One-Step Self-Assembly Synthesis α-Fe2O3 with Carbon-Coated Nanoparticles for Stabilized and Enhanced Supercapacitors Electrode
Abstract
:1. Introduction
2. Experimental Section
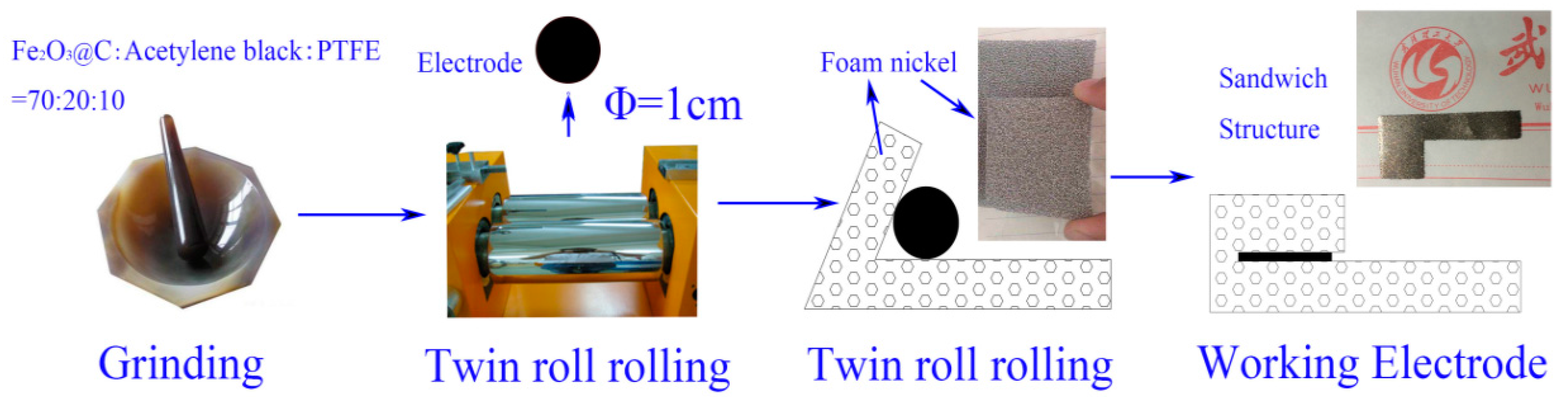
2.1. Synthesis of α-Fe2O3@C Composite
2.2. Materials Characterization
2.3. Electrochemical Measurements
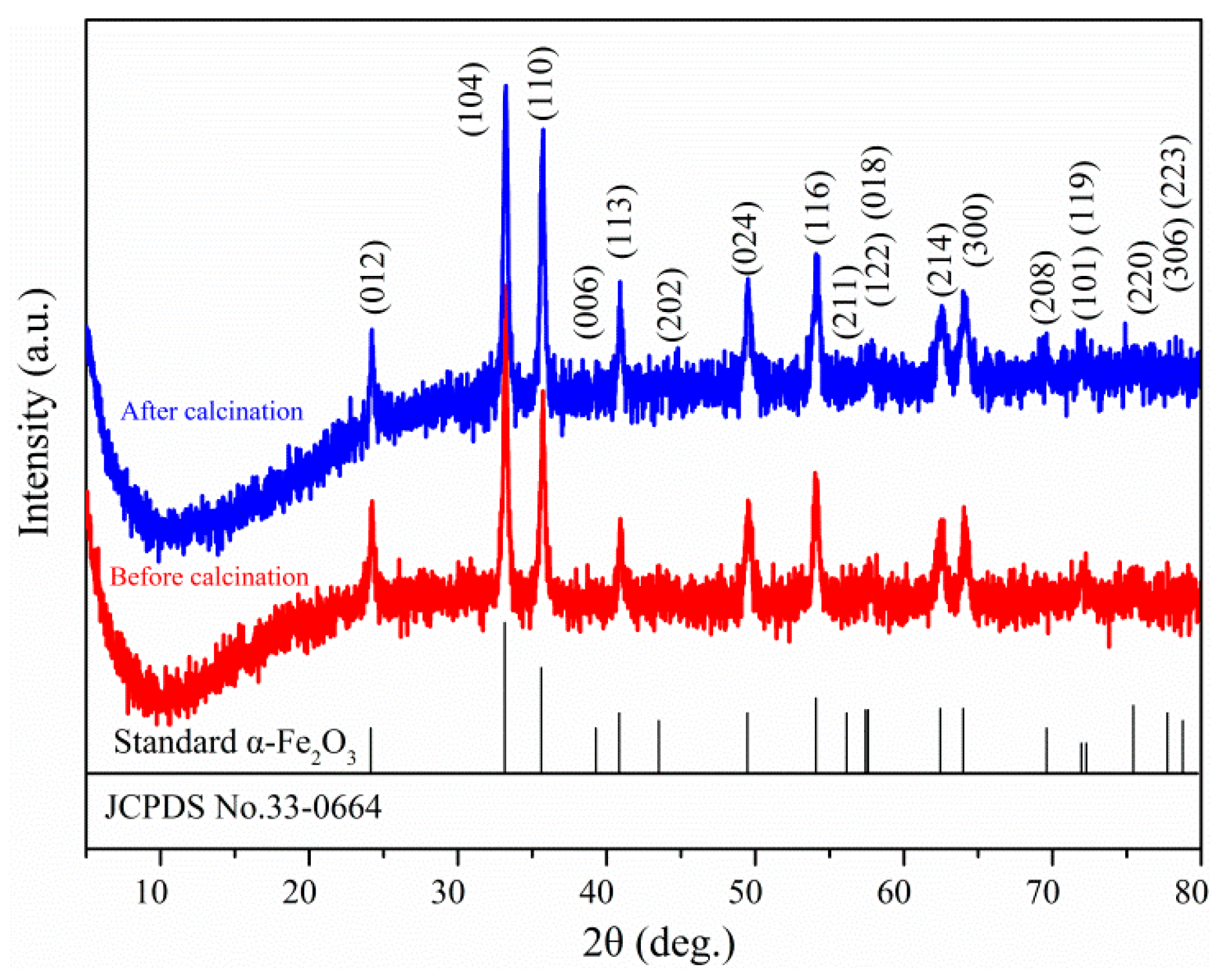
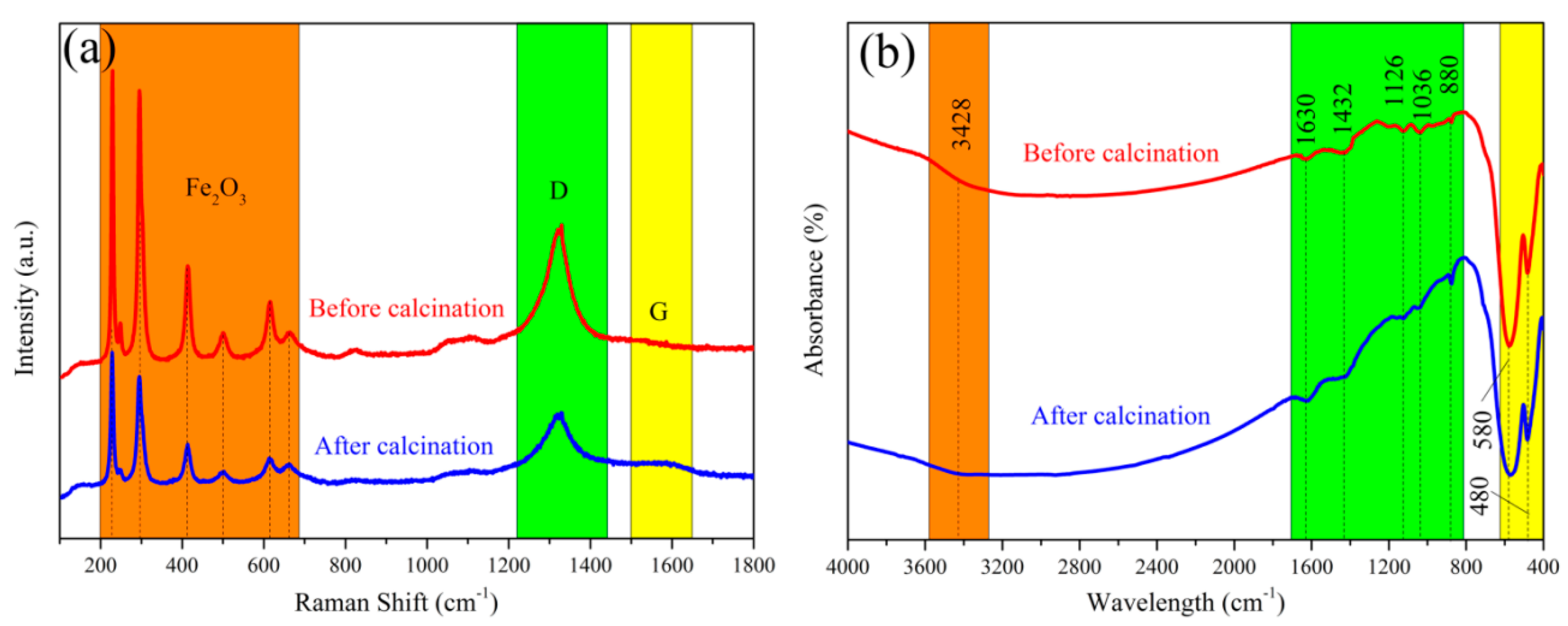
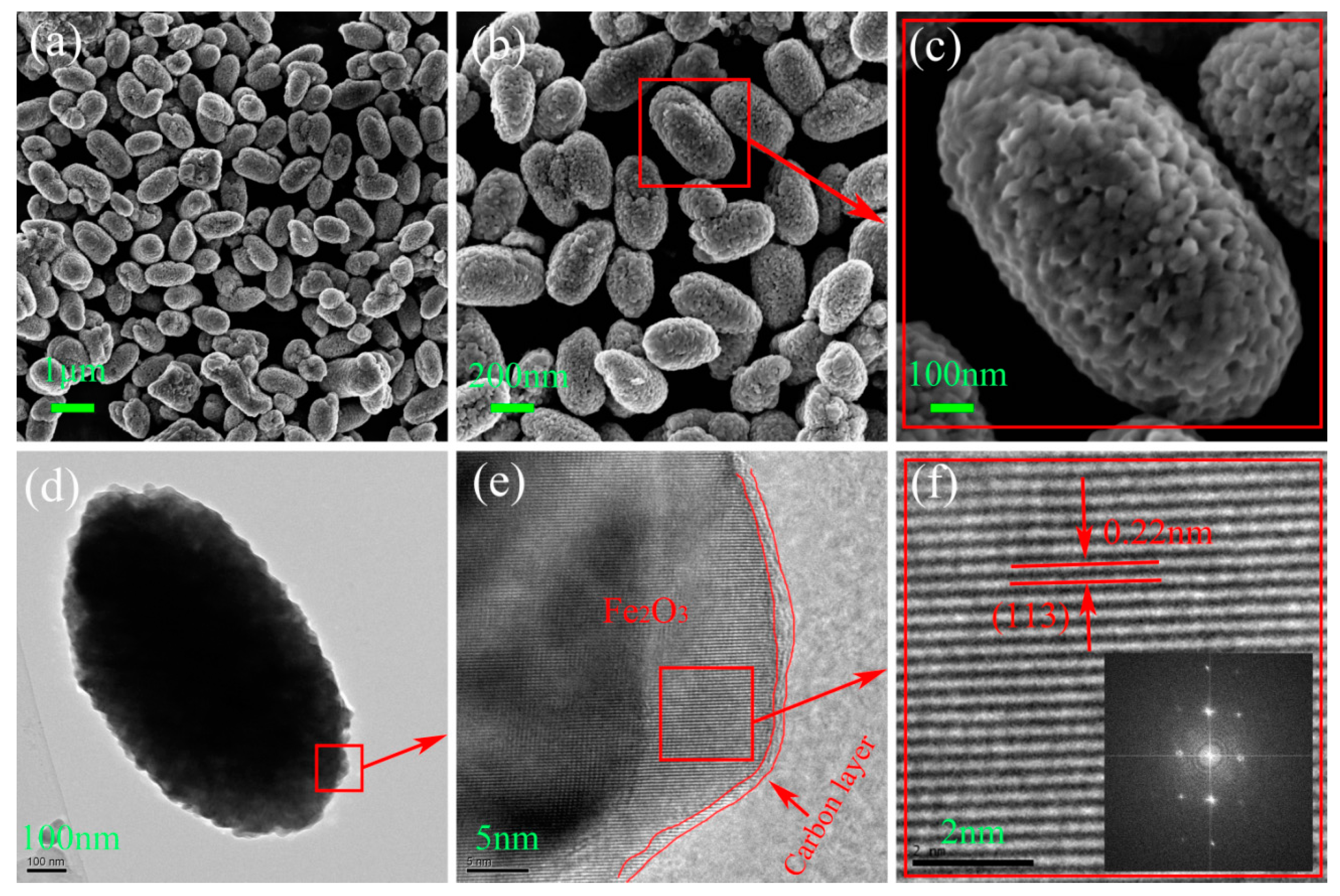
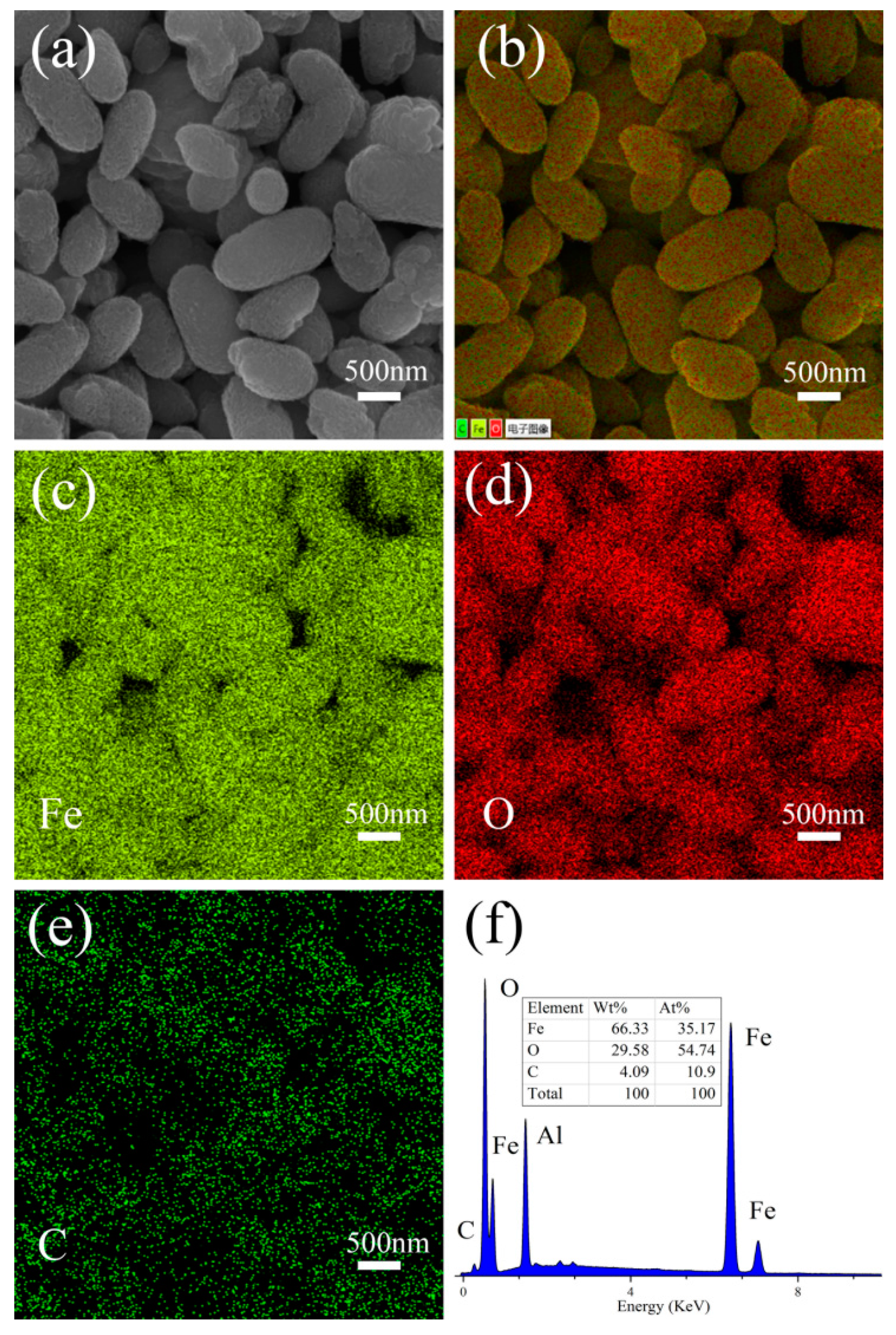
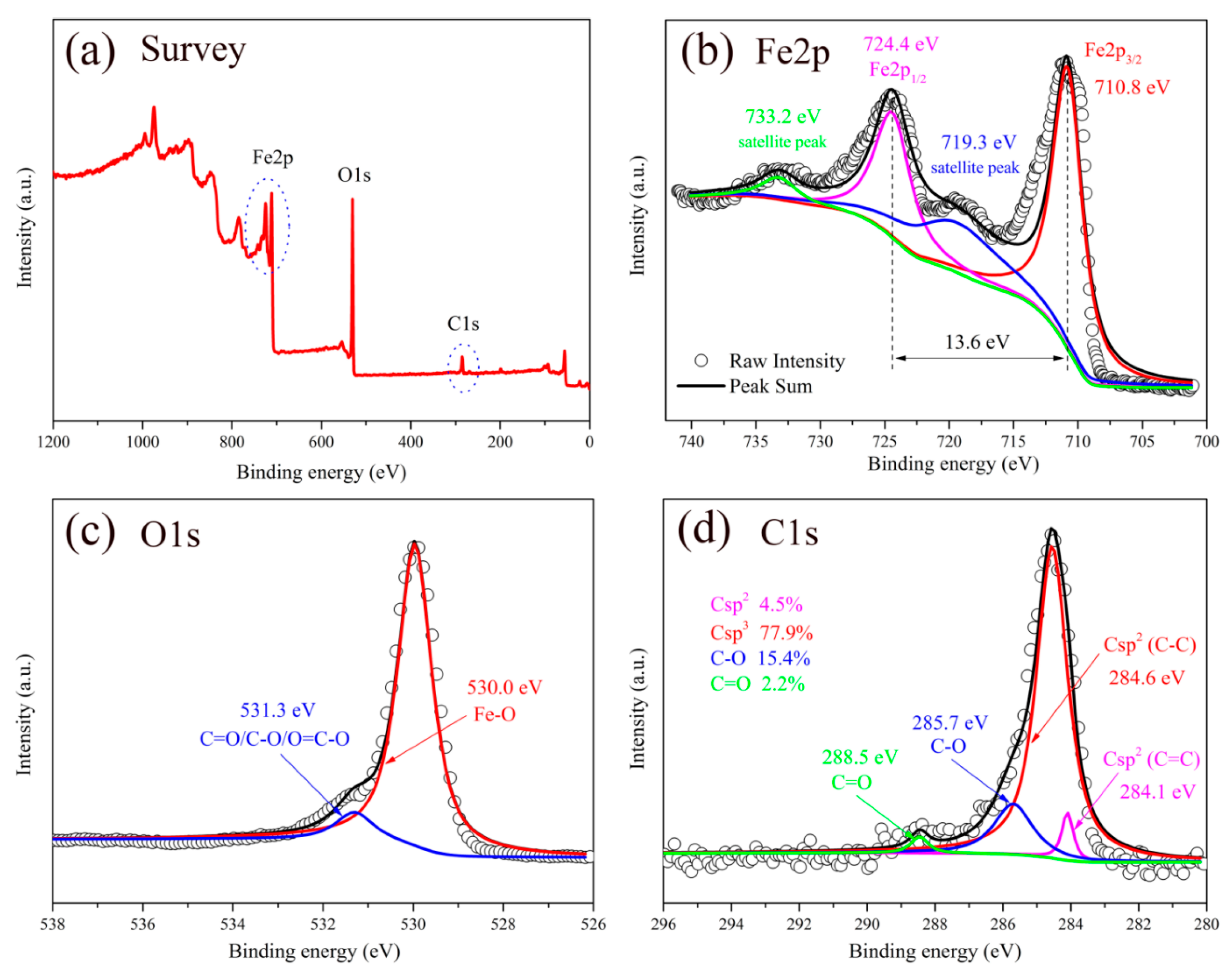
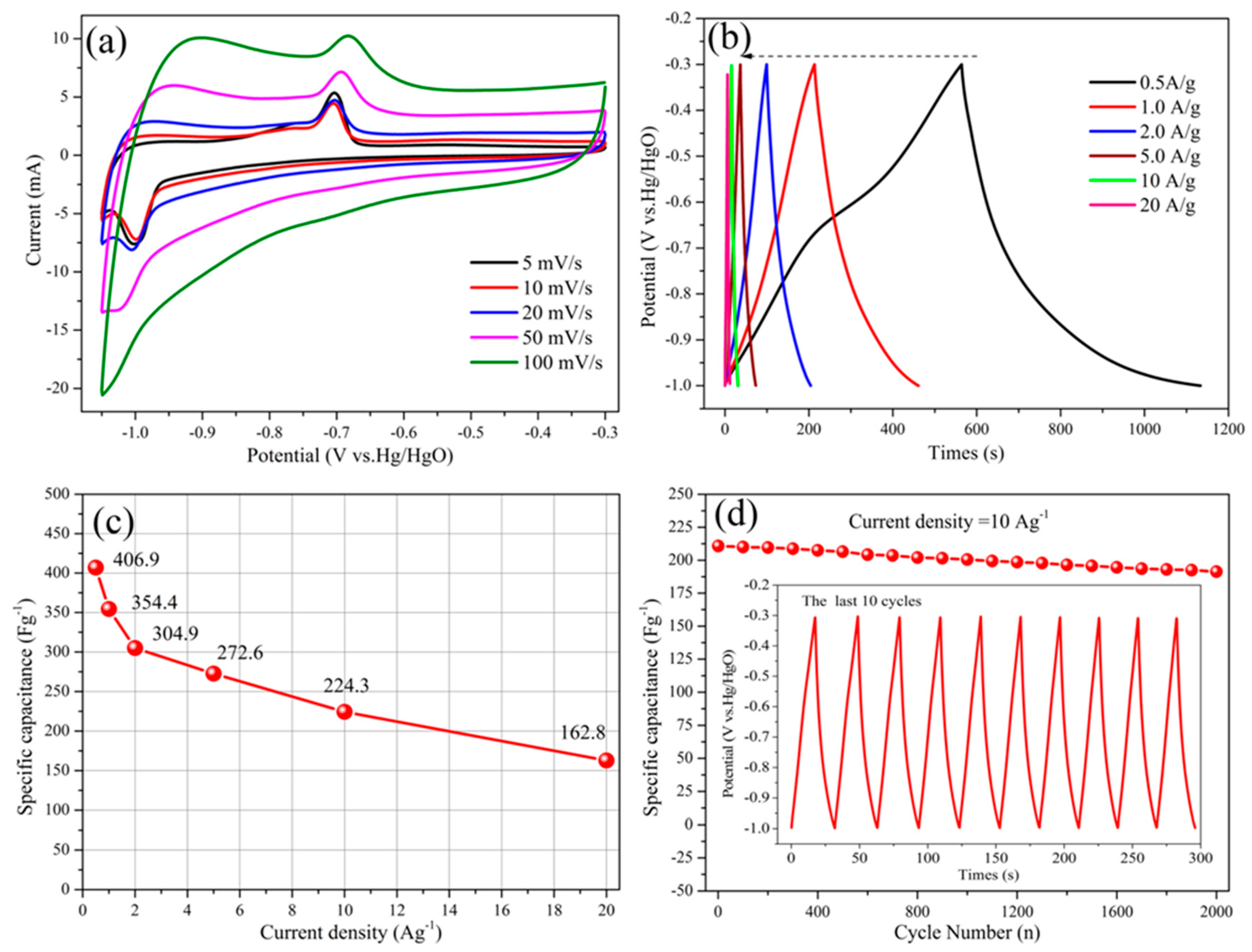
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Nithya, V.D.; Arul, N.S. Review on α-Fe2O3 based negative electrode for high performance supercapacitors. J. Power Sources 2016, 327, 297–318. [Google Scholar] [CrossRef]
- Xiong, G.; Meng, C.; Reifenberger, R.G.; Irazoqui, P.P.; Fisher, T.S. Graphitic Petal Electrodes for All-Solid-State Flexible Supercapacitors. Adv. Energy Mater. 2013, 4, 1300515. [Google Scholar] [CrossRef]
- Xiang, D.; Yin, L.; Wang, C.; Zhang, L. High electrochemical performance of RuO2–Fe2O3 nanoparticles embedded ordered mesoporous carbon as a supercapacitor electrode material. Energy 2016, 106, 103–111. [Google Scholar] [CrossRef]
- Peng, X.; Peng, L.; Wu, C.; Xie, Y. Two dimensional nanomaterials for flexible supercapacitors. Chem. Soc. Rev. 2014, 43, 3303–3323. [Google Scholar] [CrossRef] [PubMed]
- Shivakumara, S.; Penki, T.R.; Munichandraiah, N. High specific surface area α-Fe2O3 nanostructures as high performance electrode material for supercapacitors. Mater. Lett. 2014, 131, 100–103. [Google Scholar] [CrossRef]
