Impact of Thermal Pretreatment Temperatures on Woody Biomass Chemical Composition, Physical Properties and Microstructure
Abstract
:1. Introduction
2. Experimental
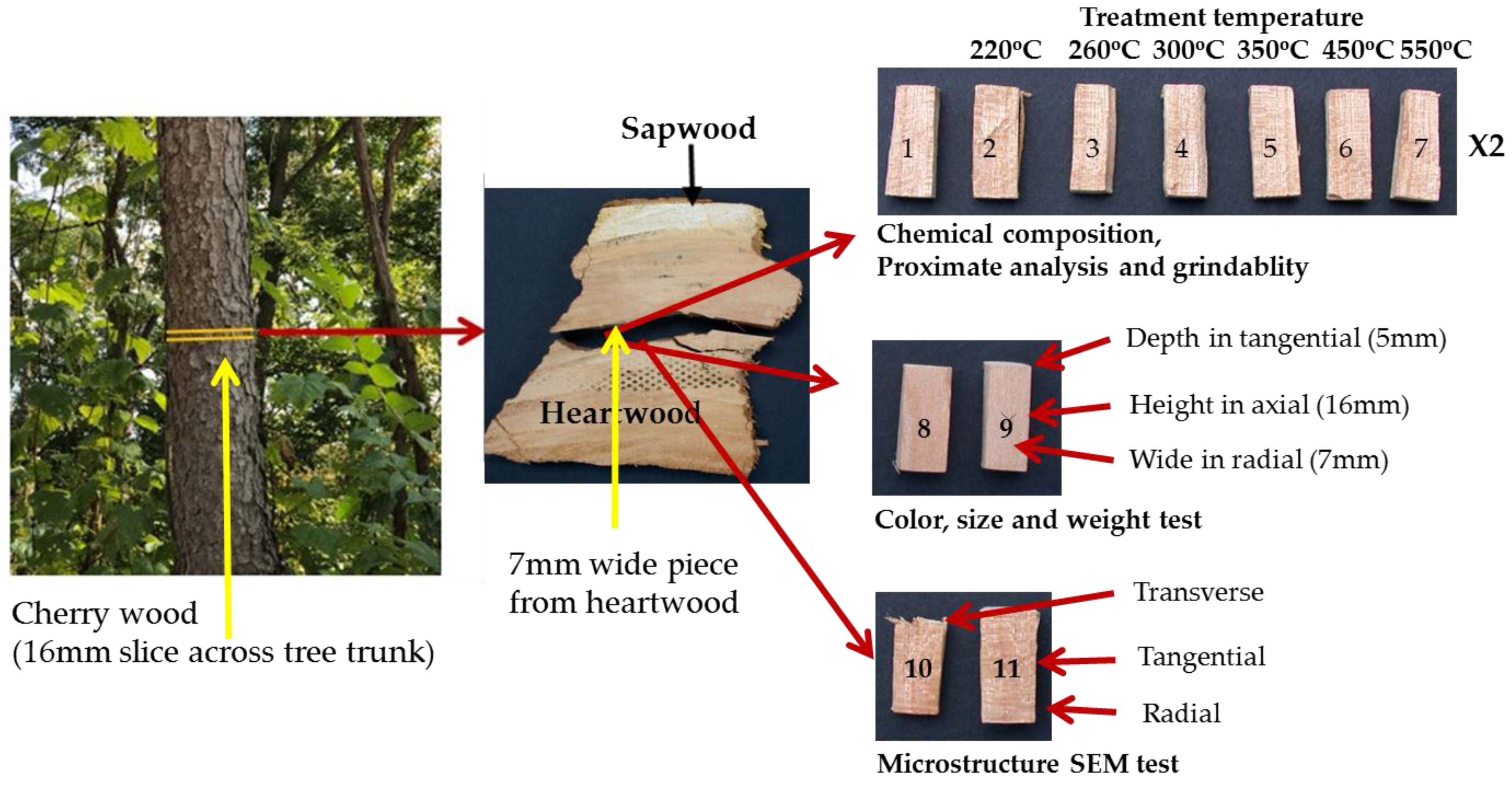
2.1. Sample Preparation
2.2. Thermal Treatment
2.3. Characterization and Analysis of the Chars Generated from Thermal Reatments
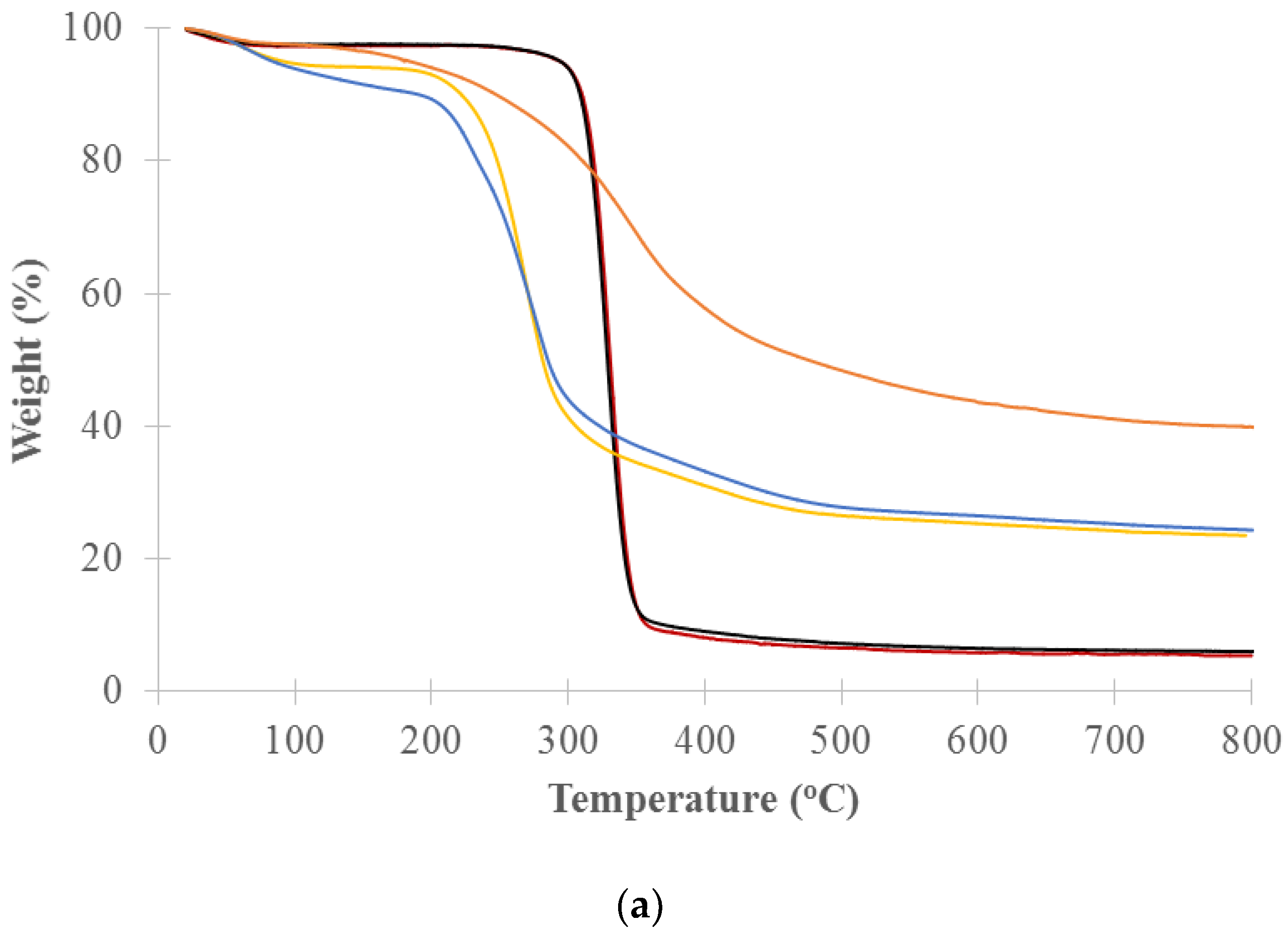
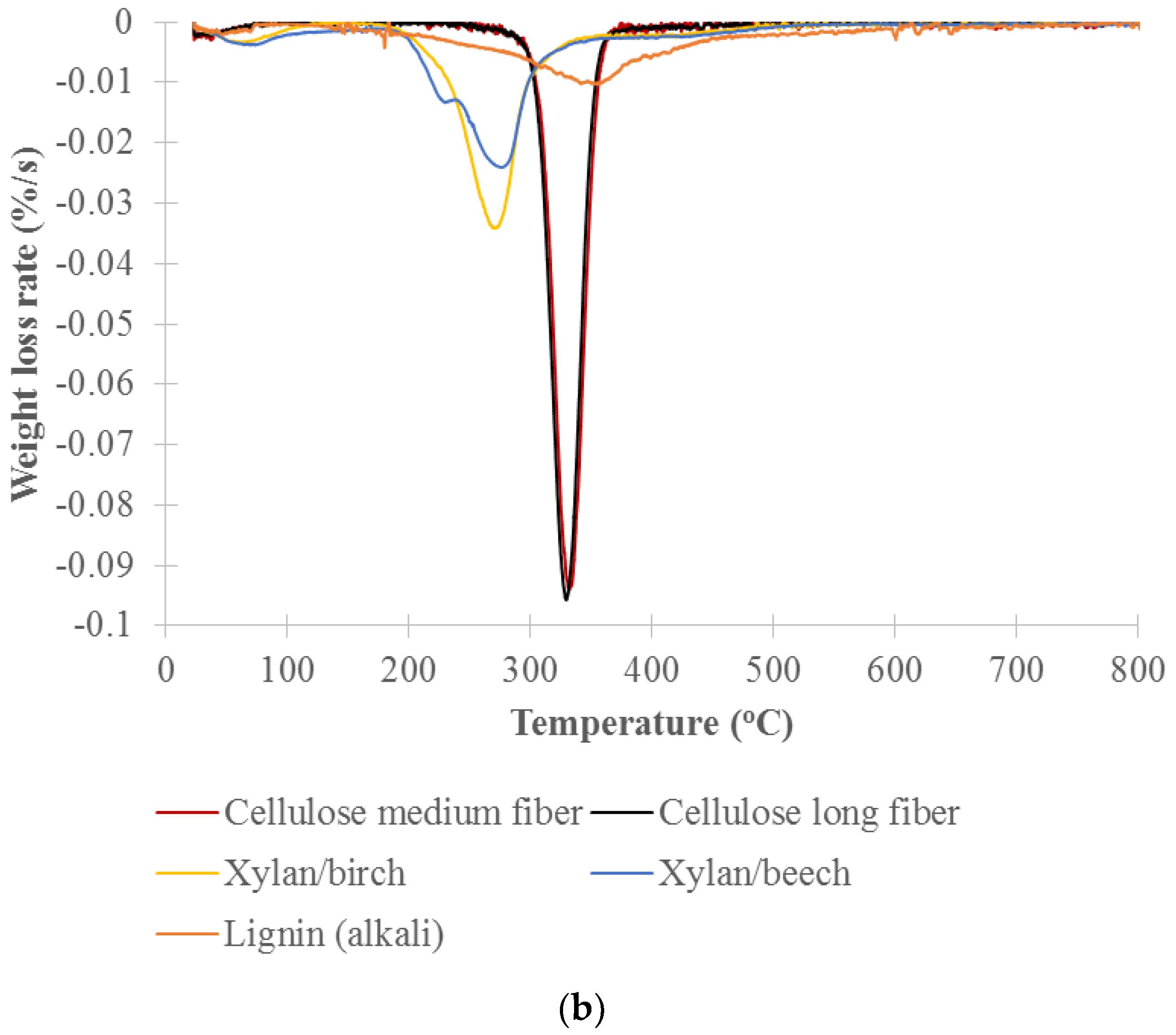
2.3.1. Cell Wall Component Decomposition Study of Untreated and Treated Woods by TGA and Differential Scanning Calorimetry (DSC)
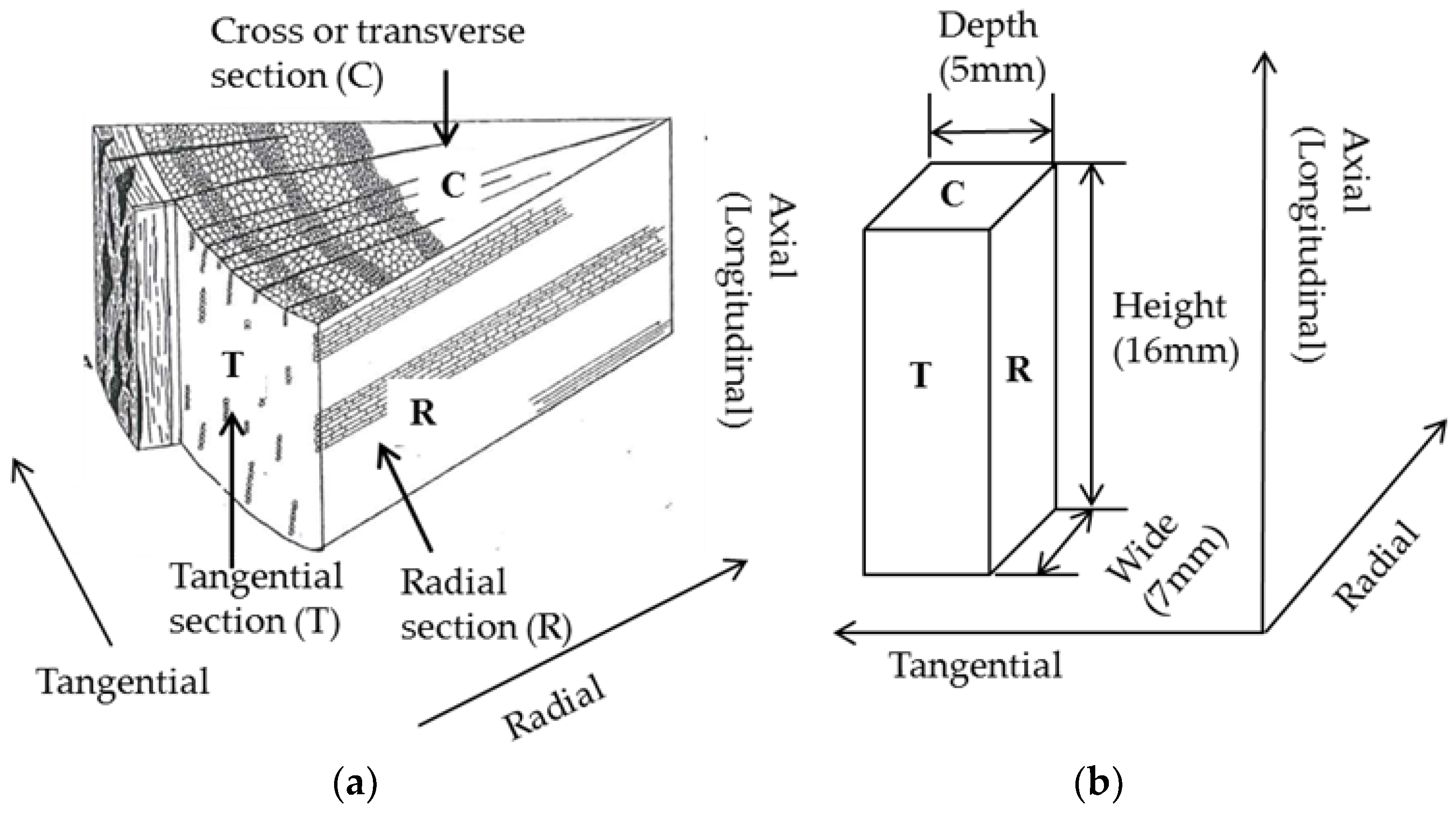
2.3.2. Physical Properties (Color, Weight, Dimensions, Bulk Density and Grindbility) of Untreated and Treated Woods
2.3.3. Proximate Analysis and High Heating Value of Untreated and Treated Woods
