Novel Receiver-Enhanced Solar Vapor Generation: Review and Perspectives
Abstract
:1. Introduction
2. Volumetric Solar Receivers for Vapor Generation
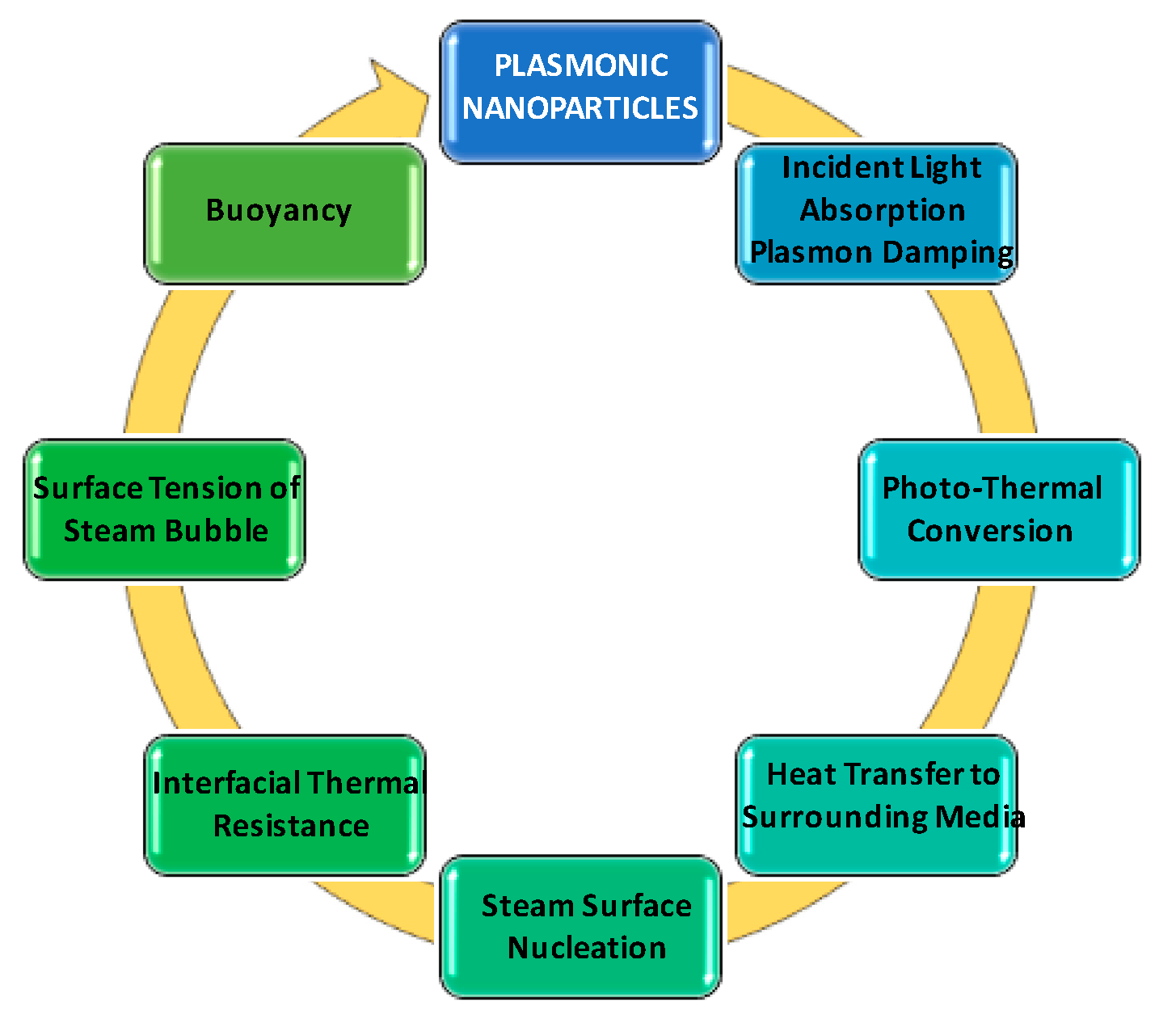
- Incident light absorption to produce heat by plasmonic nanoparticles, and rapid transfer of heat to the water in the immediate vicinity.
- Heating of thin water layers around the plasmonic nanoparticles to its boiling point to produce steam.
- Owing to the poor thermal conductivity of steam, inhibition of heat transfer from the heated nanoparticle to the bulk water.
- Gradual growth of thickness of the steam shell to several hundred nanometers upon further heating of nanoparticle by sunlight. Decrease in weight of the steam/nanoparticle assembly as compared with equivalent volume of water to creates buoyancy force.
- Lastly, the steam bubble reaches the surface and steam escapes from the water.
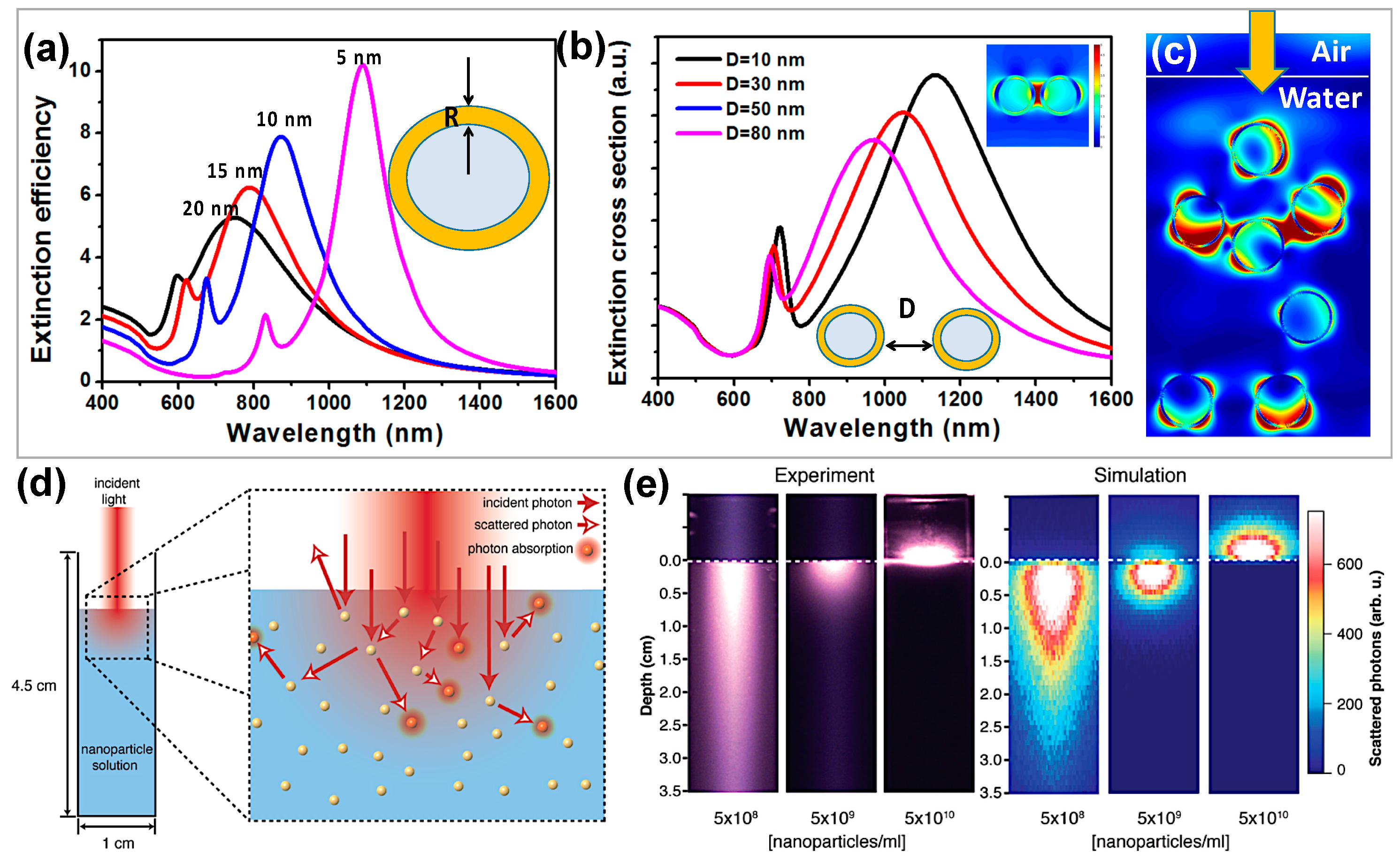
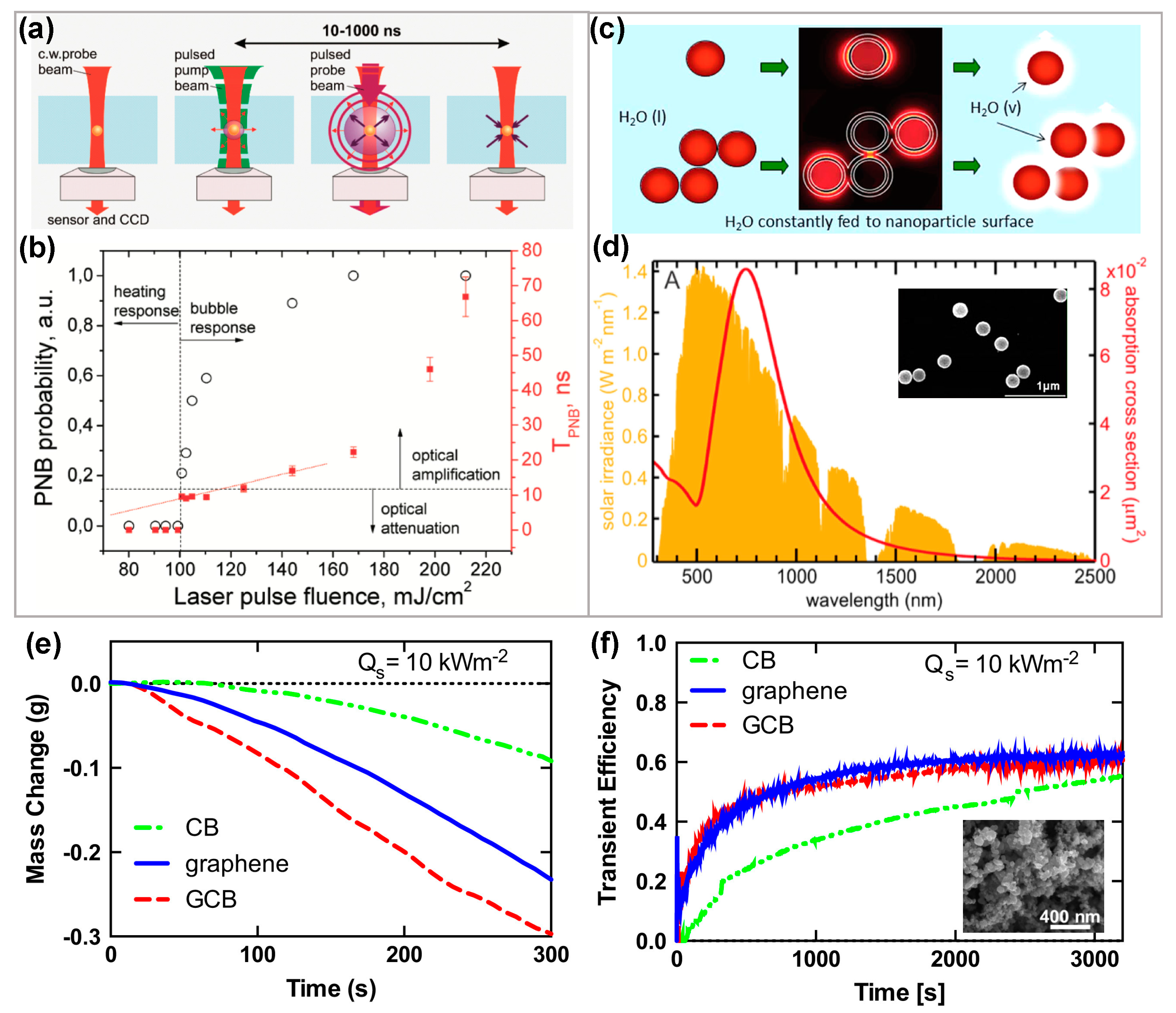
2.1. Nanobubbles Formation Using Conductive Nanoparticle under Laser Irradiance
2.2. Vapor Generation Using Core Shell Nanoparticles under Solar Irradiance
2.3. Vapor Generation Using Carbon Nanofluids under Solar Irradiance
2.4. Challenges of Volumetric Solar Receiver
3. Floating Solar Receivers for Vapor Generation
3.1. Mechanismof Solar Thermal Energy Transport
3.1.1. Light Absorption
3.1.2. Thermal Management
3.1.3. Liquid Propagation
3.1.4. Antifouling and Durability
3.1.5. Condensation and Collection
3.2. Recent Development in Floating Solar Receivers for Vapor Generation
3.2.1. Carbon/Graphene Based Floating Solar Receiver
3.2.2. Organic-Inorganic Nanocomposite Based Floating Solar Receivers
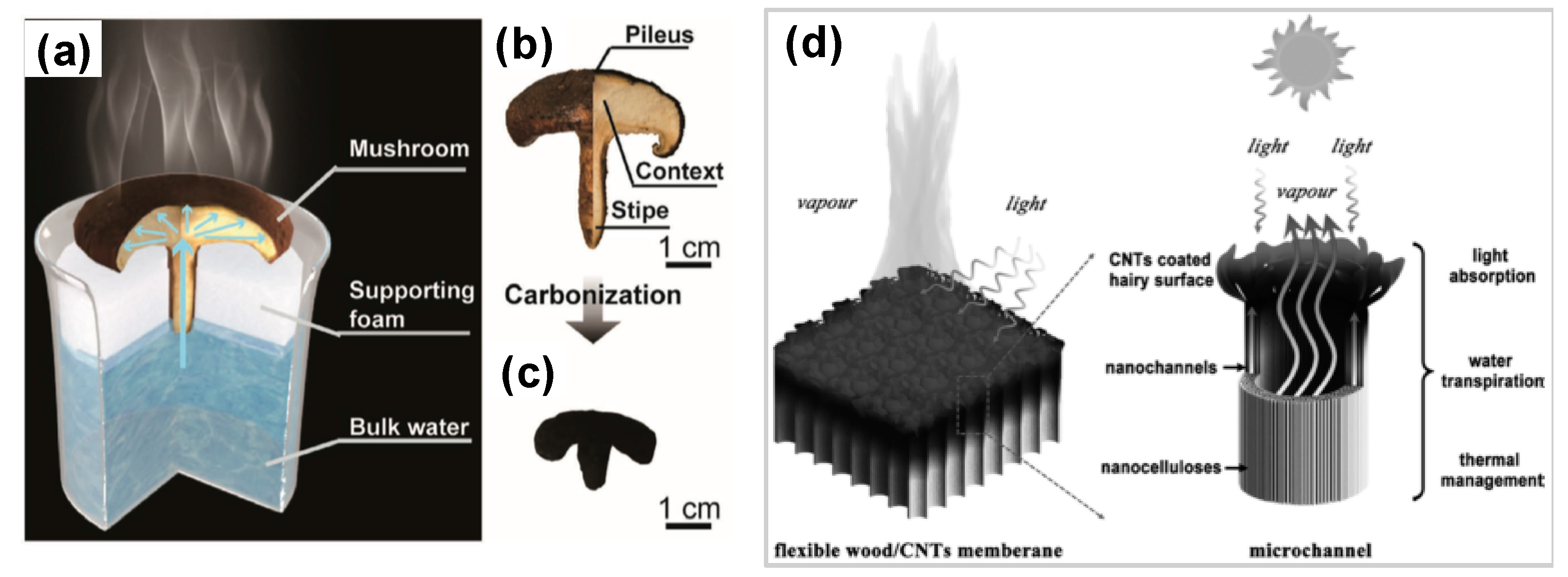
3.2.3. Bio-Composites Based Floating Solar Receivers
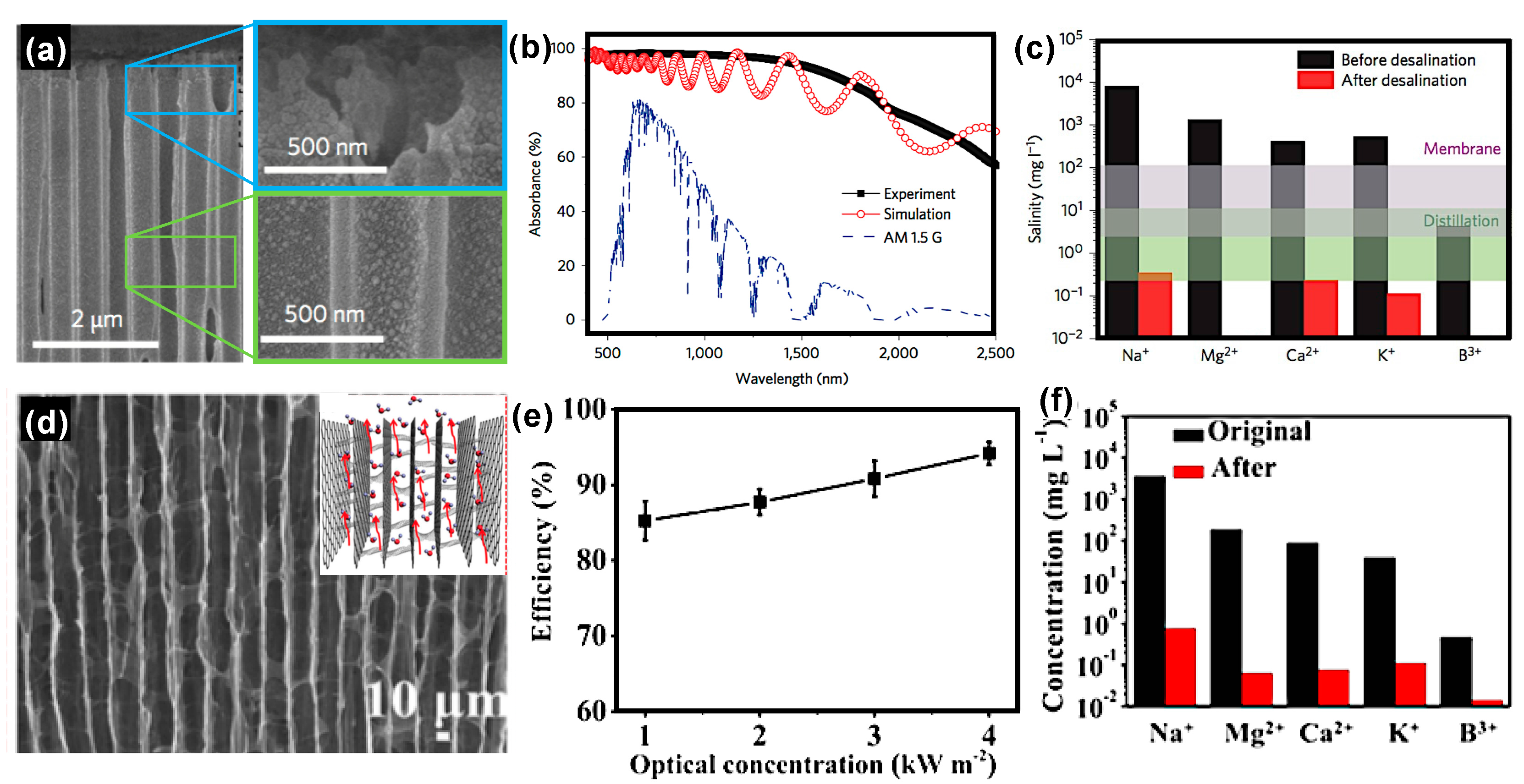
3.3. Solar Assisted Water Desalination Using Floating Solar Receivers
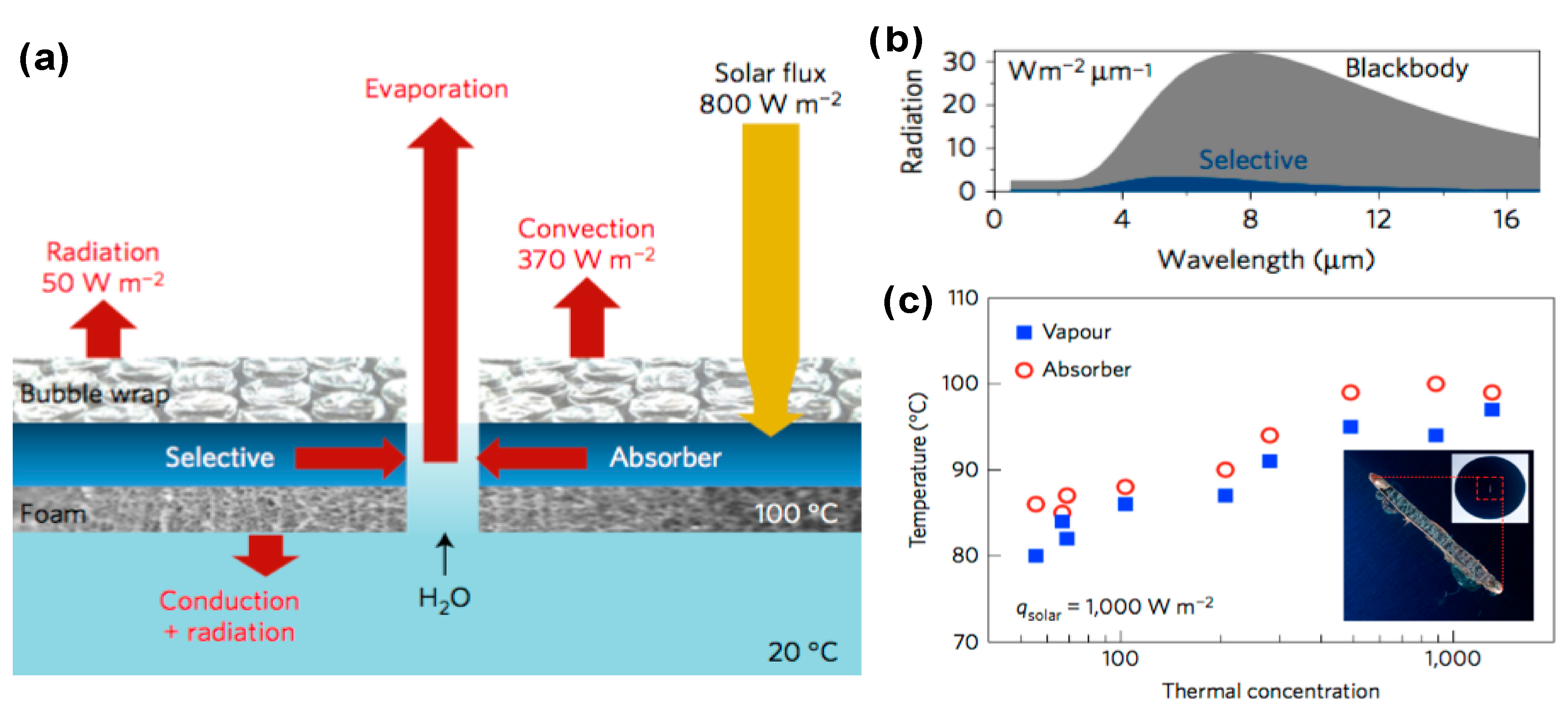
4. High-Temperature Steam Generation
4.1. High Temperature Steam Using Optical Concentrator
4.2. High Temperature Steam Using Thermal Concentrator
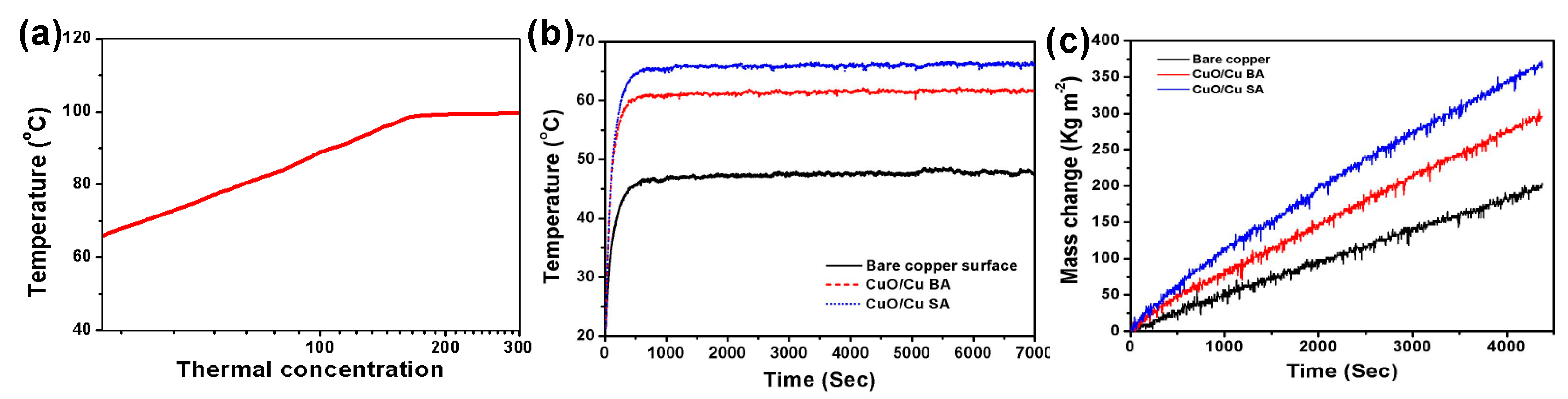
4.3. Scalable Thermal Concentration Design for Steam Generation
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tian, Y.; Zhao, C.Y. A review of solar collectors and thermal energy storage in solar thermal applications. Appl. Energy 2013, 104, 538–553. [Google Scholar] [CrossRef] [Green Version]
- Tritt, T.M.; Böttner, H.; Chen, L. Thermoelectrics: Direct Solar Thermal Energy Conversion. MRS Bull. 2008, 33, 366–368. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Sorrell, S.; Speirs, J.; Bentley, R.; Miller, R.; Thompson, E. Shaping the global oil peak: A review of the evidence on field sizes, reserve growth, decline rates and depletion rates. Energy 2012, 37, 709–724. [Google Scholar] [CrossRef]
- Thirugnanasambandam, M.; Iniyan, S.; Goic, R. A review of solar thermal technologies. Renew. Sustain. Energy Rev. 2010, 14, 312–322. [Google Scholar] [CrossRef]
- Water, Sanitation and Hygiene. Available online: https://www.unicef.org/wash/3942_resources.html (accessed on 12 July 2017).
- Van der Bruggen, B.; Vandecasteele, C. Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut. 2003, 122, 435–445. [Google Scholar] [CrossRef]
- Raza, A.; Ding, B.; Zainab, G.; El-Newehy, M.; Al-Deyab, S.S.; Yu, J. In situ cross-linked superwetting nanofibrous membranes for ultrafast oil-water separation. J. Mater. Chem. A 2014, 2, 10137–10145. [Google Scholar] [CrossRef]
- Shang, Y.; Si, Y.; Raza, A.; Yang, L.; Mao, X.; Ding, B.; Yu, J. An in situ polymerization approach for the synthesis of superhydrophobic and superoleophilic nanofibrous membranes for oil-water separation. Nanoscale 2012, 4, 7847–7854. [Google Scholar] [CrossRef] [PubMed]
- Ghernaout, D.; Naceur, M.W.; Aouabed, A. On the dependence of chlorine by-products generated species formation of the electrode material and applied charge during electrochemical water treatment. Desalination 2011, 270, 9–22. [Google Scholar] [CrossRef]