- Lin, F.; Li, X.; Zhao, Y.; Yang, Z. Control Strategies with Dynamic Threshold Adjustment for Supercapacitor Energy Storage System Considering the Train and Substation Characteristics in Urban Rail Transit. Energies 2016, 9, 257. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon Nanotubes—The Route Toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Zhou, G.; Yin, L.C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Nan, H.; Yu, L.; Ma, W.; Geng, B.; Zhang, X. Flexible superior electrode architectures based on three-dimensional porous spinous α-Fe2O3 with a high performance as a supercapacitor. Dalton Trans. 2015, 44, 9581–9587. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Han, Z.; Chai, F.; Qu, F.; Xia, H.; Wu, X. Flexible heterostructured supercapacitor electrodes based on α-Fe2O3 nanosheets with excellent electrochemical performances. Dalton Trans. 2016, 45, 12862–12870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, C.J. Preparation and application of iron oxide/graphene based composites for electrochemical energy storage and energy conversion devices: Current status and perspective. Nano Energy 2015, 11, 277–293. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Liu, X.; Zeng, R.; Li, M.; Huang, Y.; Hu, X. Constructing Hierarchical Tectorum-like α-Fe2O3/PPy Nanoarrays on Carbon Cloth for Solid-State Asymmetric Supercapacitors. Angew. Chem. Int. Ed. 2017, 129, 1125–1130. [Google Scholar] [CrossRef]
- Yang, X.; Sun, H.; Zhang, L.; Zhao, L.; Lian, J.; Jiang, Q. High Efficient Photo-Fenton Catalyst of α-Fe2O3/MoS2 Hierarchical Nanoheterostructures: Reutilization for Supercapacitors. Sci. Rep. 2016, 6, 31591. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, S.; You, X. Inorganic nanostructured materials for high performance electrochemical supercapacitors. Nanoscale 2014, 6, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, D.; Wang, T.; Jia, D.; Xia, W.; Lv, Y.; Cao, Y.; Tan, Y.; Liu, P. One-step solvothermal synthesis of quasi-hexagonal Fe2O3 nanoplates/graphene composite as high performance electrode material for supercapacitor. Electrochim. Acta 2016, 191, 275–283. [Google Scholar] [CrossRef]
