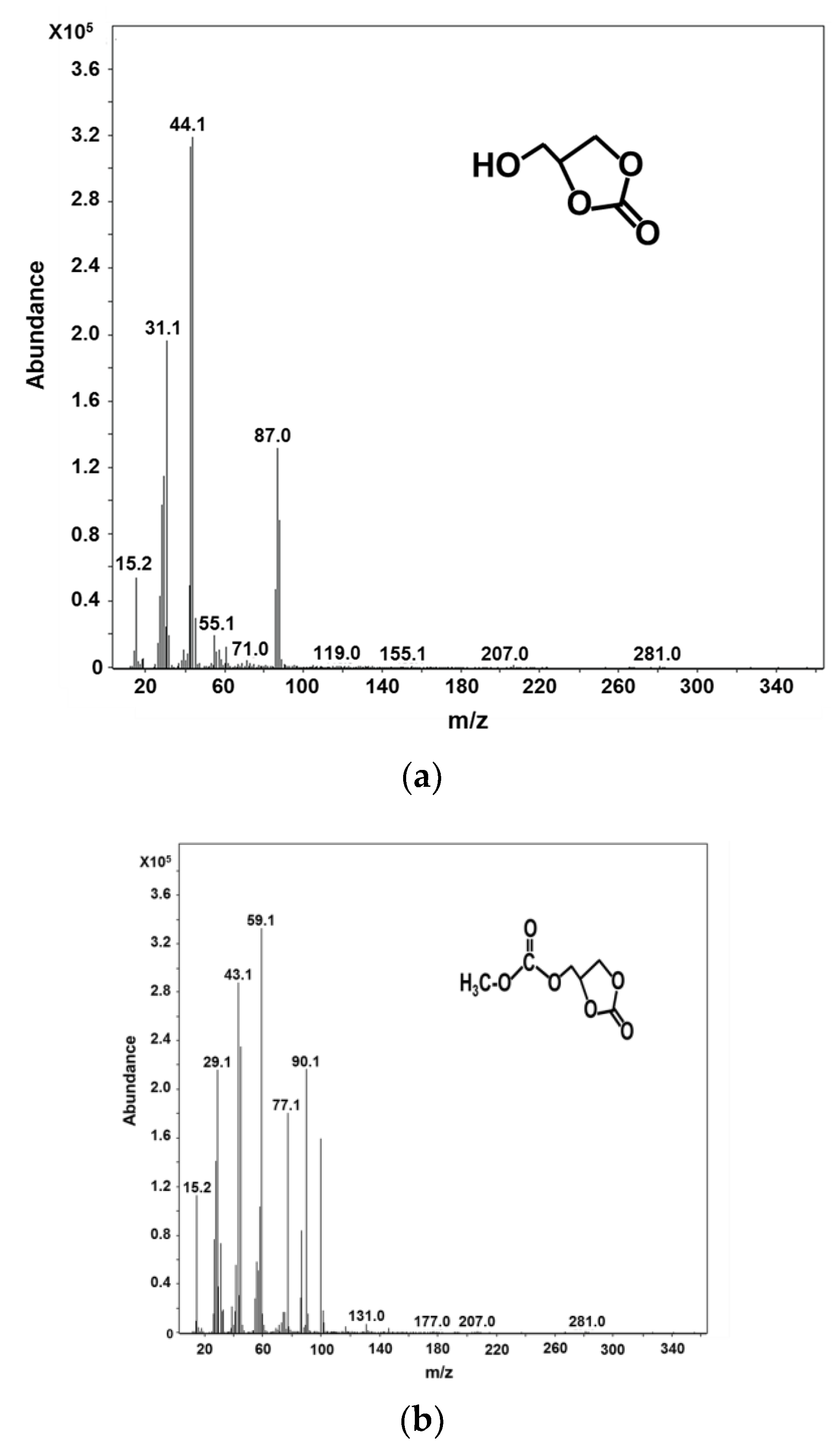
2.3. Characterization of Glycerol Carbonate and Glycerol Dicarbonate
2.4. Transformer Insulating Oil Properties
3. Materials and Methods
3.1. Materials
3.2. Simultaneous Biosynthesis of Transformer Insulating Oil and Glycerol Derivatives
3.2.1. Optimization of Transesterification
3.2.2. Scaling-Up of Transesterification
3.3. Analyses
3.4. Determination of Transformer Insulating Oil Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gwon, M.; Baek, J.H.; Kim, M.H.; Park, D.W.; Lee, M.S. Insulation properties and evaluation of diglycerol ester synthesized by solid acid catalysts. Appl. Chem. Eng. 2014, 253, 254–261. [Google Scholar] [CrossRef]
- Azis, N.; Jasni, J.; Ab Kadir, M.Z.A.; Mohtar, M.N. Suitability of palm based oil as dielectric insulating fluid in transformers. J. Electr. Eng. Technol. 2014, 9, 662–669. [Google Scholar] [CrossRef]
- Kanoh, T.; Iwabuchi, H.; Hoshida, T.Y.; Yamada, J.; Hikosaka, T.; Hatta, A.; Koide, H. Analyses of electro-chemical characteristics of palm fatty acid esters as insulating oil. In Proceedings of the 16th International Conference on Dielectric Liquids, Futuroscope-Chasseneuil, France, 30 June–3 July 2008; pp. 1–4. [Google Scholar]
- Rafiq, M.; Lv, Y.Z.; Zhou, Y.; Ma, K.B.; Wang, W.; Li, C.R.; Wang, Q. Use of vegetable oils as transformer oils—A review. Renew. Sustain. Energy Rev. 2015, 52, 308–324. [Google Scholar] [CrossRef]
- Radhika, R.V.; Iruthayarajan, M.W.; Pakianathan, P.S. Investigation of critical parameters of mixed insulating fluids. In Proceedings of the International Conference on Circuit, Power and Computing Technologies [ICCPCT], Nagercoil, India, 20–21 March 2014; pp. 357–362. [Google Scholar]
- Li, J.; Zhang, Z.; Grzybowski, S.; Liu, Y. Characteristics of moisture diffusion in vegetable oil-paper insulation. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1650–1656. [Google Scholar] [CrossRef]
- Muhamad, N.A.; Phung, B.T.; Blackburn, T.R. Dissolved gas analysis (DGA) of arcing faults in biodegradable oil insulation systems. In Proceedings of the 2008 International Symposium on Electrical Insulating Materials, Mie, Japan, 7–11 September 2008; pp. 24–27. [Google Scholar] [CrossRef]
- Cigré Working Group A2.35: Technical Brochure 436—Experiences in Service with New Insulating Liquids; Cigré: Paris, France, 2010.
- Marx, S. Glycerol-free biodiesel production through transesterification: A review. Fuel Process. Technol. 2016, 151, 139–147. [Google Scholar] [CrossRef]
- Leão, R.A.C.; Souza, S.P.; Nogueira, D.O.; Silva, G.M.A.; Silva, M.V.; Gutarra, M.L.E.; Miranda, L.S.M.; Castro, A.M.; Junior, I.I.; Souza, R.O.M.A. Consecutive lipase immobilization and glycerol carbonate production under continuous-flow conditions. Catal. Sci. Technol. 2016, 6, 4743–4748. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Production of biodiesel with glycerol carbonate by non-catalytic supercritical dimethyl carbonate. Lipid Technol. 2011, 23, 10–13. [Google Scholar] [CrossRef]