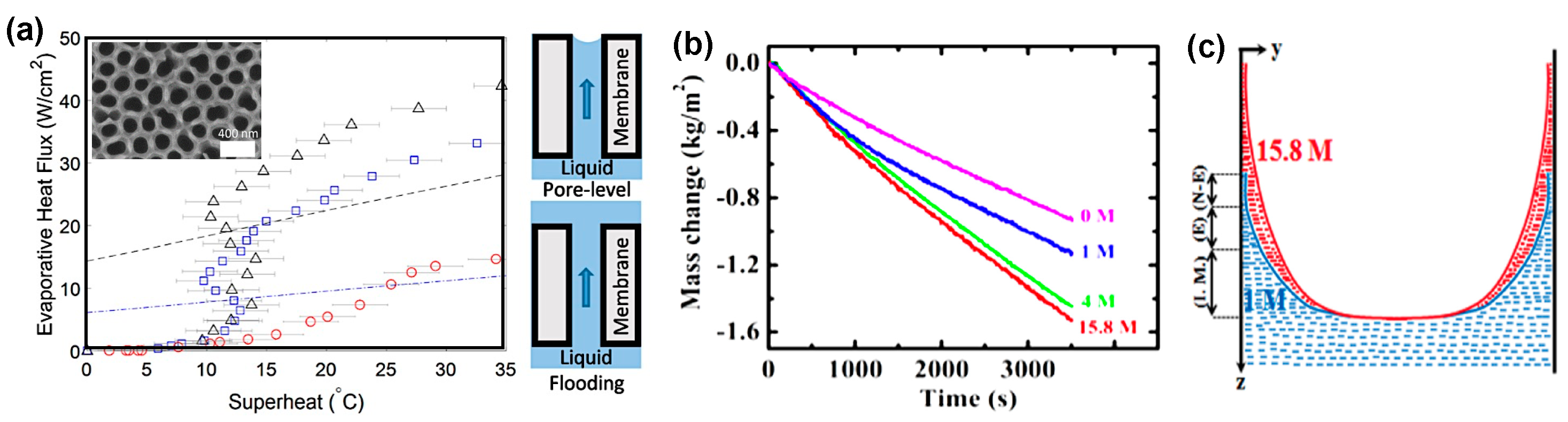
- Wilke, K.L.; Barabadi, B.; Zhang, T.; Wang, E.N. Controlled Wetting in Nanoporous Membranes for Thin Film Evaporation. J. Heat Transf. 2016, 138, 80906. [Google Scholar] [CrossRef]
- Adera, S.; Antao, D.; Raj, R.; Wang, E.N. Design of micropillar wicks for thin-film evaporation. Int. J. Heat Mass Transf. 2016, 101, 280–294. [Google Scholar] [CrossRef]
- Goto, K.; Yogo, K.; Higashii, T. A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture. Appl. Energy 2013, 111, 710–720. [Google Scholar] [CrossRef]
- Chugh, Y.P.; Patwardhan, A. Mine-mouth power and process steam generation using fine coal waste fuel. Resour. Conserv. Recycl. 2004, 40, 225–243. [Google Scholar] [CrossRef]
- Paul, P.; Al Tenaiji, A.K.; Braimah, N. A Review of the Water and Energy Sectors and the Use of a Nexus Approach in Abu Dhabi. Int. J. Environ. Res. Public Health 2016, 13, 364. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Malato, S.; Fernández-Ibañez, P.; Alarcón, D.; Gernjak, W.; Maldonado, M.I. Review of feasible solar energy applications to water processes. Renew. Sustain. Energy Rev. 2009, 13, 1437–1445. [Google Scholar] [CrossRef]
- Collado, F.J.; Guallar, J. A review of optimized design layouts for solar power tower plants with campo code. Renew. Sustain. Energy Rev. 2013, 20, 142–154. [Google Scholar] [CrossRef]
- Neumann, O.; Neumann, A.D.; Tian, S.; Thibodeaux, C.; Shubhankar, S.; Müller, J.; Silva, E.; Alabastri, A.; Bishnoi, S.W.; Nordlander, P.; Halas, N.J. Combining Solar Steam Processing and Solar Distillation for Fully Off-Grid Production of Cellulosic Bioethanol. ACS Energy Lett. 2017, 2, 8–13. [Google Scholar] [CrossRef]
- Qiblawey, H.M.; Banat, F. Solar thermal desalination technologies. Desalination 2008, 220, 633–644. [Google Scholar] [CrossRef]
- El-Sebaii, A.A.; Ramadan, M.R.I.; Aboul-Enein, S.; Khallaf, A.M. History of the solar ponds: A review study. Renew. Sustain. Energy Rev. 2011, 15, 3319–3325. [Google Scholar] [CrossRef]
- Kaushal, A. Varun Solar stills: A review. Renew. Sustain. Energy Rev. 2010, 14, 446–453. [Google Scholar] [CrossRef]
- Compain, P. Solar Energy for Water Desalination. Procedia Eng. 2012, 46, 220–227. [Google Scholar] [CrossRef]
- Is Solar Hot Water Cost Effective? Available online: http://solargroup.co.nz/is-solar-hot-water-cost-effective/ (accessed on 10 March 2014).
- Stack Ventilation and Bernoulli’s Principle. Available online: https://sustainabilityworkshop.autodesk.com/buildings/stack-ventilation-and-bernoullis-principle (accessed on 10 July 2014).
- Available online: http://mitzuki.co/service/solar-water-weaters/ (accessed on 08 August 2015).
- Solar Cooker. Available online: https://www.indiamart.com/essential-equipments/solar-cooker.html (accessed on 14 November 2017).
- Available online: https://homeplaceearth.files.wordpress.com/2011/05/solar-food-dryers-5-30-11.jpg (accessed on 3 May 2011).
- A Gigantic Floating Pipe Covered in Solar Panels Could Help Save California from Drought. Available online: http://www.businessinsider.com/solar-powered-desalinization-tube-2016-8 (accessed on 10 August 2016).
- Available online: https://www.slideshare.net/kanhaiya123654/solar-pond-67227225 (accessed on 14 November 2017).
- Aquamate Solar Still. Available online: http://www.landfallnavigation.com/memss.html (accessed on 14 November 2017).
- Serrano, E.; Rus, G.; García-Martínez, J. Nanotechnology for sustainable energy. Renew. Sustain. Energy Rev. 2009, 13, 2373–2384. [Google Scholar] [CrossRef]
- Halas, N.J.; Lal, S.; Chang, W.-S.; Link, S.; Nordlander, P. Plasmons in Strongly Coupled Metallic Nanostructures. Chem. Rev. 2011, 111, 3913–3961. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Raza, A.; Fang, N.X.; Chen, G.; Zhang, T. Effective dielectric constants and spectral density analysis of plasmonic nanocomposites. J. Appl. Phys. 2016. [Google Scholar] [CrossRef]