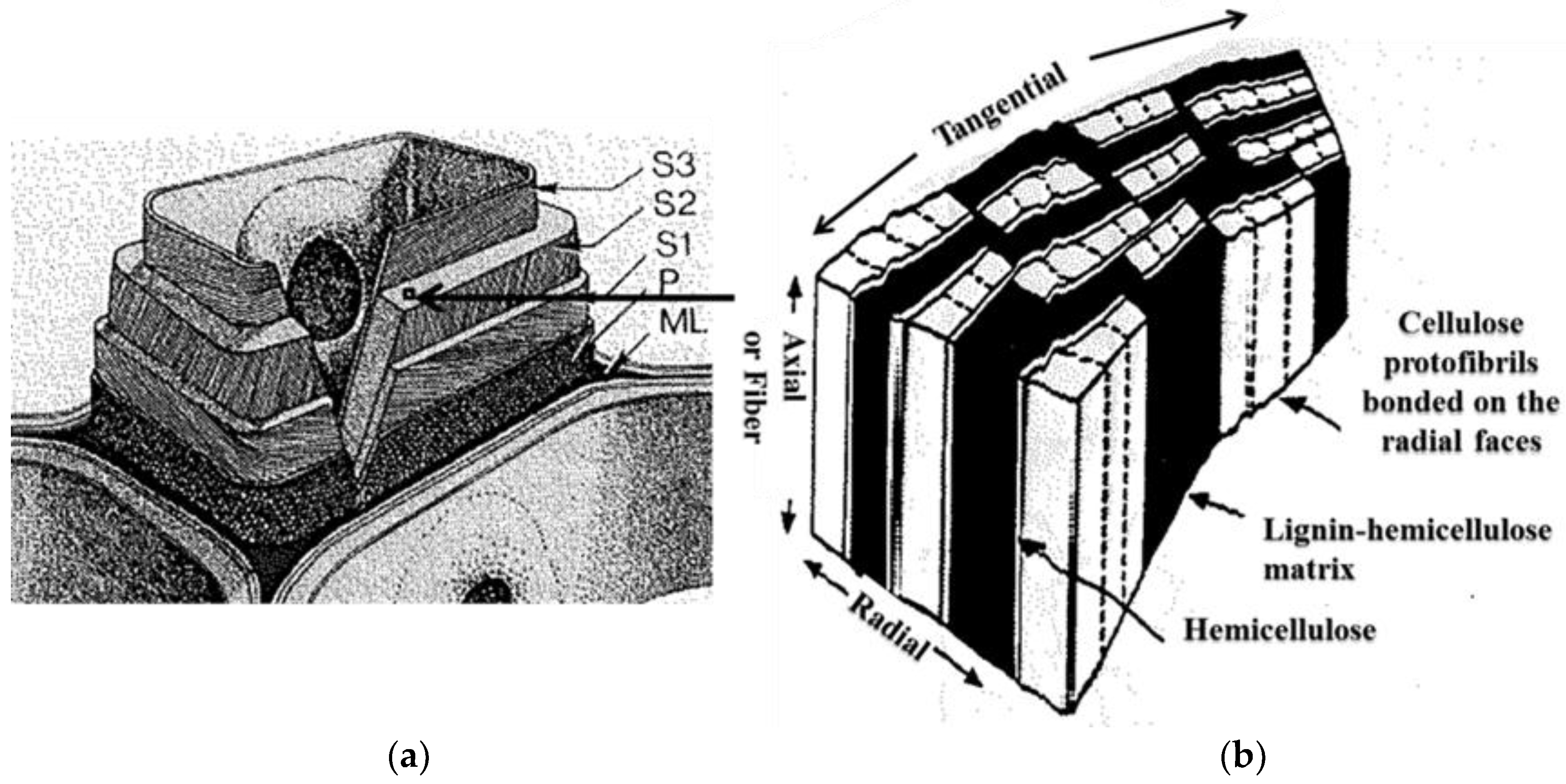
2.3.4. Microstructual Transformations Study Using SEM
3. Results and Discussions
3.1. Cell Wall Component Composition Study of Untreated and Treated Woods by TGA and DSC
3.2. Physical Properties of Untreated and Treated Woods
3.2.1. Physic Properties of Color, Weight, Dimensions, and Bulk Density
3.2.2. Grindbility of Untreated and Treated Woods
3.3. Proximate Analysis and High Heating Value of Untreated and Treated Woods
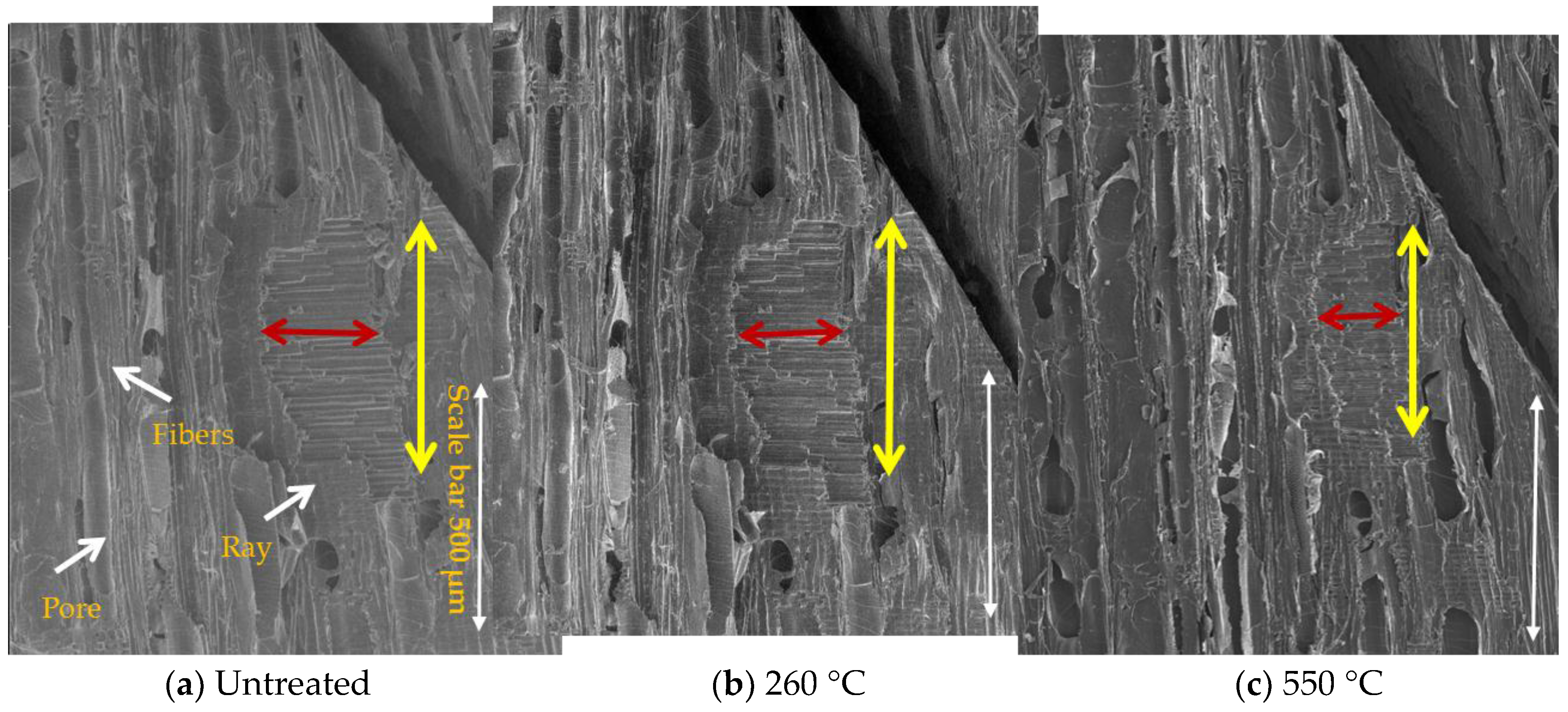
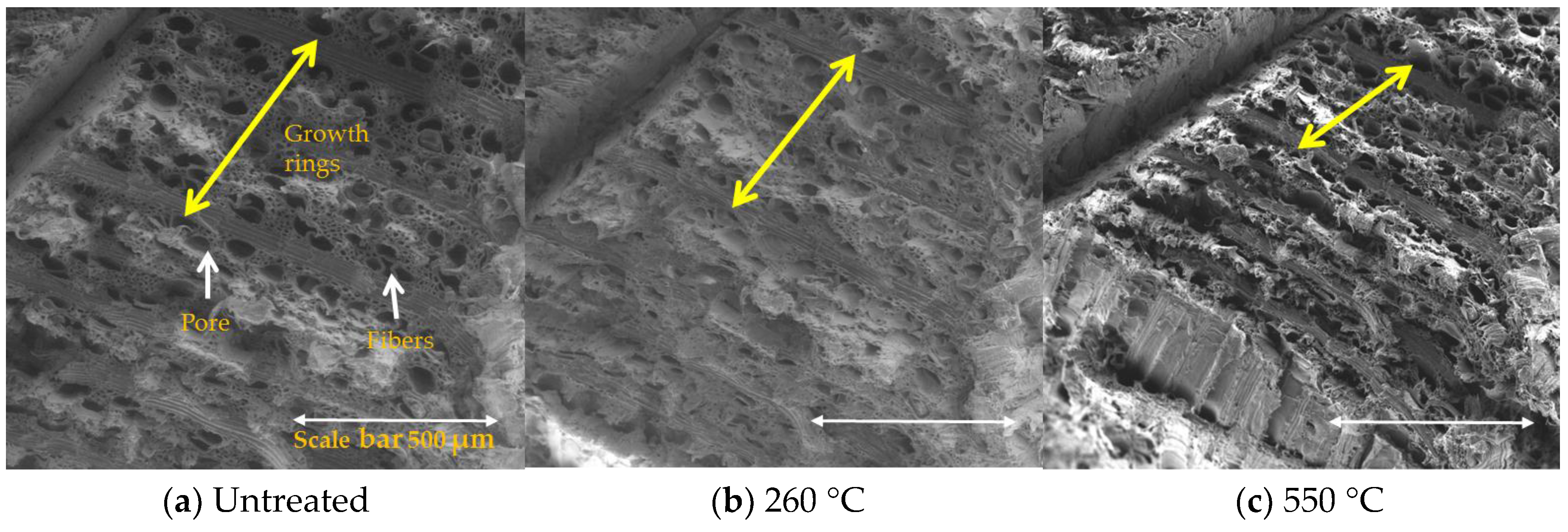
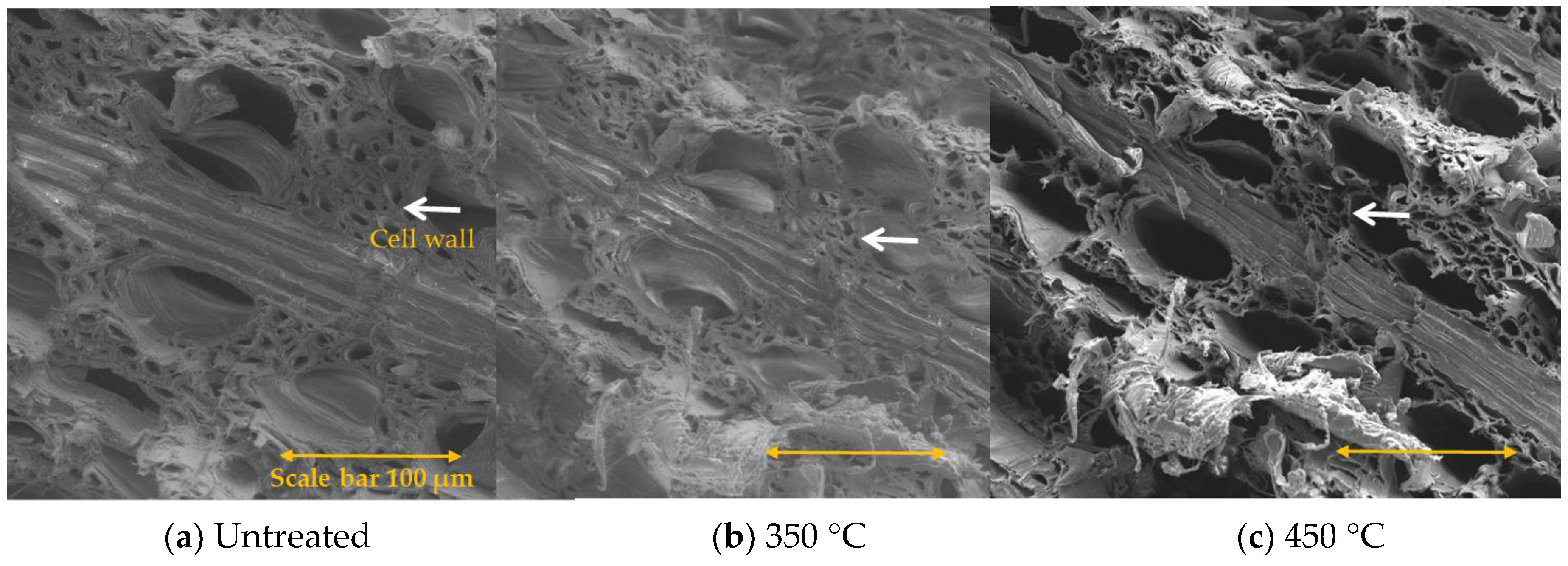
3.4. Morphological Study of Untreated and Treated Woods by SEM
