Hydrothermal Carbonization: Modeling, Final Properties Design and Applications: A Review
Abstract
:1. Introduction
- (a)
- Process knowledge, including how experimental parameters can affect the reactions taking place during HTC and final characteristics of the obtained HCs, as well as the reaction kinetics. Both experimental and modeling studies have been reviewed. To the authors’ knowledge, no previous HTC reviews have addressed the study of the models associated to the process itself, and this is a strong point of this review.
- (b)
- Applications of HCs, as biofuels in thermochemical processes, adsorbents in liquid solutions, material for electrode in supercondensers, and catalysts.
2. HTC Modeling
2.1. Kinetics Models
- increased model application for full-scale plant design, operation and optimization;
- common basis for further model development and validation studies to make outcomes more comparable and compatible; and,
- assisting technology transfer from research to industry.
2.1.1. Basic Kinetic Models
2.1.2. Statistical Models
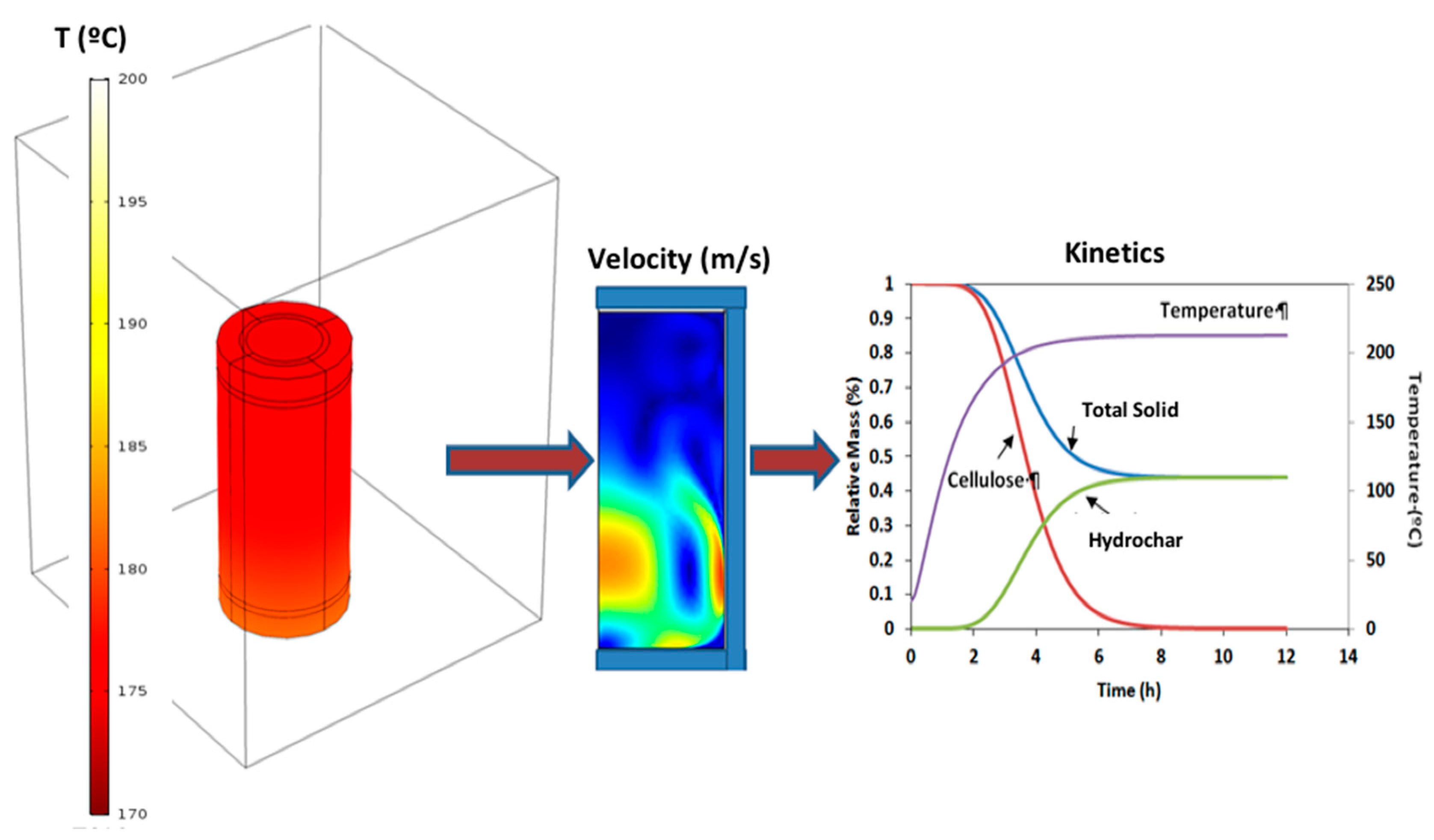
2.1.3. Computational Models
2.2. Hydrochar Properties
2.2.1. Influence of Process Conditions on HC Properties
2.2.2. Influence of Feedstock Properties on HC Characteristics
2.2.3. Review of Models Developed to Predict HC Properties
3. Applications
3.1. Biofuel
3.2. Adsorbent
3.2.1. Sorption Capacities
Heavy Metals
Organics
3.2.2. Surface and Bulk Properties of HCs
3.2.3. Adsorption Applications
3.3. Energy Storage Applications
3.4. Catalyst
4. Conclusions
- The influence of experimental variables (temperature, time, and biomass type and loading) on hydrothermal carbonization has been studied extensively. In general, the most commonly reported HC property is HC yield and the second most reported property is HC carbon content.Generally, reaction temperature has the greatest influence on the process, while the others can have a lower or greater relevance in the process depending on the particular reaction conditions; a great interdependence between variables exists. As temperature increases, solid yields and HC oxygen contents decrease and HC carbon content increases, with an enhancement of the heating value. This variable has also been shown to influence carbonization rates, with higher temperatures being correlated with the accelerating of carbonization reactions.Reaction time, less studied, is also important, primarily at early times and its influence on carbonization product characteristics is also likely dependent on reactor heating time and/or heating rate, although the wide range of experimental conditions shown in the bibliography and the participation of complex reactions whose relevance changes with time hinders the comprehension of the process.