- Ann, S.; Shirley, A.M. Undefined Plasmon. Polar Rec. (Gr. Brit.). 2012, 48, 198. [Google Scholar] [CrossRef]
- Lu, J.Y.; Chang, Y.H. Optical singularities associated with the energy flow of two closely spaced core-shell nanocylinders. Opt. Express 2009, 17, 19451–19458. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Chang, Y.H. Implementation of an efficient dielectric function into the finite difference time domain method for simulating the coupling between localized surface plasmons of nanostructures. Superlattices Microstruct. 2010, 47, 60–65. [Google Scholar] [CrossRef]
- Lu, J.Y.; Chao, H.Y.; Wu, J.C.; Wei, S.Y.; Chang, Y.H.; Chen, S.C. Retardation-effect-induced plasmon modes in a silica-core gold-shell nanocylinder pair. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 42, 2583–2587. [Google Scholar] [CrossRef]
- Hogan, N.J.; Urban, A.S.; Ayala-Orozco, C.; Pimpinelli, A.; Nordlander, P.; Halas, N.J. Nanoparticles heat through light localization. Nano Lett. 2014, 14, 4640–4645. [Google Scholar] [CrossRef] [PubMed]
- Neumann, O.; Urban, A.S.; Day, J.; Lal, S.; Nordlander, P.; Halas, N.J. Solar vapor generation enabled by nanoparticles. ACS Nano 2013, 7, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Phelan, P.E.; Adrian, R.J.; Gunawan, A.; Otanicar, T.P. Characterization of light-induced, volumetric steam generation in nanofluids. Int. J. Therm. Sci. 2012, 56, 1–11. [Google Scholar] [CrossRef]
- Alashkar, A.; Gadalla, M. Thermo-economic analysis of an integrated solar power generation system using nanofluids. Appl. Energy 2017, 191, 469–491. [Google Scholar] [CrossRef]
- Lukianova-Hleb, E.; Hu, Y.; Latterini, L.; Tarpani, L.; Lee, S.; Drezek, R.A.; Hafner, J.H.; Lapotko, D.O. Plasmonic nanobubbles as transient vapor nanobubbles generated around plasmonic nanoparticles. ACS Nano 2010, 4, 2109–2123. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Miljkovic, N.; Ghasemi, H.; Huang, X.; Boriskina, S.V.; Lin, C.-T.; Wang, J.; Xu, Y.; Rahman, M.M.; Zhang, T.; Chen, G. Volumetric solar heating of nanofluids for direct vapor generation. Nano Energy 2015, 17, 290–301. [Google Scholar] [CrossRef]
- Jin, H.; Lin, G.; Bai, L.; Zeiny, A.; Wen, D. Steam generation in a nanoparticle-based solar receiver. Nano Energy 2016, 28, 397–406. [Google Scholar] [CrossRef]
- Amjad, M.; Raza, G.; Xin, Y.; Pervaiz, S.; Xu, J.; Du, X.; Wen, D. Volumetric solar heating and steam generation via gold nanofluids. Appl. Energy 2017, 206, 393–400. [Google Scholar] [CrossRef]
- Rahman, M.M.; Younes, H.; Ni, G.; Lu, J.Y.; Raza, A.; Zhang, T.J.; Fang, N.X.; Ghaferi, A. Al Plasmonic nanofluids enhanced solar thermal transfer liquid. AIP Conf. Proc. 2017, 1850. [Google Scholar] [CrossRef]
- Ortac, I.; Simberg, D.; Yeh, Y.; Yang, J.; Messmer, B.; Trogler, W.C.; Tsien, R.Y.; Esener, S. Dual-Porosity Hollow Nanoparticles for the Immunoprotection and Delivery of Nonhuman Enzymes. Nano Lett. 2014, 14, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Mayers, B.T.; Xia, Y. Template-Engaged Replacement Reaction: A One-Step Approach to the Large-Scale Synthesis of Metal Nanostructures with Hollow Interiors. Nano Lett. 2002, 2, 481–485. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.S.; Choi, J.W.; La Grange, T.; Modestino, M.; Hashemi, S.M.H.; Pu, Y.; Birkhold, S.; Hubbell, J.A.; Psaltis, D. Hollow Mesoporous Plasmonic Nanoshells for Enhanced Solar Vapor Generation. Nano Lett. 2016, 16, 2159–2167. [Google Scholar] [CrossRef] [PubMed]
- Theye, M.-L.; Paret, V. Spatial organization of the sp2-hybridized carbon atoms and electronic density of states of hydrogenated amorphous carbon films. Carbon N. Y. 2002, 40, 1153–1166. [Google Scholar] [CrossRef]
- Godet, C.; Adamopoulos, G.; Kumar, S.; Katsuno, T. Optical and electronic properties of plasma-deposited hydrogenated amorphous carbon nitride and carbon oxide films. Thin Solid Films 2005, 482, 24–33. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A Review on Nanofluids: Preparation, Stability Mechanisms, and Applications. J. Nanomater. 2012, 2012, 17. [Google Scholar] [CrossRef]