- Ang, G.T.; Tan, K.T.; Lee, K.T. Recent development and economic analysis of glycerol-free processes via supercritical fluid transesterification for biodiesel production. Renew. Sustain. Energy Rev. 2014, 31, 61–70. [Google Scholar] [CrossRef]
- Lee, O.K.; Kim, Y.H.; Na, J.G.; Oh, Y.K.; Lee, E.Y. Highly efficient extraction and lipase-catalyzed transesterification of triglycerides from Chlorella sp. KR-1 for production of biodiesel. Bioresour. Technol. 2013, 147, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, O.K.; Kim, C.H.; Seo, J.W.; Oh, B.R.; Lee, E.Y. Lipase-catalyzed in-situ biosynthesis of glycerol-free biodiesel from heterotrophic microalgae, Aurantiochytrium sp. KRS101 biomass. Bioresour. Technol. 2016, 211, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Su, E.; You, P.; Wei, D. In situ lipase-catalyzed reactive extraction of oilseeds with short-chained dialkyl carbonates for biodiesel production. Bioresour. Technol. 2009, 100, 5813–5817. [Google Scholar] [CrossRef] [PubMed]
- Kai, T.; Mak, G.L.; Wada, S.; Nakazato, T.; Takanashi, H.; Uemura, Y. Production of biodiesel fuel from canola oil with dimethyl carbonate using an active sodium methoxide catalyst prepared by crystallization. Bioresour. Technol. 2014, 163, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.G.; Chimentão, R.J.; Figueras, F.; Medina, F. Tunable basic and textural properties of hydrotalcite derived materials for transesterification of glycerol. Appl. Clay Sci. 2012, 58, 16–24. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, C.H.; Lee, E.Y. Biosynthesis of glycerol carbonate from glycerol by lipase in dimethyl carbonate as the solvent. Bioprocess Biosyst. Eng. 2010, 33, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Su, E.Z.; Zhang, M.J.; Zhang, J.G.; Gao, J.F.; Wei, D.Z. Lipase-catalyzed irreversible transesterification of vegetable oils for fatty acid methyl esters production with dimethyl carbonate as the acyl acceptor. Biochem. Eng. J. 2007, 36, 167–173. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Ayodele, O.O.; Xin, J.; Zhang, S. Dimethyl carbonate mediated production of biodiesel at different reaction temperatures. Renew. Energy 2014, 68, 581–587. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, S.; Xin, Z.; Sheng, B.; Liu, Q. Synthesis and component confirmation of biodiesel from palm oil and dimethyl carbonate catalyzed by immobilized-lipase in solvent-free system. Fuel 2010, 89, 3960–3965. [Google Scholar] [CrossRef]