- Liu, H.D.; Zhang, J.L.; Xu, D.D.; Huang, L.H.; Tan, S.Z.; Mai, W.J. Easy one-step hydrothermal synthesis of nitrogen-doped reduced graphene oxide/iron oxide hybrid as efficient supercapacitor material. J. Solid State Electrochem. 2014, 19, 135–144. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, L.; Hou, P.; Li, F.; Liu, C.; Cheng, H. High Reversible Lithium Storage Capacity and Structural Changes of Fe2O3 Nanoparticles Confined inside Carbon Nanotubes. Adv. Energy Mater. 2016, 6, 1501755. [Google Scholar] [CrossRef]
- Wang, S.; Hu, L.; Hu, Y.; Jiao, S. Conductive polyaniline capped Fe2O3 composite anode for high rate lithium ion batteries. Mater. Chem. Phys. 2014, 146, 289–294. [Google Scholar] [CrossRef]
- Xu, L.; Xia, J.; Xu, H.; Yin, S.; Wang, K.; Huang, L.; Wang, L.; Li, H. Reactable ionic liquid assisted solvothermal synthesis of graphite-like C3N4 hybridized α-Fe2O3 hollow microspheres with enhanced supercapacitive performance. J. Power Sources 2014, 245, 866–874. [Google Scholar] [CrossRef]
- Shou, Q.; Cheng, J.; Zhang, L.; Nelson, B.J.; Zhang, X. Synthesis and characterization of a nanocomposite of goethite nanorods and reduced graphene oxide for electrochemical capacitors. J. Solid State Chem. 2012, 185, 191–197. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and Enhanced Visible-Light Photocatalytic H2-Production Activity of Graphene/C3N4 Composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Meher, S.K.; Justin, P.; Rao, G.R. Microwave-mediated synthesis for improved morphology and pseudocapacitance performance of nickel oxide. ACS Appl. Mater. Interfaces 2011, 3, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, L.; Zhao, C.; Liu, H.-K.; Guo, Z. Controllable synthesis of RGO/FexOy nanocomposites as high-performance anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 9844–9850. [Google Scholar] [CrossRef]