- Statistic (linear and non-linear models), kinetic models (assuming first-order relationships), shrinking core, and severity factor-based relationships have been developed to predict HC properties. Universal application of the majority of these models may be limited because they are based on data from individual studies. The development of a model, including temperature as a function of time, while considering heat transfer mechanisms, is very promising since it might allow the study of any installation.
- Biomass hydrochars have the potential to be used as adsorbent for wastewater treatment. Despite their scant porosity, the tunable surface chemistry can enhance adsorption selectivity and some promising results have been obtained for organic compounds, heavy metals, and emerging contaminants. Moreover, the joint of chemical and physical activation can improve their performance as a result of pore development. In general, equilibrium adsorption data fit well Langmuir and Freundlich models, and values of adsorption capacity of the same order of magnitude than those obtained using commercial activated carbons are obtained.
- The use of biomass hydrochars as fuels in combustion and gasification processes has also been studied. In this frame, most of the studies have been performed by thermogravimetry, and little literature is found on real installations. Even so, it has been assessed that hydrothermal carbonization as pretreatment can improve the production of syngas and also some important combustion (and co-combustion) features, such as activation energy, ash related issues, and harmful emissions. Moreover, demonstrated improved pelletization as a result of hydrothermal carbonization is very important for this application.
- Finally, some works on the use of biomass hydrochars as low cost catalysts and as suitable as electrode material in energy storage applications, such as supercondensers, have been reported. In general, HTC alone was not enough to develop competitive materials and additional processes (pyrolysis, chemical activation, blending…) were needed prior, during, or after the process to alter the surface functionality or the structure of the hydrochars. While the incorporation of these steps involved the use of chemicals or energy, and therefore downgrades the green character of the whole process, the method still provides important advantages, such as the use of waste, which is already a highlighting and inspiring advantage. However, more research aimed to further include more sustainable production pathways in this field should be addressed.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V. Hydrothermal Carbonization of Municipal Waste Streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ma, X.; Peng, X.; Hu, S.; Yu, Z.; Fang, S. Effect of hydrothermal carbonization temperature on combustion behavior of HC fuel from paper sludge. Appl. Therm. Eng. 2015, 91, 574–582. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Laginhas, C.; Ledesma, B.; González, J.F. Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process. Technol. 2012, 103, 78–83. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Ledesma, B.; González, J.F.; Laginhas, C.; Titirici, M.M. Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Microporous Mesoporous Mater. 2013, 165, 127–133. [Google Scholar] [CrossRef]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Coronella, C.J. Engineered pellets from dry torrefied and HTC biochar blends. Biomass Bioenergy 2014, 63, 229–238. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Holdich, R.G.; Shama, G.; Wheatley, A.D.; Sohail, M.; Martin, S.J. Kinetics of faecal biomass hydrothermal carbonisation for HC production. Appl. Energy 2013, 111, 351–357. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Fierro, V.; Izquierdo, M.T.; Parmentier, J.; Pizzi, A.; Celzard, A. Kinetics of the hydrothermal treatment of tannin for producing carbonaceous microspheres. Bioresour. Technol. 2015, 151, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Knežević, D.; van Swaaij, W.; Kersten, S. Hydrothermal Conversion of Biomass. I. Glucose Conversion in Hot Compressed Water. Ind. Eng. Chem. Res. 2009, 48, 4731–4743. [Google Scholar] [CrossRef]
- Reza, M.T.; Yan, W.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J.; Vásquez, V.R. Reaction kinetics of hydrothermal carbonization of loblolly pine. Bioresour. Technol. 2013, 139, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Iryani, D.A.; Kumagai, S.; Nonaka, M.; Sasaki, K.; Hirajima, T. Hydrothermal carbonization kinetics of sugarcane bagasse treated by hot compressed water under variable temperature conditions. ARPN J. Eng. Appl. Sci. 2016, 11, 4833–4839. [Google Scholar]