- Oehlenschläger, J.; Gercken, G. Synthesis and mass spectrometry of 1-acyl and 3-acyl-sn-glycerol carbonates. Lipids 1978, 13, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sheng, B.; Xin, Z.; Liu, Q.; Sun, S. Kinetics of transesterification of palm oil and dimethyl carbonate for biodiesel production at the catalysis of heterogeneous base catalyst. Bioresour. Technol. 2010, 101, 8144–8150. [Google Scholar] [CrossRef] [PubMed]
- Jegannathan, K.R.; Abang, S.; Poncelet, D.; Chan, E.S.; Ravindra, P. Production of biodiesel using immobilized lipase—A critical review. Crit. Rev. Biotechnol. 2008, 28, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Seong, P.J.; Jeon, B.W.; Lee, M.; Cho, D.H.; Kim, D.K.; Jung, K.S.; Han, S.O.; Kim, Y.H.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil and dimethyl carbonate. Enzyme Microb. Technol. 2011, 48, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.J.; Lee, O.K.; Lee, E.Y. Dimethyl carbonate-mediated lipid extraction and lipase-catalyzed in situ transesterification for simultaneous preparation of fatty acid methyl esters and glycerol carbonate from Chlorella sp. KR-1 biomass. Bioresour. Technol. 2014, 158, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Batan, L.; Quinn, J.; Willson, B.; Bradley, T. Net energy and greenhouse gas emission evaluation of biodiesel derived from microalgae. Environ. Sci. Technol. 2010, 44, 7975–7980. [Google Scholar] [CrossRef] [PubMed]

| Properties | Unit | Mineral Oil | Natural Ester a | In This Study | |
|---|---|---|---|---|---|
| Density | g/cm3 | <0.91 | 0.92 | 0.92 | |
| Viscosity | 40 °C | mm2/s | <13.0 | 33.0 | 6.66 |
| 100 °C | <4.0 | - | 2.29 | ||
| Pour point | °C | <−27.5 | - | 0 | |
| Flash point | °C | 140.0 | 316.0 | 124.0 | |
| Dielectric breakdown voltage | kV | >40.0 | 56.0 | 82.0 | |
| Moisture | (m/m) % | <0.003 | - | 0.017 | |
| Oxidation stability | Sludge | % | - | - | <0.01 |
| Acid value | mg KOH/g | <0.40 | - | 0.30 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.H.; Lee, E.Y. Simultaneous Production of Transformer Insulating Oil and Value-Added Glycerol Carbonates from Soybean Oil by Lipase-Catalyzed Transesterification in Dimethyl Carbonate. Energies 2018, 11, 82. https://doi.org/10.3390/en11010082
Kim KH, Lee EY. Simultaneous Production of Transformer Insulating Oil and Value-Added Glycerol Carbonates from Soybean Oil by Lipase-Catalyzed Transesterification in Dimethyl Carbonate. Energies. 2018; 11(1):82. https://doi.org/10.3390/en11010082
Chicago/Turabian StyleKim, Keon Hee, and Eun Yeol Lee. 2018. "Simultaneous Production of Transformer Insulating Oil and Value-Added Glycerol Carbonates from Soybean Oil by Lipase-Catalyzed Transesterification in Dimethyl Carbonate" Energies 11, no. 1: 82. https://doi.org/10.3390/en11010082