- Sani, E.; Barison, S.; Pagura, C.; Mercatelli, L.; Sansoni, P.; Fontani, D.; Jafrancesco, D.; Francini, F. Carbon nanohorns-based nanofluids as direct sunlight absorbers. Opt. Express 2000, 18, 5179–5187. [Google Scholar] [CrossRef] [PubMed]
- Sani, E.; Mercatelli, L.; Barison, S.; Pagura, C.; Agresti, F.; Colla, L.; Sansoni, P. Potential of carbon nanohorn-based suspensions for solar thermal collectors. Sol. Energy Mater. Sol. Cells 2011, 95. [Google Scholar] [CrossRef]
- Wang, X.; Ou, G.; Wang, N.; Wu, H. Graphene-based Recyclable Photo-Absorbers for High-Efficiency Seawater Desalination. ACS Appl. Mater. Interfaces 2016, 8, 9194–9199. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Mei, T.; Wang, G.; Guo, A.; Dai, G.; Wang, S.; Wang, J.; Li, J.; Wang, X. Investigation on enhancing effects of Au nanoparticles on solar steam generation in graphene oxide nanofluids. Appl. Therm. Eng. 2017, 114, 961–968. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Deng, L.; Wei, N.; Weng, Y.; Dong, S.; Qi, D.; Qiu, J.; Chen, X.; Wu, T. High-Performance Photothermal Conversion of Narrow-Bandgap Ti2O3 Nanoparticles. Adv. Mater. 2017, 29, 1603730. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.F.; Moussa, S.; Terner, J.; Atkinson, G.; El-Shall, M.S. Ultrasmall Gold Nanoparticles Anchored to Graphene and Enhanced Photothermal Effects by Laser Irradiation of Gold Nanostructures in Graphene Oxide Solutions. ACS Nano 2013, 7, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Colombara, D.; Dale, P.J.; Kissling, G.P.; Peter, L.M.; Tombolato, S. Photoelectrochemical Screening of Solar Cell Absorber Layers: Electron Transfer Kinetics and Surface Stabilization. J. Phys. Chem. C 2016, 120, 15956–15965. [Google Scholar] [CrossRef]
- Paquez, X.; Amiard, G.; de Combarieu, G.; Boissière, C.; Grosso, D. Resistant RuO2/SiO2 Absorbing Sol–Gel Coatings for Solar Energy Conversion at High Temperature. Chem. Mater. 2015, 27, 2711–2717. [Google Scholar] [CrossRef]
- Tsujimoto, K.; Nguyen, D.-C.; Ito, S.; Nishino, H.; Matsuyoshi, H.; Konno, A.; Kumara, G.R.A.; Tennakone, K. TiO2 Surface Treatment Effects by Mg2+, Ba2+, and Al3+ on Sb2S3 Extremely Thin Absorber Solar Cells. J. Phys. Chem. C 2012, 116, 13465–13471. [Google Scholar] [CrossRef]
- Steinmann, V.; Chakraborty, R.; Rekemeyer, P.H.; Hartman, K.; Brandt, R.E.; Polizzotti, A.; Yang, C.; Moriarty, T.; Gradečak, S.; Gordon, R.G.; Buonassisi, T. A Two-Step Absorber Deposition Approach To Overcome Shunt Losses in Thin-Film Solar Cells: Using Tin Sulfide as a Proof-of-Concept Material System. ACS Appl. Mater. Interfaces 2016, 8, 22664–22670. [Google Scholar] [CrossRef] [PubMed]
- García-Vidal, F.J.; Pitarke, J.M.; Pendry, J.B. Effective Medium Theory of the Optical Properties of Aligned Carbon Nanotubes. Phys. Rev. Lett. 1997, 78, 4289–4292. [Google Scholar] [CrossRef]
- Chou, J.B.; Yeng, Y.X.; Lee, Y.E.; Lenert, A.; Rinnerbauer, V.; Celanovic, I.; Soljačić, M.; Fang, N.X.; Wang, E.N.; Kim, S.-G. Enabling Ideal Selective Solar Absorption with 2D Metallic Dielectric Photonic Crystals. Adv. Mater. 2014, 26, 8041–8045. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.-Q.; Schubert, M.F.; Kim, J.K.; Schubert, E.F.; Chen, M.; Lin, S.-Y.; Liu, W.; Smart, J.A. Optical thin-film materials with low refractive index for broadband elimination of Fresnel reflection. Nat. Photonics 2007, 1, 176–179. [Google Scholar] [CrossRef]
- Ramsurn, H.; Gupta, R.B. Nanotechnology in Solar and Biofuels. ACS Sustain. Chem. Eng. 1982, 1, 779–797. [Google Scholar] [CrossRef]