- Jatzwauck, M.; Schumpe, A. Kinetics of hydrothermal carbonization (HTC) of soft rush. Biomass Bioenergy 2015, 75, 94–100. [Google Scholar] [CrossRef]
- Ro, K.; Lima, I.; Reddy, G.; Jackson, M.; Gao, B. Removing Gaseous NH3 Using Biochar as an Adsorbent. Agriculture 2015, 5, 991–1002. [Google Scholar] [CrossRef]
- Jung, D.; Kruse, A. Evaluation of Arrhenius-type overall kinetic equations for hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2017, 127, 286–291. [Google Scholar] [CrossRef]
- Ruyter, H.P. Coalification model. Fuel 1982, 61, 1182–1187. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Chornet, E.; Belkacemi, K.; Overend, R.P. Phenomenological kinetics of complex systems: The development of a generalized severity parameter and its application to lignocellulosics fractionation. Chem. Eng. Sci. 1992, 47, 1109–1122. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, B.; Wu, K.; Yuan, Q.X.; Wang, X.H.; Chen, H.P. Physicochemical, pyrolytic, and combustion characteristics of HC obtained by hydrothermal carbonization of biomass. Bioresources 2016, 11, 4113–4133. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Felix, L.; Farthing, W. Hydrothermal carbonization (HTC) of loblolly pine using a continuous, reactive twin-screw extruder. Energy Convers. Manag. 2017, 134, 247–259. [Google Scholar] [CrossRef]
- Janga, K.K.; Øyaas, K.; Hertzberg, T.; Moe, S.T. Application of a pseudo-kinetic generalized severity model to the concentrated sulfuric acid hydrolysis of pinewood and aspenwood. BioResources 2012, 7, 2728–2741. [Google Scholar]
- Suwelack, K.U.; Wüst, D.; Fleischmann, P.; Kruse, A. Prediction of gaseous, liquid and solid mass yields from hydrothermal carbonization of biogas digestate by severity parameter. Biomass Convers. Biorefinery 2016, 6, 151–160. [Google Scholar] [CrossRef]
- Gallifuoco, A.; Taglieri, L.; Scimia, F.; Papa, A.A.; Di Giacomo, G. Hydrothermal carbonization of Biomass: New experimental procedures for improving the industrial Processes. Bioresour. Technol. 2017, 244, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Mäkela, M. Experimental design and response surface methodology in energy applications: A tutorial review. Energy Convers. Manag. 2017, 151, 630–640. [Google Scholar] [CrossRef]
- Heilmann, S.M.; Davis, H.T.; Jader, L.R.; Lefebvre, P.A.; Sadowsky, M.J.; Schendel, F.J.; von Keitz, M.G.; Valentas, K.J. Hydrothermal carbonization of microalgae. Biomass Bioenergy 2010, 34, 875–882. [Google Scholar] [CrossRef]
- Álvarez-Murillo, A.; Román, S.; Ledesma, B.; Sabio, E. Study of variables in energy densification of olive stone by hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 307–314. [Google Scholar] [CrossRef]
- Sabio, E.; Álvarez-Murillo, A.; Román, S.; Ledesma, B. Conversion of tomato-peel waste into solid fuel by hydrothermal carbonization: Influence of the processing variables. Waste Manag. 2016, 47, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal carbonisation of sewage sludge: Effect of process conditions on product characteristics and methane production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Gariepy, Y.; Vijaya Raghavan, G.S. Optimization and characterization of hydrochar produced from microwave hydrothermal carbonization of fish waste. Waste Manag. 2017, 65, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal carbonization of industrial mixed sludge from a pulp and paper mill. Bioresour. Technol. 2016, 200, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Murillo, A.; Sabio, E.; Ledesma, B.; Román, S.; González-García, C.M. Generation of biofuel from hydrothermal carbonization of cellulose. Kinetics modeling. Energy 2016, 94, 600–608. [Google Scholar] [CrossRef]
- Sabzo, N.; Baloch, H.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abrod, R.; Mazari, S.A.; Alie, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar]
- Falco, C.; Baccile, N.; Titirici, M.-M. Green Chemistry Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Hwang, I.-H.; Aoyama, H.; Matsuto, T.; Nakagishi, T.; Matsuo, T. Recovery of solid fuel from municipal solid waste by hydrothermal treatment using subcritical water. Waste Manag. 2012, 32, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Kieseler, S.; Neubauer, Y.; Zobel, N. Ultimate and Proximate Correlations for Estimating the Higher Heating Value of Hydrothermal Solids. Energy Fuels 2013, 27, 908–918. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Lu, X.; Pellechia, P.J.; Flora, J.R.V.; Berge, N.D. Influence of reaction time and temperature on product formation and characteristics associated with the hydrothermal carbonization of cellulose. Bioresour. Technol. 2013, 138, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Shi, W.; Chen, X.; Ni, W.; Strong, P.J.; Jia, Y.; Wang, H. Hydrothermal conversion of water lettuce biomass at 473 or 523 K. Biomass Bioenergy 2011, 35, 4855–4861. [Google Scholar] [CrossRef]