- Cao, K.; Jiao, L.; Liu, H.; Liu, Y.; Wang, Y.; Guo, Z.; Yuan, H. 3D Hierarchical Porous α-Fe2O3 Nanosheets for High-Performance Lithium-Ion Batteries. Adv. Energy Mater. 2014, 5, 1401421. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, H.; Liu, Z.; Han, B.; Zhang, X. A Highly Efficient Chemical Sensor Material for H2S: α-Fe2O3 Nanotubes Fabricated Using Carbon Nanotube Templates. Adv. Mater. 2005, 17, 2993–2997. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Liu, E.; He, F.; Shi, C.; He, C.; Li, J.; Zhao, N. Three-dimensional core-shell Fe2O3@carbon/carbon cloth as binder-free anode for the high-performance lithium-ion batteries. Appl. Surf. Sci. 2016, 390, 350–356. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; He, Y.S.; Gong, Q.; Che, H.; Ma, Z.F. Carbon coated SnO2 nanoparticles anchored on CNT as a superior anode material for lithium-ion batteries. Nanoscale 2016, 8, 4121–4126. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Qin, X. Flower-like SnO2 nanoparticles grown on graphene as anode materials for lithium-ion batteries. J. Solid State Electrochem. 2013, 18, 1031–1039. [Google Scholar] [CrossRef]
- Subramaniyam, C.M.; Islam, M.M.; Akhter, T.; Cardillo, D.; Konstantinov, K.; Liu, H.K.; Dou, S.X. A chemically modified graphene oxide wrapped porous hematite nano-architecture as a high rate lithium-ion battery anode material. RSC Adv. 2016, 6, 82698–82706. [Google Scholar] [CrossRef]
- Hu, J.; Zheng, J.; Tian, L.; Duan, Y.; Lin, L.; Cui, S.; Peng, H.; Liu, T.; Guo, H.; Wang, X.; et al. A core-shell nanohollow-γ-Fe2O3@graphene hybrid prepared through the Kirkendall process as a high performance anode material for lithium ion batteries. Chem. Commun. 2015, 51, 7855–7858. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Yi, H.; Wei, H.; Guo, Z.; Wang, X. One-step preparation of single-crystalline Fe2O3 particles/graphene composite hydrogels as high performance anode materials for supercapacitors. Nano Energy 2014, 7, 86–96. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, H.; Wu, J.; Chu, X.; He, Y.-B.; Han, C.; Miao, C.; Wang, S.; Li, B.; Kang, F. Fe3O4 nanoparticles encapsulated in electrospun porous carbon fibers with a compact shell as high-performance anode for lithium ion batteries. Carbon 2015, 87, 347–356. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, Z.; Zheng, M.; Wang, T.; Yang, J.; Yuan, F.; Lu, X.; Liu, L.; Sun, D. Amorphous Fe2O3 nanoshells coated on carbonized bacterial cellulose nanofibers as a flexible anode for high-performance lithium ion batteries. J. Power Sources 2016, 307, 649–656. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, C.; Lu, T.; Guo, Z.; Zhang, D.; Ma, J.; Zhu, S. Simple fabrication of a Fe2O3/carbon composite for use in a high-performance lithium ion battery. Carbon 2013, 52, 565–573. [Google Scholar] [CrossRef]
- Mai, L.Q.; Yang, F.; Zhao, Y.L.; Xu, X.; Xu, L.; Luo, Y.Z. Hierarchical MnMoO4/CoMoO4 heterostructured nanowires with enhanced supercapacitor performance. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Kant, R. Theory for staircase voltammetry and linear scan voltammetry on fractal electrodes: Emergence of anomalous Randles-Sevcik behavior. Electrochim. Acta 2013, 111, 223–233. [Google Scholar]
- Mundinamani, S.P.; Rabinal, M.K. Molecular modification of highly degenerate semiconductor as an active electrode to enhance the performance of supercapacitors. Mater. Res. Express 2014, 1, 045508. [Google Scholar] [CrossRef]