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Energy Agency (IEA). Technology Roadmap Bioenergy for Heat and Power; International Energy Agency: Paris, France, 2012; Available online: http://www.iea.org/publications/freepublications/publication/2012_Bioenergy_Roadmap_2nd_Edition_WEB.pdf (accessed on 10 May 2017).
- U.S. Energy Information Administration (EIA). International Energy Outlook 2016; DOE/EIA-0484 (2016); U.S. Energy Information Administration: Washington, DC, USA, 2016. Available online: https://www.eia.gov/outlooks/ieo/ (accessed on 11 May 2017).
- U.S. Energy Information Administration (EIA). June 2017 Monthly Energy Review; DOE/EIA-0035(2017/6); U.S. Energy Information Administration: Washington, DC, USA, 2017. Available online: https://www.eia.gov/totalenergy/data/monthly/pdf/mer.pdf (accessed on 11 May 2017).
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, R. A comparison of thermal behaviors of raw biomass, pyrolytic biochar and their blends with lignite. Bioresour. Technol. 2013, 146, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Van Paasen, R.H.; Kiel, J.H.A. Tar Formation in a Fluidised-Bed Gasifier: Impact of Fuel Properties and Operating Conditions; Energy Research Center of the Netherlands (ECN): Sint Maartensvlotbrug, The Netherlands, 2004. [Google Scholar]
- Higman, C.; van der Burgt, M. Gasification; Elsevier: New York, NY, USA, 2008. [Google Scholar]
- Smoot, L.D.; Smith, P.J. Coal Combustion and Gasification; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Bergman, P.; Kiel, J. Torrefaction for biomass upgrading. In Proceedings of the 14th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005. [Google Scholar]