- Akalın, M.K.; Tekin, K.; Karagoz, S. Hydrothermal liquefaction of cornelian cherry stones for bio-oil production. Bioresour. Technol. 2012, 110, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Jamari, S.S.; Howse, J.R. The effect of the hydrothermal carbonization process on palm oil empty fruit bunch. Biomass Bioenergy 2012, 47, 82–90. [Google Scholar] [CrossRef]
- Li, L.; Diederick, R.; Flora, J.R.V.; Berge, N.D. Hydrothermal carbonization of food waste and associated packaging materials for energy source generation. Waste Manag. 2013, 33, 2478–2492. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jordan, B.; Berge, N.D. Thermal conversion of municipal solid waste via hydrothermal carbonization: Comparison of carbonization products to products from current waste management techniques. Waste Manag. 2012, 32, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ro, K.S.; Libra, C.I.; Kammann, C.I.; Lima, I.; Libra, J.; Li, L.; Li, Y.; Chen, N.; Yang, J. Effects of biomass types and carbonization conditions on the chemical characteristics of hydrochars. J. Agric. Food Chem. 2013, 61, 9401–9411. [Google Scholar] [CrossRef] [PubMed]
- Garcia Alba, L.; Torri, C.; Samori, C.; van der Spek, J.; Fabbri, D.; Kersten, S.R.A.; Brilman, D.W.F. Hydrothermal Treatment (HTT) of Microalgae: Evaluation of the Process as Conversion Method in an Algae Biorefinery Concept. Energy Fuels 2012, 26, 642–657. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Solid fuel production by hydrothermal carbonization of black liquor. Bioresour. Technol. 2012, 110, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, X.H.; Yang, H.P.; Chen, H.P. Characterization of products from hydrothermal treatments of cellulose. Energy 2012, 42, 457–465. [Google Scholar] [CrossRef]
- Mumme, J.; Eckervogt, L.; Pielert, J.; Diakité, M.; Rupp, F.; Kern, J. Hydrothermal carbonization of anaerobically digested maize silage. Bioresour. Technol. 2011, 102, 9255–9260. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Berge, N. Influence of feedstock chemical composition on product formation and characteristics derived from the hydrothermal carbonization of mixed feedstocks. Bioresour. Technol. 2014, 166, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Tremel, A.; Stemann, J.; Herrmann, M.; Erlach, B.; Spliethoff, H. Entrained flow gasification of biocoal from hydrothermal carbonization. Fuel 2012, 102, 396–403. [Google Scholar] [CrossRef]
- Wiedner, K.; Naisse, C.; Rumpel, C.; Pozzi, A.; Wieczorek, P.; Glaser, B. Chemical modification of biomass residues during hydrothermal carbonization: What makes the difference, temperature or feedstock? Org. Geochem. 2013, 54, 91–100. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.-M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204–1212. [Google Scholar] [CrossRef]
- Dinjus, E.; Kruse, A.; Tröger, N. Hydrothermal Carbonization—1. Influence of Lignin in Lignocelluloses. Chem. Eng. Technol. 2011, 34, 2037–2043. [Google Scholar] [CrossRef]
- Mäkela, M.; Fullana, A.; Yoshikawa, K. Ash behaviour during hydrothermal treatment for solid fuel applications. Part 1: Overview of different feedstock. Energy Convers. Manag. 2016, 121, 402–408. [Google Scholar] [CrossRef]
- Li, L.; Flora, J.R.V.; Caicedo, J.M.; Berge, N.D. Investigating the role of feedstock properties and process conditions on products formed during the hydrothermal carbonization of organics using regression techniques. Bioresour. Technol. 2015, 187, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Gariepy, Y.; Raghavan, G.S.V. Optimization and Characterization of Hydrochar Derived from Shrimp Waste. Energy Fuels 2017, 31, 4068–4077. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Mubarak, N.M.; Tiripathi, M.; Jayakumar, N.S.; Sahu, J.N.; Ganesan, P. Chemical, dielectric and structural characterization of optimized hydrochar produced from hydrothermal carbonization of palm shell. Fuel 2016, 163, 88–97. [Google Scholar] [CrossRef]
- Guo, S.; Dong, X.; Wu, T.; Zhu, C. Influence of reaction conditions and feedstock on hydrochar properties. Energy Convers. Manag. 2016, 123, 95–103. [Google Scholar] [CrossRef]
- Titirici, M.M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, K.; Yang, Y.; Wang, J.-Y. Utilization of Sewage-Sludge-Derived Hydrochars toward Effi cient Co-combustion with Different-Rank Coals: Effects of Subcritical Water Conversion and Blending Scenarios. Energy Fuels 2014, 28, 6140–6150. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–27. [Google Scholar] [CrossRef]
- Saari, J.; Sermyagina, E.; Kaikko, J.; Vakkilainen, E.; Sergeev, V. Integration of hydrothermal carbonization and a CHP plant: Part 2—Operational and economic analysis. Energy 2016, 113, 574–585. [Google Scholar] [CrossRef]