- Nie, G.; Lu, X.; Lei, J.; Jiang, Z.; Wang, C. Electrospun V2O5-doped α-Fe2O3 composite nanotubes with tunable ferromagnetism for high-performance supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 15495–15501. [Google Scholar] [CrossRef]
- Nie, G.; Lu, X.; Chi, M.; Zhu, Y.; Yang, Z.; Song, N.; Wang, C. Hierarchical α-Fe2O3@MnO2 core-shell nanotubes as electrode materials for high-performance supercapacitors. Electrochim. Acta 2017, 231, 36–43. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, M.C.; Kong, L.B.; Wang, D.; Hu, Y.M.; Han, W.; Kang, L. Advanced asymmetric supercapacitors based on Ni3(PO4)2@GO and Fe2O3@GO electrodes with high specific capacitance and high energy density. RSC Adv. 2015, 5, 41721–41728. [Google Scholar] [CrossRef]
- Tian, W.; Wang, X.; Zhi, C.; Zhai, T.; Liu, D.; Zhang, C.; Golberg, D.; Bando, Y. Ni(OH)2 nanosheet@Fe2O3 nanowire hybrid composite arrays for high-performance supercapacitor electrodes. Nano Energy 2013, 2, 754–763. [Google Scholar] [CrossRef]
- Xing, W.; Qiao, S.; Wu, X.; Gao, X.; Zhou, J.; Zhuo, S.; Hartono, S.B.; Hulicova-Jurcakova, D. Exaggerated capacitance using electrochemically active nickel foam as current collector in electrochemical measurement. J. Power Sources 2011, 196, 4123–4127. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Murali, S.; Stoller, M.D.; Ruoff, R.S. Nanostructured Reduced Graphene Oxide/Fe2O3 Composite As a High-Performance Anode Material for Lithium Ion Batteries. ACS Nano 2011, 5, 3333–3338. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yan, J.; Zhi, L.; Zhang, Q.; Wei, T.; Feng, J.; Zhang, M.; Qian, W.; Wei, F. A three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors. Adv. Mater. 2010, 22, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Y.; Wang, Q.; Wang, T. Nanostructured Fe2O3-graphene composite as a novel electrode material for supercapacitors. J. Solid State Electrochem. 2011, 16, 2095–2102. [Google Scholar] [CrossRef]