- Abdullah, H.; Wu, H. Biochar as a fuel: 1. Properties and grindability of biochars produced from the pyrolysis of mallee wood under slow-heating conditions. Energy Fuels 2009, 23, 4174–4181. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Syu, F.-S.; Chiueh, P.-T.; Lo, S.-L. Life cycle assessment of biochar cofiring with coal. Bioresour. Technol. 2013, 131, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-W.; Jang, C.-H.; Baek, K.-R.; Yang, J.-K. Torrefaction and low-temperature carbonization of woody biomass: Evaluation of fuel characteristics of the products. Energy 2012, 45, 676–685. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Chew, J.J.; Doshi, V. Recent advances in biomass pretreatment—Torrefaction fundamentals and technology. Renew. Sustain. Energy Rev. 2011, 15, 4212–4222. [Google Scholar] [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Madanayake, B.N.; Gan, S.Y.; Eastwick, C.; Ng, H.K. Biomass as an energy source in coal co-firing and its feasibility enhancement via pre-treatment techniques. Fuel Process. Technol. 2017, 159, 287–305. [Google Scholar] [CrossRef]
- Koppejan, J.; Cremers, M.; Middelkamp, J.; Witkamp, J.; Sokhansanj, S.; Melin, S.; Madrali, S. Status Overview of Torrefaction Technologies: A Review of the Commercialisation Status of Biomass Torrefaction; International Energy Agency Bioenergy: Paris, France, 2015; Available online: http://www.ieabcc.nl/publications/IEA_Bioenergy_T32_Torrefaction_update_2015b.pdf (accessed on 9 October 2017).