- Ueno, K.; Oshikiri, T.; Sun, Q.; Shi, X.; Misawa, H. Solid-State Plasmonic Solar Cells. Chem. Rev. 1982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jie, J.; Zhang, X.; Ou, X.; Zhang, X. Large-Scale Fabrication of Silicon Nanowires for Solar Energy Applications. ACS Appl. Mater. Interfaces 1982, 9, 34527–34543. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.Y.; Cheng, J.H.; Lu, J.Y.; Chang, Y.H.; Cheng, C.L.; Chen, Y.F. Growth and characterization of type-II ZnO/ZnTe core-shell nanowire arrays for solar cell applications. Superlattices Microstruct. 2010, 47, 160–164. [Google Scholar] [CrossRef]
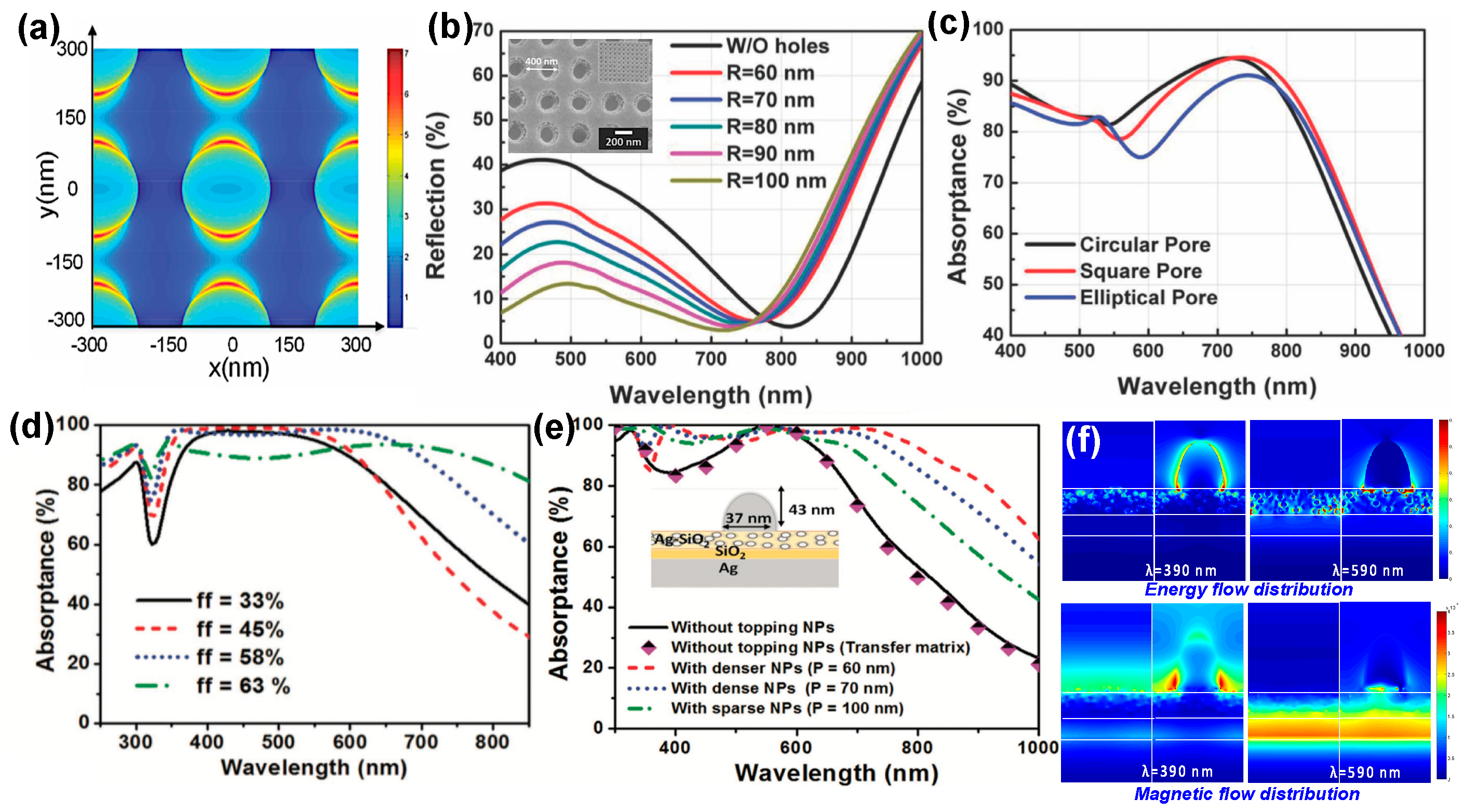
- Lu, J.Y.; Nam, S.H.; Wilke, K.; Raza, A.; Lee, Y.E.; AlGhaferi, A.; Fang, N.X.; Zhang, T.J. Localized Surface Plasmon-Enhanced Ultrathin Film Broadband Nanoporous Absorbers. Adv. Opt. Mater. 2016, 4, 1255–1264. [Google Scholar] [CrossRef]
- Lu, J.Y.; Raza, A.; Noorulla, S.; Alketbi, A.S.; Fang, N.X.; Chen, G.; Zhang, T.J. Near-Perfect Ultrathin Nanocomposite Absorber with Self-Formed Topping Plasmonic Nanoparticles. Adv. Opt. Mater. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Cao, F.; McEnaney, K.; Chen, G.; Ren, Z. A review of cermet-based spectrally selective solar absorbers. Energy Environ. Sci. 2014, 7, 1615–1627. [Google Scholar] [CrossRef]
- Duta, A.; Isac, L.; Milea, A.; Ienei, E.; Perniu, D. Coloured Solar-thermal Absorbers – A Comparative Analysis of Cermet Structures. Energy Procedia 2014, 48, 543–553. [Google Scholar] [CrossRef]
- Selvakumar, N.; Krupanidhi, S.B.; Barshilia, H.C. Carbon Nanotube-Based Tandem Absorber with Tunable Spectral Selectivity: Transition from Near-Perfect Blackbody Absorber to Solar Selective Absorber. Adv. Mater. 2014, 26, 2552–2557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Li, Z.; Cheng, H.; Zhang, Y.; Jia, G.; Chen, S.; Tian, J. Ultrathin polarization-insensitive wide-angle broadband near-perfect absorber in the visible regime based on few-layer MoS2 films. Appl. Phys. Lett. 2017, 111, 111109. [Google Scholar] [CrossRef]
- Wang, H.; Alshehri, H.; Su, H.; Wang, L. Design, fabrication and optical characterizations of large-area lithography-free ultrathin multilayer selective solar coatings with excellent thermal stability in air. Sol. Energy Mater. Sol. Cells 2018, 174, 445–452. [Google Scholar] [CrossRef]
- Ranjan, R.; Murthy, J.Y.; Garimella, S.V. A microscale model for thin-film evaporation in capillary wick structures. Int. J. Heat Mass Transf. 2011, 54, 169–179. [Google Scholar] [CrossRef]
- Wilke, K.L.; Barabadi, B.; Lu, Z.; Zhang, T.; Wang, E.N. Parametric study of thin film evaporation from nanoporous membranes. Appl. Phys. Lett. 2017, 111, 111. [Google Scholar] [CrossRef]
- Alhosani, M.H.; Zhang, T. Dynamics of Microscale Liquid Propagation in Micropillar Arrays. Langmuir 2017, 33, 6620–6629. [Google Scholar] [CrossRef] [PubMed]
- Canbazoglu, F.M.; Fan, B.; Kargar, A.; Vemuri, K.; Bandaru, P.R. Enhanced solar evaporation of water from porous media, through capillary mediated forces and surface treatment. AIP Adv. 2016, 6. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Choi, W. Pure and modified TiO2 photocatalysts and their environmental applications. Catal. Surv. Asia 2006, 10, 16–28. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Gao, L.; Gan, W.; Cao, G.; Zhan, X.; Qiang, T.; Li, J. Visible-light activate Ag/WO3 films based on wood with enhanced negative oxygen ions production properties. Appl. Surf. Sci. 2017, 425, 889–895. [Google Scholar] [CrossRef]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; Pastrana-Martínez, L.M.; Likodimos, V.; Falaras, P.; Silva, A.M.T.; Hiskia, A. Photocatalytic Degradation of Microcystin-LR and Off-Odor Compounds in Water under UV-A and Solar Light with a Nanostructured Photocatalyst Based on Reduced Graphene Oxide–TiO2 Composite. Identification of Intermediate Products. Ind. Eng. Chem. Res. 2013, 52, 13991–14000. [Google Scholar] [CrossRef]
- Liu, H.; Raza, A.; Aili, A.; Lu, J.; AlGhaferi, A.; Zhang, T. Sunlight-Sensitive Anti-Fouling Nanostructured TiO2 coated Cu Meshes for Ultrafast Oily Water Treatment. Sci. Rep. 2016, 6, 25414. [Google Scholar] [CrossRef] [PubMed]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Analysis of electronic structures of 3d transition metal-doped TiO2 based on band calculations. J. Phys. Chem. Solids 2002, 63, 1909–1920. [Google Scholar] [CrossRef]