- Shivakumara, S.; Penki, T.R.; Munichandraiah, N. Preparation and electrochemical performance of porous hematite (α-Fe2O3) nanostructures as supercapacitor electrode material. J. Solid State Electrochem. 2013, 18, 1057–1066. [Google Scholar] [CrossRef]
- Zhao, P.; Li, W.; Wang, G.; Yu, B.; Li, X.; Bai, J.; Ren, Z. Facile hydrothermal fabrication of nitrogen-doped graphene/Fe2O3 composites as high performance electrode materials for supercapacitor. J. Alloys Compd. 2014, 604, 87–93. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Zan, G.; Wu, Q. A high-performance dual-function material: Self-assembled super long α-Fe2O3 hollow tubes with multiple heteroatom (C-, N- and S-) doping. Dalton Trans. 2016, 45, 12790–12799. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lang, J.; Zhang, P.; Hu, B.; Yan, X. Facile Synthesis of Fe2O3 Nano-Dots@Nitrogen-Doped Graphene for Supercapacitor Electrode with Ultralong Cycle Life in KOH Electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sha, J.; Miao, X.; Liu, E.; Shi, C.; Li, J.; He, C.; Li, Q.; Zhao, N. Three-dimensional graphene anchored Fe2O3@C core-shell nanoparticles as supercapacitor electrodes. J. Alloys Compd. 2017, 696, 956–963. [Google Scholar] [CrossRef]
- Nathan, D.M.G.T.; Boby, S.J.M. Hydrothermal preparation of hematite nanotubes/reduced graphene oxide nanocomposites as electrode material for high performance supercapacitors. J. Alloys Compd. 2017, 700, 67–74. [Google Scholar] [CrossRef]
- Wang, D.; Dong, H.; Zhang, H.; Zhang, Y.; Xu, Y.; Zhao, C.; Sun, Y.; Zhou, N. Enabling a High Performance of Mesoporous α-Fe2O3 Anodes by Building a Conformal Coating of Cyclized-PAN Network. ACS Appl. Mater. Interfaces 2016, 8, 19524–19532. [Google Scholar] [CrossRef] [PubMed]

| Fe2O3-Based Electrode | Synthesis Method | Electrolyte | Potential Range (V) | Specific Capacitance (Fg−1)/(Ag−1) | Reference (year) |