- Licursi, D.; Antonetti, C.; Fulignati, S.; Vitolo, S.; Puccini, M.; Ribechini, E.; Bernazzani, L.; Raspolli Galletti, A.M. In-depth characterization of valuable char obtained from hydrothermal conversion of hazelnut shells to levulinic acid. Bioresour. Technol. 2017, 244, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Mun, T.-Y.; Yang, W.; Lee, U.; Hwang, J.; Jang, E.; Choi, C. Effects of hydrothermal treatment of sewage sludge on pyrolysis and steam gasification. Energy Convers. Manag. 2015, 103, 401–407. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z.; Fang, S.; Lin, Y.; Fan, Y. Combustion, pyrolysis and char CO2-gasification characteristics of hydrothermal carbonization solid fuel from municipal solid wastes. Fuel 2016, 181, 905–915. [Google Scholar] [CrossRef]
- Gunarathne, D.S.; Mueller, A.; Fleck, S.; Kolb, T.; Chmielewski, J.K.; Yang, W.; Blasiak, W. Gasification characteristics of hydrothermal carbonized biomass in an updraft pilot-scale gasifier. Energy Fuels 2014, 28, 1992–2002. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tang, J.; Gong, Y.; Zhang, H. Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ. Sci. Pollut. Res. 2015, 22, 16640–16651. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meas, A.; Shan, S.; Yang, R.; Gai, X. Production and optimization of bamboo hydrochars for adsorption of Congo red and 2-naphthol. Bioresour. Technol. 2016, 207, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Garcia Moscoso, J.L.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ro, K.S.; Sun, K.; Sun, H.; Wang, Z.; Libra, J.A.; Xing, B. New Evidence for High Sorption Capacity of Hydrochar for Hydrophobic Organic Pollutants. Environ. Sci. Technol. 2016, 50, 13274–13282. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Sun, H.; Ro, K.S.; Sun, K.; Libra, J.A.; Xing, B. Removal of antimony (III) and cadmium (II) from aqueous solution using animal manure-derived hydrochars and pyrochars. Bioresour. Technol. 2017, 234, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Pellera, F.M.; Giannis, A.; Kalderis, D.; Anastasiadou, K.; Stegmann, R.; Wang, J.Y.; Gidarakos, E. Adsorption of Cu(II) ions from aqueous solutions on biochars prepared from agricultural by-products. J. Environ. Manag. 2012, 96, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Kirschhöfer, F.; Sahin, O.; Becker, G.C.; Meffert, F.; Nusser, M.; Anderer, G.; Kusche, S.; Klaeusli, T.; Kruse, A.; Brenner-Weiss, G. Wastewater treatment—Adsorption of organic micropollutants on activated HTC-carbon derived from sewage sludge. Water Sci. Technol. 2016, 73, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Koottatep, T.; Fakkaew, K.; Tajai, N.; Polprasert, C. Isotherm models and kinetics of copper adsorption by using hydrochar produced from hydrothermal carbonization of faecal sludge. J. Water Sanit. Hyg. Dev. 2017. [Google Scholar] [CrossRef]
- Sun, K.; Gao, B.; Ro, K.S.; Novak, J.M.; Wang, Z.; Herbert, S.; Xing, B. Assessment of herbicide sorption by biochars and organic matter associated with soil and sediment. Environ. Pollut. 2012, 163, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Eibisch, N.; Schroll, R.; Fuß, R.; Mikutta, R.; Helfrich, M.; Flessa, H. Pyrochars and hydrochars differently alter the sorption of the herbicide isoproturon in an agricultural soil. Chemosphere 2015, 119, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Y.; Zhou, C.; Luo, G.; Zhang, S.; Chen, J. A novel porous carbon derived from hydrothermal carbon for efficient adsorption of tetracycline. Carbon 2014, 77, 627–636. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Ro, K.; Guo, M.; Novak, J.; Mashayekhi, H.; Xing, B. Sorption of bisphenol A, 17α-ethinyl estradiol and phenanthrene on thermally and hydrothermally produced biochars. Bioresour. Technol. 2011, 102, 5757–5763. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Gao, B.; Chen, J.; Zimmerman, A.R. Hydrochars derived from plant biomass under various conditions: Characterization and potential applications and impacts. Chem. Eng. J. 2015, 267, 253–259. [Google Scholar] [CrossRef]
- Fang, J.; Gao, B.; Zimmerman, A.R.; Ro, K.S.; Chen, J. Physically (CO2) activated hydrochars from hickory and peanut hull: Preparation, characterization, and sorption of methylene blue, lead, copper, and cadmium. RSC Adv. 2016, 6, 24906–24911. [Google Scholar] [CrossRef]