- Bae, E.; Choi, W.; Park, J.; Shin, H.S.; Kim, S.B.; Lee, J.S. Effects of Surface Anchoring Groups (Carboxylate vs Phosphonate) in Ruthenium-Complex-Sensitized TiO2 on Visible Light Reactivity in Aqueous Suspensions. J. Phys. Chem. B 2004, 108, 14093–14101. [Google Scholar] [CrossRef]
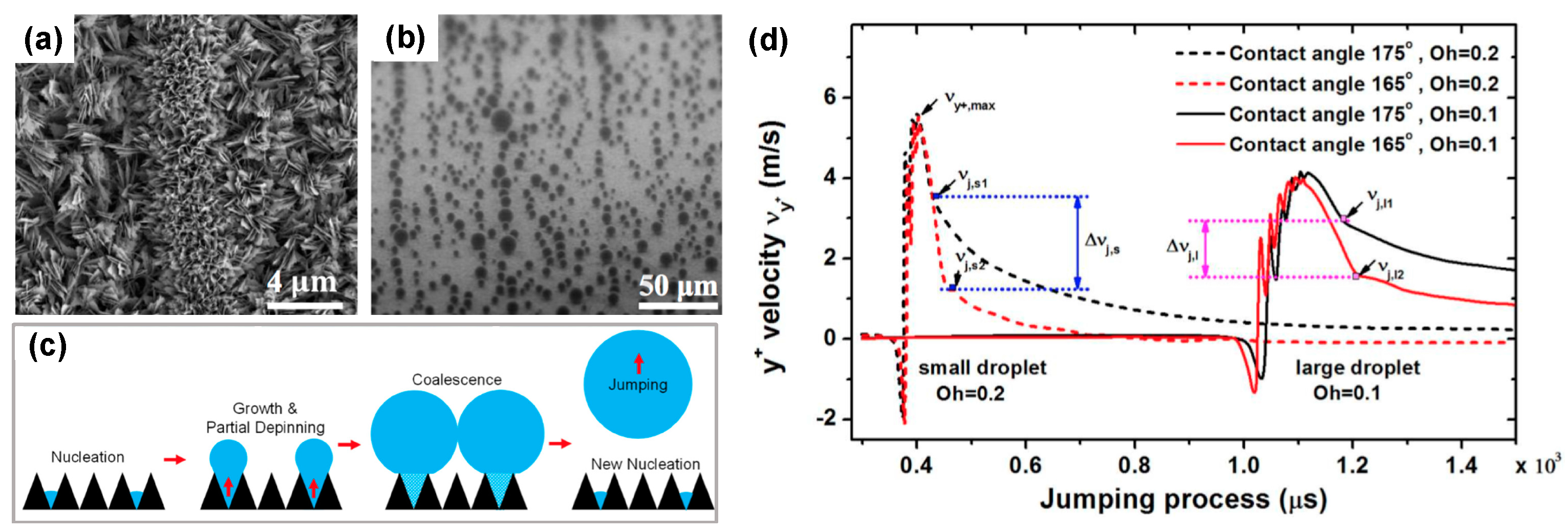
- Aili, A.; Li, H.; Alhosani, M.H.; Zhang, T. Unidirectional Fast Growth and Forced Jumping of Stretched Droplets on Nanostructured Microporous Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 21776–21786. [Google Scholar] [CrossRef] [PubMed]
- Aili, A.; Ge, Q.; Zhang, T. How Nanostructures Affect Water Droplet Nucleation on Superhydrophobic Surfaces. J. Heat Transf. 2017, 139, 112401. [Google Scholar] [CrossRef]
- Li, H.; Yang, W.; Aili, A.; Zhang, T. Insights into the Impact of Surface Hydrophobicity on Droplet Coalescence and Jumping Dynamics. Langmuir 2017, 33, 8574–8581. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xu, J.; Wang, K. Solar water evaporation by black photothermal sheets. Nano Energy 2017. [Google Scholar] [CrossRef]
- Ito, Y.; Tanabe, Y.; Han, J.; Fujita, T.; Tanigaki, K.; Chen, M. Multifunctional Porous Graphene for High-Efficiency Steam Generation by Heat Localization. Adv. Mater. 2015, 27, 4302–4307. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Kozbial, A.; Shenoy, G.; Zhou, F.; McGinley, R.; Ireland, P.; Morganstein, B.; Kunkel, A.; Surwade, S.P.; Li, L.; Liu, H. Effect of airborne contaminants on the wettability of supported graphene and graphite. Nat. Mater. 2013, 12, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pang, Y.; Huang, W.; Shaw, S.K.; Schiffbauer, J.; Pillers, M.A.; Mu, X.; Luo, S.; Zhang, T.; Huang, Y.; Li, G.; Ptasinska, S.; Lieberman, M.; Luo, T. Functionalized Graphene Enables Highly Efficient Solar Thermal Steam Generation. ACS Nano 2017, 11, 5510–5518. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, W.; Zhou, L.; Tan, Y.; Wang, Y.; Zhu, S.; Zhu, J. Tailoring Graphene Oxide-Based Aerogels for Efficient Solar Steam Generation under One Sun. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Ming, X.; Fu, Y.; Wang, G.; Wang, X. Fiber-Based, Double-Sided, Reduced Graphene Oxide Films for Efficient Solar Vapor Generation. ACS Appl. Mater. Interfaces 2017, 9, 29958–29964. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, G.; Mei, T.; Li, J.; Wang, J.; Wang, X. Accessible Graphene Aerogel for Efficiently Harvesting Solar Energy. ACS Sustain. Chem. Eng. 2017, 5, 4665–4671. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, H.; Jian, M.; Li, Y.; Xia, K.; Zhang, M.; Wang, C.; Wang, Q.; Ma, M.; Zheng, Q.; Zhang, Y. Extremely Black Vertically Aligned Carbon Nanotube Arrays for Solar Steam Generation. ACS Appl. Mater. Interfaces 2017, 9, 28596–28603. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, T.; Yang, Z.; Chen, C.; Luo, W.; Song, J.; Hitz, E.; Jia, C.; Zhou, Y.; Liu, B.; Yang, B.; Hu, L. 3D-Printed, All-in-One Evaporator for High-Efficiency Solar Steam Generation under 1 Sun Illumination. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
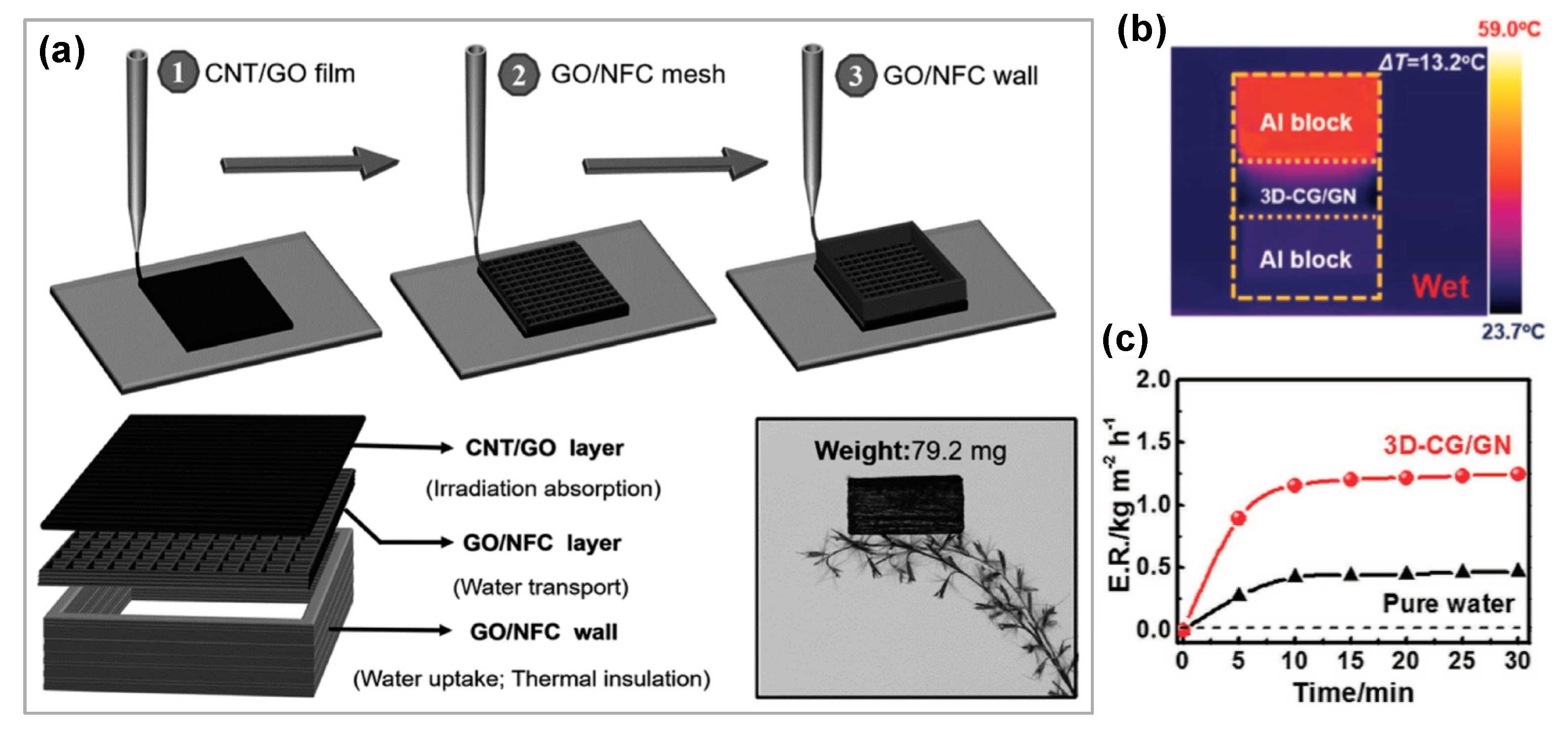
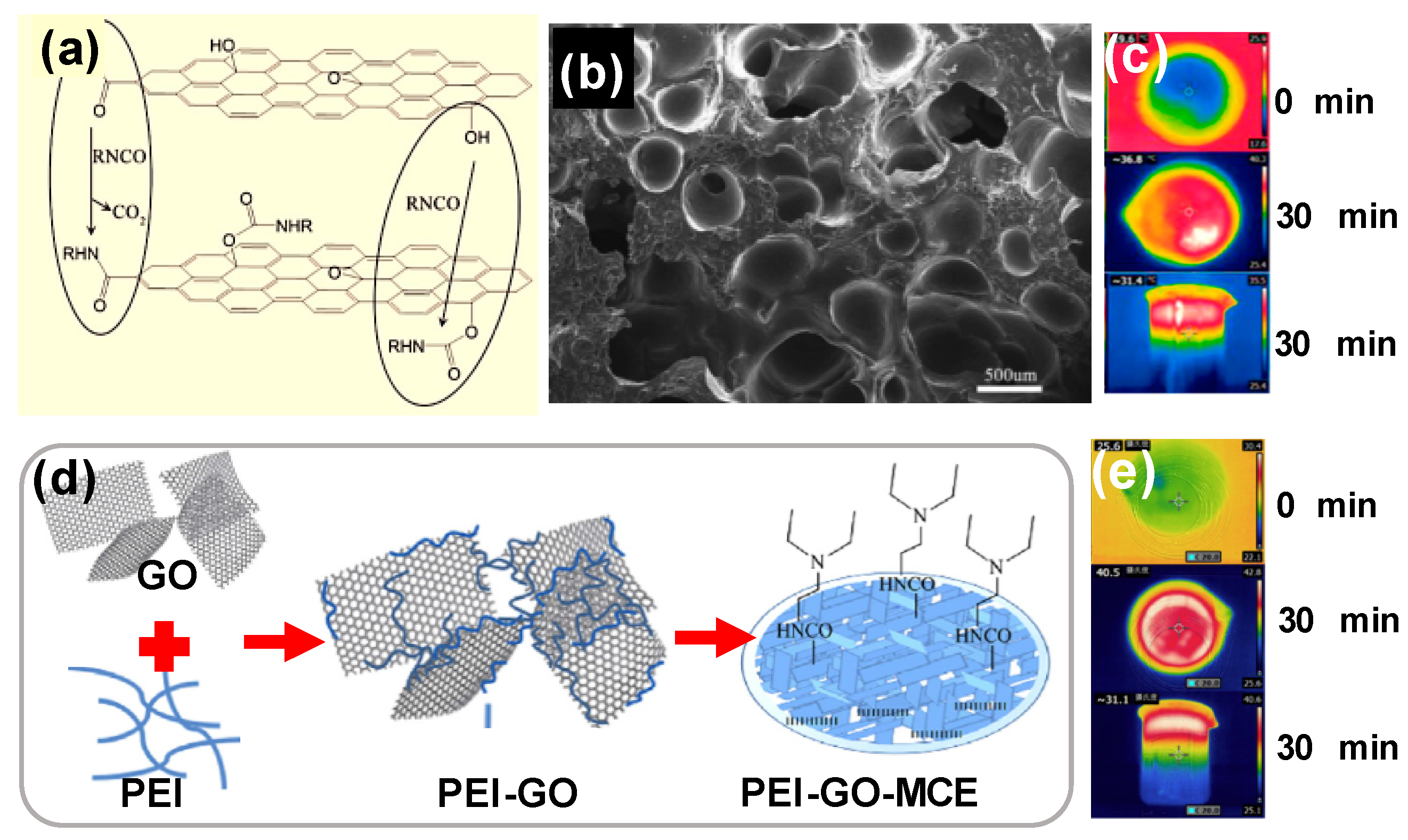
- Wang, G.; Fu, Y.; Guo, A.; Mei, T.; Wang, J.; Li, J.; Wang, X. Reduced Graphene Oxide-Polyurethane Nanocomposite Foam as a Reusable Photoreceiver for Efficient Solar Steam Generation. Chem. Mater. 2017, 29, 5629–5635. [Google Scholar] [CrossRef]
- Wang, G.; Fu, Y.; Ma, X.; Pi, W.; Liu, D.; Wang, X. Reusable reduced graphene oxide based double-layer system modified by polyethylenimine for solar steam generation. Carbon N. Y. 2017, 114, 117–124. [Google Scholar] [CrossRef]