|---|---|---|---|---|---|
| Fe2O3-graphene | solution-based hydrothermal | 2 M KOH | −0.85–0 | 151.8/1.0 94/16 | [46] (2012) |
| Porous α-Fe2O3 | Sol-gel route | 0.5 M Na2SO3 | −0.8–0 | 193/1.0 90/5.0 | [47] (2013) |
| g-C3N4/α-Fe2O3 | ionic liquid assisted solvothermal | 2.5 M Li2SO4 | −1–0 | 260/0.5 87/5.0 | [20] (2014) |
| N-doped grphene/Fe2O3 | hydrothermal | 1 M Na2SO4 | −0.85–(−0.1) | 260.1/2.0 145.1/5.0 | [48] (2014) |
| N-rGO/Fe2O3 | hydrothermal | 1 M KOH | −1~0 | 268.4/2.0 137/5.0 | [17] (2015) |
| α-Fe2O3 | Template method | 6 M KOH | −1.2–(−0.5) | 330/0.5 | [49] (2016) |
| Fe2O3 NDs@N-graphene | one-pot solvothermal | 2 M KOH | −1.0–0 | 274/1.0 201/5.0 | [50] (2016) |
| Fe2O3@MnO2 | two-step method | 3 M KOH | −0.4–0.4 | 289.9/1.0 118.3/5.0 | [40] (2017) |
| Fe2O3@C-rGO | hydrothermal | 1 M Na2SO4 | −0.5–0.5 | 211.4/0.5 177.2/20 | [51] (2017) |
| α-Fe2O3 NTs/rGO | hydrothermal | 1 M Na2SO4 | −0.8–0 | 262/1.0 196.3/5.0 | [52] (2017) |
| Fe2O3@C | hydrothermal self-assembly | 6 M KOH | −1.0–(−0.3) | 304.9/2.0 162.8/20 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Tang, H.; Wu, F.; Wang, R.; Pan, M. One-Step Self-Assembly Synthesis α-Fe2O3 with Carbon-Coated Nanoparticles for Stabilized and Enhanced Supercapacitors Electrode. Energies 2017, 10, 1296. https://doi.org/10.3390/en10091296
Yan Y, Tang H, Wu F, Wang R, Pan M. One-Step Self-Assembly Synthesis α-Fe2O3 with Carbon-Coated Nanoparticles for Stabilized and Enhanced Supercapacitors Electrode. Energies. 2017; 10(9):1296. https://doi.org/10.3390/en10091296
Chicago/Turabian StyleYan, Yizhi, Haolin Tang, Fan Wu, Rui Wang, and Mu Pan. 2017. "One-Step Self-Assembly Synthesis α-Fe2O3 with Carbon-Coated Nanoparticles for Stabilized and Enhanced Supercapacitors Electrode" Energies 10, no. 9: 1296. https://doi.org/10.3390/en10091296