- Kim, H.-W.; Bae, S.; Lee, J.-Y. A Study on the Removal of Heavy Metals in Soil by Sewage Sludge Biochar. J. Soil Groundw. Environ. 2013, 18, 58–64. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Rocher, V.; Kyriakou, G.; Greenway, G.M. Removal of Pb2+ and Cd2+ from aqueous solution using chars from pyrolysis and microwave-assisted hydrothermal carbonization of Prosopis africana shell. J. Ind. Eng. Chem. 2014, 20, 3467–3473. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N.D. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Nartey, D.; Zhao, B. Biochar Preparation, Characterization, and Adsorptive Capacity and Its Effect on Bioavailability of Contaminants: An Overview. Adv. Mater. Sci. Eng. 2014, 2014, 715398. [Google Scholar] [CrossRef]
- Baccile, N.; Laurent, G.; Babonneau, F.; Fayon, F.; Titirici, M.-M.; Antonietti, M. Structural Characterization of Hydrothermal Carbon Spheres by Advanced Solid-State MAS 13C NMR Investigations. J. Phys. Chem. C 2009, 113, 9644–9654. [Google Scholar] [CrossRef]
- Falco, C.; Perez Caballero, F.; Babonneau, F.; Gervais, C.; Laurent, G.; Titirici, M.-M.; Baccile, N. Hydrothermal Carbon from Biomass: Structural Differences between Hydrothermal and Pyrolyzed Carbons via 13C Solid State NMR. Langmuir 2011, 27, 14460–14471. [Google Scholar] [CrossRef] [PubMed]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nie, P.; Ding, B.; Dong, S.; Hao, X.; Dou, H.; Zhang, X. Biomass derived carbon for energy storage devices. J. Mater. Chem. A 2017, 5, 2411–2428. [Google Scholar] [CrossRef]
- Wei, T.; Wei, X.; Gao, Y.; Li, H. Large scale production of biomass-derived nitrogen-doped porous carbon materials for supercapacitors. Electrochim. Acta 2015, 169, 186–194. [Google Scholar] [CrossRef]
- Liu, X.; Giordano, C.; Antonietti, M. A Facile Molten-Salt Route to Graphene Synthesis. Small 2014, 10, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Manyala, N.; Bello, A.; Barzegar, F.; Khaleed, A.A.; Momodu, D.Y.; Dangbegnon, J.K. Coniferous pine biomass: A novel insight into sustainable carbon materials for supercapacitors electrode. Mater. Chem. Phys. 2016, 182, 139–147. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Fuertes, A.B.; Sevilla, M. From Soybean residue to advanced supercapacitors. Sci. Rep. 2015, 5, 16618. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zou, B.; Li, Y.; Wang, Z.; Zhao, C.; Su, Y.; Guo, Y. The production of hydrochar-based hierarchical porous carbons for use as electrochemical supercapacitor electrode materials. Colloids Surf. A Phys. Eng. Aspects 2013, 423, 104–111. [Google Scholar] [CrossRef]
- Jain, A.; Xu, C.; Jayaraman, S.; Balasubramanian, R.; Lee, J.Y.; Srinivasan, M.P. Mesoporous activated carbons with enhanced porosity by optimal hydrothermal pre-treatment of biomass for supercapacitor applications. Microporous Mesoporous Mater. 2015, 218, 55–61. [Google Scholar] [CrossRef]
- Tan, J.; Chen, H.; Gao, Y.; Li, H. Nitrogen-doped porous carbons derived from citric acid and urea with outstanding supercapacitance performance. Electrochim. Acta 2015, 178, 144–152. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. A Green Approach to High-Performance Supercapacitor Electrodes: The Chemical Activation of Hydrochar with Potassium Bicarbonate. ChemSusChem 2016, 9, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Brun, N.; Edembe, L.; Gounel, S.; Mano, N.; Titirici, M.M. Emulsion-Templated Macroporous Carbons Synthesized by Hydrothermal Carbonization and Their Application for the Enzymatic Oxidation of Glucose. ChemSusChem 2013, 6, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Flexer, V.; Donose, B.C.; Lefebvre, C.; Pozo, G.; Boone, M.N.; Van Hoorebeke, L.; Baccour, M.; Bonnet, L.; Calas-Etienne, S.; Galarneau, A.; et al. Microcellular Electrode Material for Microbial Bioelectrochemical Systems Synthesized by Hydrothermal Carbonization of Biomass Derived Precursors. ACS Sustain. Chem. Eng. 2016, 4, 2508–2516. [Google Scholar] [CrossRef]
- Safari, F.; Norouzi, O.; Tavasoli, A. Hydrothermal gasification of Cladophora glomerata macroalgae over its HC as a catalyst for hydrogen-rich gas production. Bioresour. Technol. 2016, 222, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Zhou, X.; Feng, C.; Gao, S.; Wu, Q.; Wang, C. Synergetic catalysis of Ni Pd nanoparticles supported on biomass-derived carbon spheres for hydrogen production from ammonia borane at room temperature. Int. J. Hydrogen Energy 2017, 42, 5733–5740. [Google Scholar] [CrossRef]
- Gai, C.; Zhang, F.; Lang, Q.; Liu, T.; Peng, N.; Liu, Z. Facile one-pot synthesis of iron nanoparticles immobilized into theporous hydrochar for catalytic decomposition of phenol. Appl. Catal. B Environ. 2017, 204, 566–576. [Google Scholar] [CrossRef]
- Prasannan, A.; Imae, T. One-Pot Synthesis of Fluorescent Carbon Dots from Orange Waste Peels. Ind. Eng. Chem. Res. 2013, 52, 15673–15678. [Google Scholar] [CrossRef]