- Jiang, Q.; Tian, L.; Liu, K.K.; Tadepalli, S.; Raliya, R.; Biswas, P.; Naik, R.R.; Singamaneni, S. Bilayered Biofoam for Highly Efficient Solar Steam Generation. Adv. Mater. 2016, 28, 9400–9407. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Luan, J.; Liu, K.K.; Jiang, Q.; Tadepalli, S.; Gupta, M.K.; Naik, R.R.; Singamaneni, S. Plasmonic Biofoam: A Versatile Optically Active Material. Nano Lett. 2016, 16, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Hu, X.; Xu, W.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Mushrooms as Efficient Solar Steam-Generation Devices. Adv. Mater. 2017, 29, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Y.; Song, J.; Yang, Z.; Kuang, Y.; Hitz, E.; Jia, C.; Gong, A.; Jiang, F.; Zhu, J.Y.; Yang, B.; Xie, J.; Hu, L. Highly Flexible and Efficient Solar Steam Generation Device. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Liu, K.; Chen, Q.; Yang, P.; Li, J.; Ding, T.; Duan, J.; Qi, B.; Zhou, J. Robust and Low-Cost Flame-Treated Wood for High-Performance Solar Steam Generation. ACS Appl. Mater. Interfaces 2017, 9, 15052–15057. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.K.; Jiang, Q.; Tadepalli, S.; Raliya, R.; Biswas, P.; Naik, R.R.; Singamaneni, S. Wood-Graphene Oxide Composite for Highly Efficient Solar Steam Generation and Desalination. ACS Appl. Mater. Interfaces 2017, 9, 7675–7681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, J.; Lv, L.; Zhao, Y.; Qu, L. Vertically Aligned Graphene Sheets Membrane for Highly Efficient Solar Thermal Generation of Clean Water. ACS Nano 2017, 11, 5087–5093. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, R.; Ni, G.; Xu, N.; Hu, X.; Zhu, B.; Lv, G.; Li, J.; Zhu, S.; Zhu, J. Three-dimensional artificial transpiration for efficient solar waste-water treatment. Natl. Sci. Rev. 2017, 1–8. [Google Scholar] [CrossRef]
- Morciano, M.; Fasano, M.; Salomov, U.; Ventola, L.; Chiavazzo, E.; Asinari, P. Efficient steam generation by inexpensive narrow gap evaporation device for solar applications. Sci. Rep. 2017, 7, 11970. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tan, Y.; Wang, J.; Xu, W.; Yuan, Y.; Cai, W.; Zhu, S.; Zhu, J. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photonics 2016, 10, 393–398. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Ji, D.; Zhu, B.; Zhang, P.; Xu, J.; Gan, Q.; Yu, Z.; Zhu, J. Self-assembly of highly efficient, broadband plasmonic absorbers for solar steam generation. Sci. Adv. 2016, 2, e1501227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, B.; Wu, J.; Li, R.; Wang, P. Hydrophobic Light-to-Heat Conversion Membranes with Self-Healing Ability for Interfacial Solar Heating. Adv. Mater. 2015, 27, 4889–4894. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, S.; Feng, R.; Bernard, A.; Liu, Y.; Zhang, Y.; Duan, H.; Shang, W.; Tao, P.; Song, C.; Deng, T. A Bioinspired, Reusable, Paper-Based System for High-Performance Large-Scale Evaporation. Adv. Mater. 2015, 27, 2768–2774. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Z.; Zhai, X.Q. Development of solar thermal technologies in China. Energy 2010, 35, 4407–4416. [Google Scholar] [CrossRef]
- Jebasingh, V.K.; Herbert, G.M.J. A review of solar parabolic trough collector. Renew. Sustain. Energy Rev. 2016, 54, 1085–1091. [Google Scholar] [CrossRef]
- Coco-Enríquez, L.; Muñoz-Antón, J.; Martínez-Val, J.M. Integration between direct steam generation in linear solar collectors and supercritical carbon dioxide Brayton power cycles. Int. J. Hydrog. Energy 2015, 40, 15284–15300. [Google Scholar] [CrossRef]
- Hijazi, H.; Mokhiamar, O.; Elsamni, O. Mechanical design of a low cost parabolic solar dish concentrator. Alex. Eng. J. 2016, 55, 1–11. [Google Scholar] [CrossRef]
- Bierman, B.; O’Donnell, J.; Burke, R.; McCormick, M.; Lindsay, W. Construction of an Enclosed trough EOR System in South Oman. Energy Procedia 2014, 49, 1756–1765. [Google Scholar] [CrossRef]
- First Enclosed-Trough Solar Steam Generation Pilot for EOR Applications. Available online: https://www.glasspoint.com/media/2014/06/WorldOil_May2014_SPESolarEOR.pdf (accessed on 14 November 2017).