- Bergman, P.C.A.; Boersma, A.R.; Zwart, R.W.R.; Kiel, J.H.A. Torrefation for Biomass Co-Fring in Existing Coal-Fired Power Stations “Biocoal”; ECN-C-05-013; Energy Research Centre of the Netherlands: Sint Maartensvlotbrug, The Netherlands, 2005. [Google Scholar]
- Du, S.-W.; Chen, W.-H.; Lucas, J.A. Pretreatment of biomass by torrefaction and carbonization for coal blend used in pulverized coal injection. Bioresour. Technol. 2014, 161, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Phanphanich, M.; Mani, S. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Repellin, V.; Govin, A.; Rolland, M.; Guyonnet, R. Energy requirement for fine grinding of torrefied wood. Biomass Bioenergy 2010, 34, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Liu, Z.G.; Han, G.H. Production of solid fuel biochar from waste biomass by low temperature pyrolysis. Fuel 2015, 158, 159–165. [Google Scholar] [CrossRef]
- Davidsson, K.O.; Pettersson, J.B.C. Birch wood particle shrinkage during rapid pyrolysis. Fuel 2002, 81, 263–270. [Google Scholar] [CrossRef]
- Kumar, R.R.; Kolar, A.K.; Leckner, B. Shrinkage characteristics of casuarina wood during devolatilization in a fluidized bed combustor. Biomass Bioenergy 2006, 30, 153–165. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Bajer, K.; Celinska, A.; Dudynski, M.; Korotko, J.; Sosnowska, M. Pyrolysis and gasification of a thermally thick wood particle—Effect of fragmentation. Fuel 2014, 132, 125–134. [Google Scholar] [CrossRef]
- Chen, W.H.; Cheng, W.Y.; Lu, K.M.; Huang, Y.P. An evaluation on improvement of pulverized biomass property for solid fuel through torrefaction. Appl. Energy 2011, 88, 3636–3644. [Google Scholar] [CrossRef]
- Mafu, L.D.; Neomagus, H.; Everson, R.C.; Carrier, M.; Strydom, C.A.; Bunt, J.R. Structural and chemical modifications of typical south african biomasses during torrefaction. Bioresour. Technol. 2016, 202, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shuster, E.; Matuszewski, M.; Tarka, T.; VanEssendelft, D.; Berry, D. Selection of biomass type for co-gasification studies. In Proceedings of the 35th International Technical Conference on Clean Coal & Fuel Systems, Clearwater, FL, USA, 6–10 June 2010. [Google Scholar]
- Rowell, R.M.; Pettersen, R.; Han, J.S.; Rowel, J.S.; Tshabalala, M.A. Cell wall chemisty. In Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; Tayer & Francis: New York, NY, USA, 2005. [Google Scholar]
- Winandy, J.E.; Rowell, R.M. Chemistry of wood strength. In Handbook of Wood Chemistry and Wood Composites; Rowell, R.M., Ed.; Tayor & Francis: New Yok, NY, USA, 2005. [Google Scholar]
- Hanaoka, T.; Inoue, S.; Uno, S.; Ogi, T.; Minowa, T. Effect of woody biomass components on air-steam gasification. Biomass Bioenergy 2005, 28, 69–76. [Google Scholar] [CrossRef]
- Serapiglia, M.J.; Cameron, K.D.; Stipanovic, A.J.; Smart, L.B. High-resolution thermogravimetric analysis for rapid characterization of biomass composition and selection of shrub willow varieties. Appl. Biochem. Biotechnol. 2008, 145, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Kuo, P.C. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586. [Google Scholar] [CrossRef]
- Bahng, M.-K.; Mukarakate, C.; Robichaud, D.J.; Nimlos, M.R. Current technologies for analysis of biomass thermochemical processing: A review. Anal. Chim. Acta 2009, 651, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.M.; Dupont, C.; Darvell, L.I.; Commandre, J.M.; Saddawi, A.; Jones, J.M.; Grateau, M.; Nocquet, T.; Salvador, S. Combustion and gasification characteristics of chars from raw and torrefied biomass. Bioresour. Technol. 2012, 119, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Biorefineries: Current activities and future developments. Energy Convers. Manag. 2009, 50, 2782–2801. [Google Scholar] [CrossRef]
- Friedman, J. Wood Identification by Microscopic Examination: A Guide for the Archaeologist on the Northwest Coast of North America (Heritage Record); British Columbia Provincial Museum: Victoria, BC, Canada, 1978. [Google Scholar]
- Erol, M.; Haykiri-Acma, H.; Kucukbayrak, S. Calorific value estimation of biomass from their proximate analyses data. Renew. Energy 2010, 35, 170–173. [Google Scholar] [CrossRef]