- Wen, G.; Wang, B.; Wang, C.; Wang, J.; Tian, Z.; Schlögl, R.; Su, D.S. Hydrothermal Carbon Enriched with Oxygenated Groups from Biomass Glucose as an Efficient Carbocatalyst. Angew. Chem. Int. Ed. 2017, 56, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Frusteri, L.; Cannilla, C.; Bonura, G.; Chuvilin, A.L.; Perathoner, S.; Centi, G.; Frusteri, F. Carbon microspheres preparation, graphitization and surface functionalization for glycerol etherification. Catal. Today 2016, 277, 68–77. [Google Scholar] [CrossRef]
| Model Category | Advantages | Limitations |
|---|---|---|
| Basic kinetic models |
|
|
| ||
| Statistical models |
|
|
| ||
| ||
| Computational models |
|
|
| ||
|
| Hydrochar Property | Modeling Approach | Description of Data Used in Model | Reference | ||
|---|---|---|---|---|---|
| Feedstock | Process Conditions | Data Points | |||
| Hydrochar Yield | Linear regression | Data was collected from the literature, various feedstocks were used | Data was collected from the literature, various conditions were included | 263 | [55] |
| Regression tree | Data was collected from the literature, various feedstocks were used | Data was collected from the literature, various conditions were included | 263 | [55] | |
| Quadratic model | Olive stones | Biomass/water ratio: 1.1–12.3%; Temp: 150–250 °C; Time: 3.2–36.8 h | 18 experiments, designed using response surface methodology | [24] | |
| Quadratic model | Shrimp waste | Temp: 150–210 °C; Time: 1–2 h | 13 experiments, designed using response surface methodology | [56] | |
| Linear regression | Anaerobically digested maize silage | Biomass Conc.: 42.3 g/L; pH: 3–7; Temp: 190–270 °C; Time: 2–10 h | 15 experiments, designed using Box-Behnken fractional design | [46] | |
| Quadratic model | Palm shell | Biomass/water ratio: 1.10–1.60; Temp: 180–260 °C; Time: 0.5–2 h | 20 experiments, designed using response surface methodology | [57] | |
| Quadratic model | Tomato-peel waste | Biomass/water ratio: 3.3–12.3%; Temp: 150–250 °C; Time: 1.6–18.4 h | 18 experiments, designed using response surface methodology | [25] | |
| Hydrochar carbon content | Linear regression | Data was collected from the literature, various feedstocks were used | Data was collected from the literature, various conditions were included | 248 | [55] |
| Regression tree | Data was collected from the literature, various feedstocks were used | Data was collected from the literature, various conditions were included | 248 | [55] | |
| Linear regression | Anaerobically digested maize silage | Biomass Conc.: 42.3 g/L; pH: 3–7; Temp: 190–270 °C; Time: 2–10 h | 15 experiments, designed using Box-Behnken fractional design | [46] | |
| Carbon yield (g C/g dry feedstock) | Linear regression | Data was collected from the literature, various feedstocks were used | Data was collected from the literature, various conditions were included | 244 | [55] |
| Regression tree | Data was collected from the literature, various feedstocks were used | Data was collected from the literature, various conditions were included | 244 | [55] | |
| Energy content | Linear regression | Data was collected from the literature, various feedstocks were used | Data was collected from the literature, various conditions were included | 220 | [55] |
| Regression tree | Data was collected from the literature, various feedstocks were used | Data was collected from the literature, various conditions were included | 220 | [55] | |
| Quadratic model | Olive stones | Biomass/water ratio: 1.1–12.3%; Temp: 150–250 °C; Time: 3.2–36.8 h | 18 experiments, designed using response surface methodology | [24] | |
| Quadratic model | Shrimp waste | Temp: 150–210 °C; Time: 1–2 h | 13 experiments, designed using response surface methodology | [56] | |
| Quadratic model | Tomato-peel waste | Biomass/water ratio: 3.3–12.3%; Temp: 150–250 °C; Time: 1.6–18.4 h | 18 experiments, designed using response surface methodology | [25] | |
| Feedstock | Temperature (Time) | SA-N2 | SA-CO2 | pH Optimum | Sorption Capacity qc (mg/g) | Isotherm Model * | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (m2/g) | (m2/g) | - | Pb (II) | Cu (II) | Cd (II) | Sb (III) | Zn (II) | ||||
| Hydrochars | |||||||||||
| prosopis africana shell | 200 °C (20 min, microwave) | 6.05 | Pb: 6, Cd: 8 | 45.3 | - | 38.3 | - | L > F | [87] | ||
| sawdust | 200 °C (20 h) | 4.41 | 4–8 | - | 14.5 | L > F | [69] | ||||
| wheat straw | 200 °C (20 h) | 9.14 | 4–8 | - | 13.9 | L > F | [69] | ||||
| corn stalk | 200 °C (20 h) | 8.58 | 4–8 | - | 14.5 | L > F | [69] | ||||
| sawdust (multi-metal) | 200 °C (20 h) | 3.8 | 2.2 | 1.3 | 0.7 | n.r. | [69] | ||||
| wheat straw (multi-metal) | 200 °C (20 h) | 2.8 | 2.2 | 1.3 | 0.7 | n.r. | [69] | ||||
| corn stalk (multi-metal) | 200 °C (20 h) | 2.9 | 2.7 | 1.6 | 1.3 | n.r. | [69] | ||||
| switchgrass | 300 °C (30 min) | - | 4.0 ** | 1.5 ** | [71] | ||||||
| peanut hulls | 300 °C (5 h) | 1.3 | 96.9 | - | 0.88 | L > F | [87] | ||||