- Ni, G.; Li, G.; Boriskina, S.V.; Li, H.; Yang, W.; Zhang, T.; Chen, G. Steam generation under one sun enabled by a floating structure with thermal concentration. Nat. Energy 2016, 1, 16126. [Google Scholar] [CrossRef]
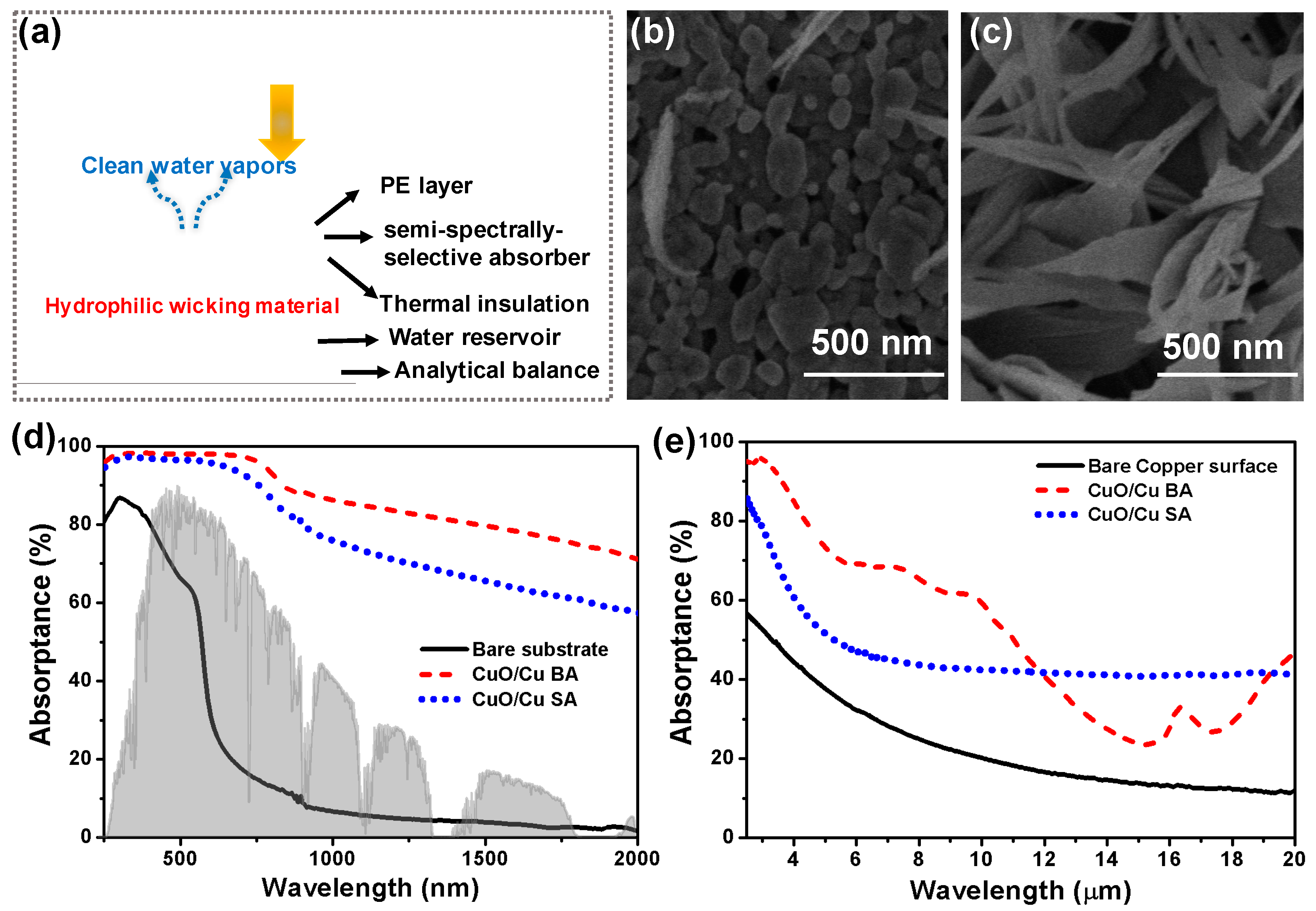
- Altaweel, A.; Filipič, G.; Gries, T.; Belmonte, T. Controlled growth of copper oxide nanostructures by atmospheric pressure micro-afterglow. J. Cryst. Growth 2014, 407, 17–24. [Google Scholar] [CrossRef]
- Li, X.; Zhu, D.; Wang, X. Evaluation on dispersion behavior of the aqueous copper nano-suspensions. J. Colloid Interface Sci 2007, 310. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, K.K.; Srinivasan, P.S.S. Experimental investigation of forced and wind assisted domestic solar hot water systems. Proc. Inst. Mech. Eng. Part. C J. Mech. Eng. Sci. 2011, 226, 154–160. [Google Scholar] [CrossRef]
- Piroozfar, P.; Pomponi, F.; Farr, E.R.P. Life cycle assessment of domestic hot water systems: A comparative analysis. Int. J. Constr. Manag. 2016, 16, 109–125. [Google Scholar] [CrossRef] [Green Version]






| Materials | Thermal Conductivity | Energy Conversion | References |
|---|---|---|---|
| W m−1 K−1 | % | ||
| Porous graphene | 94 ± 5.2 | 46 | [107] |
| Porous N-doped graphene | 9.0 ± 1.2 | 54 | [98] |
| Graphite | 119–165 | 56 | [43] |
| Activated carbon fiber | 21–125 | - | [98] |
| Carbon nanotubes-Balsa wood | 0.21 | 65 | [111] |
| Reduced graphene oxide-polyurethane | 1.0984 | 65 | [106] |
| Reduced graphene oxide- cellulose ester membranes | 4 4.466 | 52 | [107] |
| Vertically aligned graphene sheet membranes | 0.0038 | 86.5 | [114] |
| Carbonized mushrooms | 0.45 | 78 | [110] |
| Materials | Solar Irradiance (kW m−2) | Steam Temperature (°C) | Reference |
|---|---|---|---|
| Reduced graphene oxide-Fe3O4 | 1 | 47 | [56] |
| Reduced graphene oxide-Fe3O4 | 2 | 56 | [56] |
| Au nanoparticles | 220 | 100 | [44] |
| Au nanoparticles-graphene | 0.7 | 27.2 | [57] |
| Vertically aligned carbon nanotubes | 15 | 60 | [104] |
| Polyurethane-Reduced graphene oxide | 1 | 31.4 | [106] |
| Polyurethane-Reduced graphene oxide | 10 | 67.8 | [106] |
| Reduced graphene oxide +MCE | 4 | 40.5 | [107] |
| Reduced graphene oxide bilayer | 10 | 80 | [108] |
| Carbonized mushroom | 1 | 40 | [110] |
| CNTs modified balsa wood | 1 | 36 | [111] |
| CuO/Cu SA | 1 | 67 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, A.; Lu, J.-Y.; Alzaim, S.; Li, H.; Zhang, T. Novel Receiver-Enhanced Solar Vapor Generation: Review and Perspectives. Energies 2018, 11, 253. https://doi.org/10.3390/en11010253
Raza A, Lu J-Y, Alzaim S, Li H, Zhang T. Novel Receiver-Enhanced Solar Vapor Generation: Review and Perspectives. Energies. 2018; 11(1):253. https://doi.org/10.3390/en11010253
Chicago/Turabian StyleRaza, Aikifa, Jin-You Lu, Safa Alzaim, Hongxia Li, and TieJun Zhang. 2018. "Novel Receiver-Enhanced Solar Vapor Generation: Review and Perspectives" Energies 11, no. 1: 253. https://doi.org/10.3390/en11010253