- Demirbas, A. Relationships between lignin contents and heating values of biomass. Energy Convers. Manag. 2001, 42, 183–188. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.A.; Ghosal, G.K. A correlation for calculating hhv from proximate analysis of solid fuels. Fuel 2005, 84, 487–494. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Wiedenhoeft, A.C.; Miller, R.B. Structure and function of wood. In Handbook of Wood Chemistry and Wood Composites; MRowell, R.M., Ed.; Taylor & Francis: New York, NY, USA, 2005; pp. 9–33. [Google Scholar]
- Byrne, C.E.; Nagle, D.C. Carbonized wood monoliths—Characterization. Carbon 1997, 35, 267–273. [Google Scholar] [CrossRef]
- Yan, W.; Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Thermal pretreatment of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2009, 28, 435–440. [Google Scholar] [CrossRef]
- Byrne, C.E.; Nagle, D.C. Carbonization of wood for advanced materials applications. Carbon 1997, 35, 259–266. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Haas, T.J.; Nimlos, M.R.; Donohoe, B.S. Real-time and post-reaction microscopic structural analysis of biomass undergoing pyrolysis. Energy Fuels 2009, 23, 3810–3817. [Google Scholar] [CrossRef]


| Samples | Chemical Components (%) | ||
|---|---|---|---|
| Hemicellulose | Cellulose | Lignin | |
| Untreated | 24.3 | 42.8 | 32.9 |
| 220 | 21.2 | 46.1 | 32.8 |
| 260 | 10.1 | 48.3 | 41.6 |
| 300 | 7.4 | 38.7 | 53.9 |
| 350 | 0.0 | 6.8 | 93.2 |
| 450 | 0.0 | 0.0 | 100.0 |
| 550 | 0.0 | 0.0 | 100.0 |
| Physical Property | Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|
| 25 | 220 | 260 | 300 | 350 | 450 | 550 | |
| Color |  |  |  |  |  |  |  |
| Weight loss (%) | 0 | 15.84 | 27.57 | 39.21 | 62.07 | 69.70 | 73.22 |
| Height shrinkage (%) | 0 | 0.09 | 0.06 | 0.41 | 6.48 | 12.51 | 15.34 |
| Wide shrinkage (%) | 0 | 3.85 | 6.64 | 10.72 | 22.54 | 26.95 | 29.53 |
| Depth shrinkage (%) | 0 | 1.06 | 2.31 | 4.52 | 13.57 | 19.44 | 22.24 |
| Volume loss (%) | 0 | 4.96 | 8.85 | 15.11 | 37.39 | 48.52 | 53.60 |
| Bulk density (g/cm3) | 0.54 | 0.48 | 0.43 | 0.39 | 0.33 | 0.32 | 0.31 |
| Density change (%) | 0 | −11.44 | −20.54 | −28.39 | −39.43 | −41.16 | −42.29 |
| Samples | >500 µm | 500–212 µm | 212–106 µm | <106 µm |
|---|---|---|---|---|
| Untreated | 66.0 | 40.7 | 11.0 | 2.8 |
| 220 | 63.6 | 45.9 | 11.9 | 1.5 |
| 260 | 54.2 | 53.2 | 23.5 | 3.6 |
| 300 | 32.3 | 38.1 | 55.5 | 3.0 |
| 350 | 4.2 | 33.6 | 45.5 | 16.7 |
| 450 | 4.6 | 23.6 | 50.5 | 21.4 |
| 550 | 3.8 | 24.7 | 49.8 | 21.8 |
| Samples | VM | FC | Ash | High Heating Value (MJ/kg) | High Heating Value (MJ/m3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proximate | Incr. | Lignine | Incr. | Proximate | Decr. | Lignine | Decr. | ||||
| (wt%) | (wt%) | (wt%) | Eq. 2 | % | Eq. 1 | % | Eq. 2 | % | Eq. 1 | % | |
| Untreated | 88.1 | 11.5 | 0.4 | 17.8 | 19.7 | 9605.2 | 10663.2 | ||||
| 220 | 85.7 | 13.9 | 0.5 | 18.3 | 2.6 | 19.7 | 0.0 | 8760.0 | −8.8 | 9474.1 | −11.2 |
| 260 | 82.0 | 16.0 | 0.8 | 18.9 | 6.1 | 20.5 | 3.9 | 8113.0 | −15.5 | 8823.6 | −17.3 |
| 300 | 73.3 | 24.9 | 0.7 | 20.6 | 15.9 | 21.6 | 9.5 | 8041.5 | −16.3 | 8429.3 | −20.9 |
| 350 | 44.1 | 54.3 | 0.9 | 26.3 | 47.9 | 25.1 | 27.1 | 8682.0 | −9.6 | 8285.4 | −22.3 |
| 450 | 25.8 | 72.9 | 1.6 | 29.7 | 66.9 | 25.7 | 30.2 | 9500.3 | −1.1 | 8227.8 | −22.8 |
| 550 | 14.9 | 83.4 | 1.7 | 31.8 | 78.7 | -- | -- | 9856.0 | 2.6 | -- | -- |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Howard, B.H. Impact of Thermal Pretreatment Temperatures on Woody Biomass Chemical Composition, Physical Properties and Microstructure. Energies 2018, 11, 25. https://doi.org/10.3390/en11010025
Wang P, Howard BH. Impact of Thermal Pretreatment Temperatures on Woody Biomass Chemical Composition, Physical Properties and Microstructure. Energies. 2018; 11(1):25. https://doi.org/10.3390/en11010025
Chicago/Turabian StyleWang, Ping, and Bret H. Howard. 2018. "Impact of Thermal Pretreatment Temperatures on Woody Biomass Chemical Composition, Physical Properties and Microstructure" Energies 11, no. 1: 25. https://doi.org/10.3390/en11010025