| swine solids | 250 °C (4) | 1.87 | 22.38 | 27.18 | 3.98 | LL > L > F | [73] | ||||
| poultry litter | 250 °C (4) | 2.77 | 24.23 | 19.8 | 2.24 | LL > L > F | [73] | ||||
| faecal sludge | 200 °C (5 h) | 4.03 | n.r. | [78] | |||||||
| compost (MSW) | 300 °C (30 min) | - | - | 5–6 | 7.72 | - | - | - | F > L | [74] | |
| Modified or activated hydrochars | |||||||||||
| sawdust | 200 °C (20 h) + KOH | 0.69 | 40.78 | L > F | [69] | ||||||
| wheat straw | 200 °C (20 h) + KOH | 0.42 | 38.75 | L > F | [69] | ||||||
| corn stalk | 200 °C (20 h) + KOH | 1.84 | 30.4 | L > F | [69] | ||||||
| sawdust (multi-metal) | 200 °C (20 h) + KOH | 15.6 | 8.9 | 4.2 | 3.8 | n.r. | [69] | ||||
| wheat straw (multi-metal) | 200 °C (20 h) + KOH | 21.8 | 11.8 | 4.7 | 3.6 | n.r. | [69] | ||||
| corn stalk (multi-metal) | 200 °C (20 h) + KOH | 18.8 | 10.0 | 4.6 | 3.1 | n.r. | [69] | ||||
| peanut hulls | 300 °C (5 h) + H2O2 | 1.4 | 114.4 | - | 22.82 | L > F | [75] | ||||
| peanut hulls (multi-metal) | 300 °C (5 h) + H2O2 | 1.4 | 114.4 | - | 16.45 | 1.22 | 0.21 | ADM | [75] | ||
| switchgrass | 300 °C (30 min) + KOH | - | 31 ** | 34 ** | |||||||
| banana peels (fresh) | 230 °C (2 h/H3PO4) | 31.65 | 7 | 193 | L > F | [77] | |||||
| banana peels (dried) | 230 °C (2 h/H3PO4) | 15.76 | 7 | 359 | L > F | [77] | |||||
| faecal sludge | 200 °C (5 h) + KOH | 4.41 | 36.63 | L > F | [78] | ||||||
| Pyrochars | |||||||||||
| prosopis africane shell | 350 °C (10 min) | 3.11 | 31.3 | 29.9 | - | [87] | |||||
| swine solid | 250 °C (4 h) | 1.2 | 29.13 | 81.32 | 13.09 | LL > L > F | [73] | ||||
| swine solid | 450 °C (4 h) | 14.25 | 138.99 | 76.18 | 12.66 | LL > L > F | [73] | ||||
| swine solid | 600 °C (4 h) | 5.51 | 205.56 | 33.44 | 4.44 | LL > L > F | [73] | ||||
| poultry litter | 250 °C (4 h) | 2.99 | 35.11 | 57.69 | 16.28 | LL > L > F | [73] | ||||
| poultry litter | 450 °C (4 h) | 4.76 | 135.74 | 35.15 | 8.27 | LL > L > F | [73] | ||||
| poultry litter | 600 °C (4 h) | 4.2 | 150.01 | 33.48 | 6.63 | LL > L > F | [73] | ||||
| compost (MSW) | 300 °C (6 h) | 5–6 | 7.94 | F > L | [74] | ||||||
| compost (MSW) | 600 °C (6 h) | 5–6 | 3.38 | F > L | [74] | ||||||
| Feed-Stock | Process-T (Time) | N2-SA | CO2-SA | Co (mg/L) | Pyrene ** | Triclosan ** | Estrone ** | Carba-Mazepine ** | Acetamino-Phen ** | Fluridone ** | Nor-Flurazon ** | Bisphenol A * | 17a-Ethinyl Estradiol * | Phenan-Threne * | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.001–0.12 | 0.1–10 | 0.2–20 | 0.2–100 | 0.2–100 | n.r. | 0.4–18 | 0.025–12 | 0.1–4 | 0.010–1.12 | ||||||
| °C (h) | (m2/g) | (m2/g) | logKow | 5.18 | 4.76 | 3.13 | 2.45 | 0.46 | 1.87 | 2.45 | 2.20 | 4.15 | 4.57 | ||
| swine solid | H-250 (4) | 1.9 | 22.4 | logKOC * (mL/g) | 6.19 | 5.36 | 4.78 | 4.31 | 2.97 | - | - | - | - | - | [72] |
| swine solid | P-250 (4) | 1.2 | 29.1 | - | 5.56 | 4.31 | 3.19 | 2.34 | 1.55 | - | - | - | - | - | [72] |
| swine solid | P-450 (4) | 14.3 | 139 | - | 5.85 | 4.72 | 3.63 | 2.91 | 1.85 | - | - | - | - | - | [72] |
| swine solid | P-600 (4) | 5.5 | 205.6 | - | 5.71 | 4.85 | 4.24 | 3.07 | 2.12 | - | - | - | - | - | [72] |
| swine solid | H-250 (20) | 4.03 | 27.2 | - | - | - | - | - | - | 3.72 | 3.2 | 3.42 | 4.36 | 5.12 | [79] |
| poultry litter | H-250 (4) | 2.8 | 24.2 | - | 6.08 | 5.26 | 4.53 | 4.19 | 2.79 | - | - | - | - | - | [72] |
| poultry litter | P-250 (4) | 3 | 35.1 | - | 5.33 | 4.55 | 3.45 | 2.68 | 1.67 | - | - | - | - | - | [72] |
| poultry litter | P-450 (4) | 4.8 | 135.7 | - | 5.7 | 4.75 | 3.76 | 2.85 | 1.79 | - | - | - | - | - | [72] |
| poultry litter | P-600 (4) | 4.2 | 150 | - | 5.78 | 4.9 | 4.15 | 3.21 | 2.17 | - | - | - | - | - | [72] |
| poultry litter | H-250 (20) | 8.77 | 61.4 | - | - | - | - | - | - | 3.92 | 3.49 | 3.39 | 4.65 | 5.41 | [79] |
| poultry litter | P-400 (2–7) | 6.71 | 110.2 | - | - | - | - | - | - | 3.46 | 3.2 | 3.08 | 3.71 | 5.48 | [79] |
| wheat straw | P-400 (2–7) | 2.08 | 229.8 | - | - | - | - | - | - | 3.33 | 2.78 | 2.29 | 3.66 | 4.96 | [79] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Álvarez, A.; Bae, S. Hydrothermal Carbonization: Modeling, Final Properties Design and Applications: A Review. Energies 2018, 11, 216. https://doi.org/10.3390/en11010216
Román S, Libra J, Berge N, Sabio E, Ro K, Li L, Ledesma B, Álvarez A, Bae S. Hydrothermal Carbonization: Modeling, Final Properties Design and Applications: A Review. Energies. 2018; 11(1):216. https://doi.org/10.3390/en11010216
Chicago/Turabian StyleRomán, Silvia, Judy Libra, Nicole Berge, Eduardo Sabio, Kyoung Ro, Liang Li, Beatriz Ledesma, Andrés Álvarez, and Sunyoung Bae. 2018. "Hydrothermal Carbonization: Modeling, Final Properties Design and Applications: A Review" Energies 11, no. 1: 216. https://doi.org/10.3390/en11010216







