Studies on the Effect of Nano-Sized MgO in Magnesium-Ion Conducting Gel Polymer Electrolyte for Rechargeable Magnesium Batteries
Abstract
:1. Introduction
2. Results and Discussion
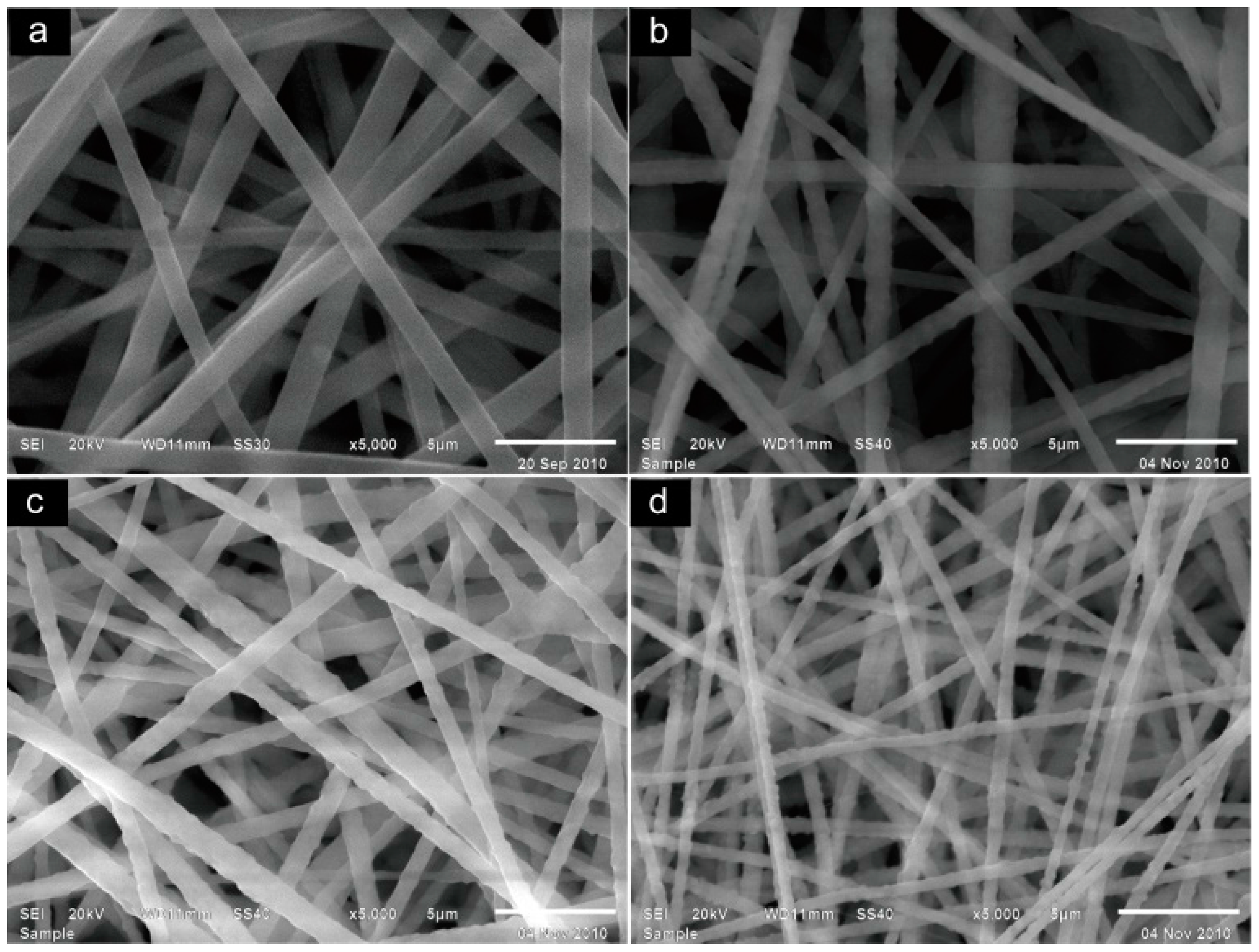
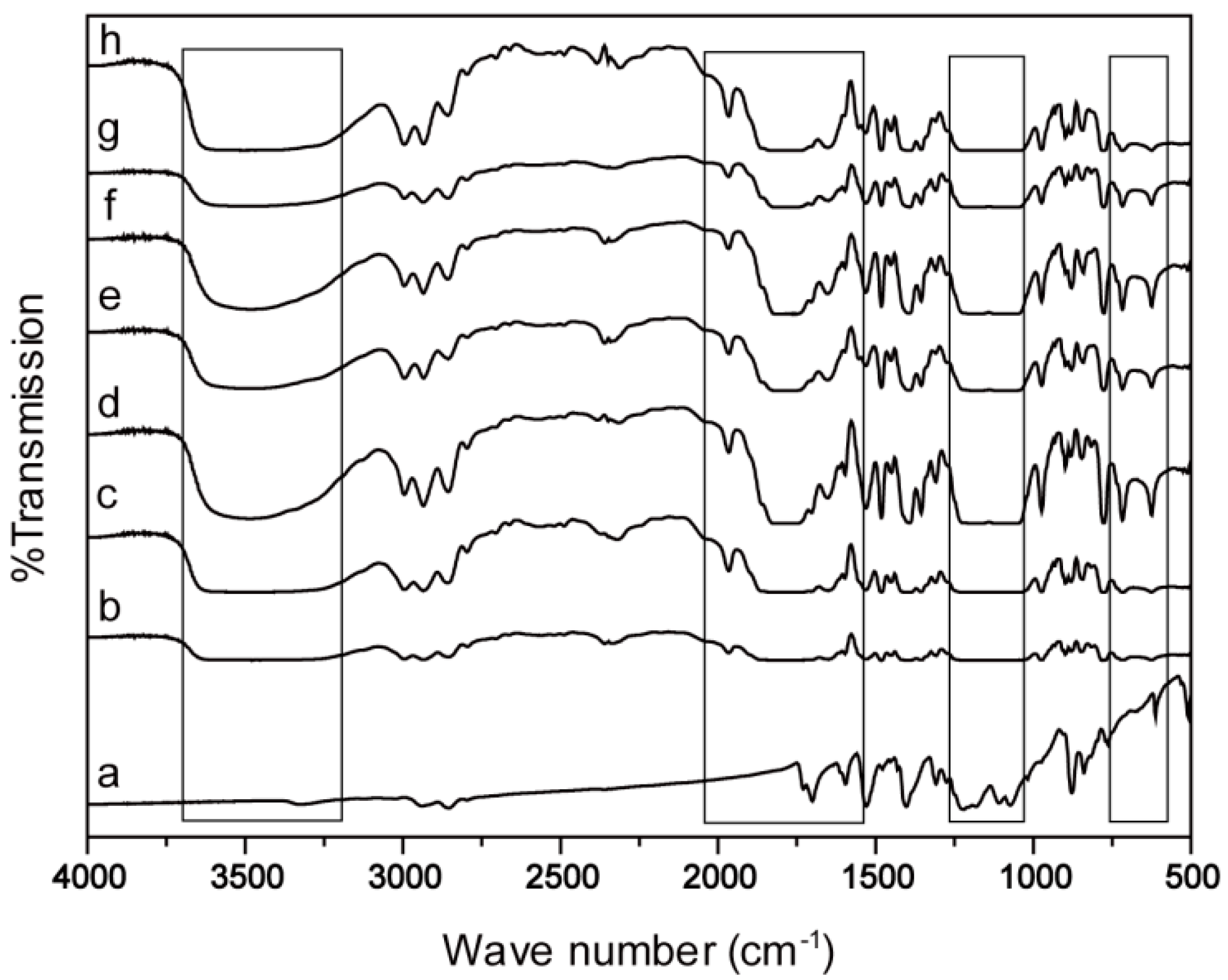
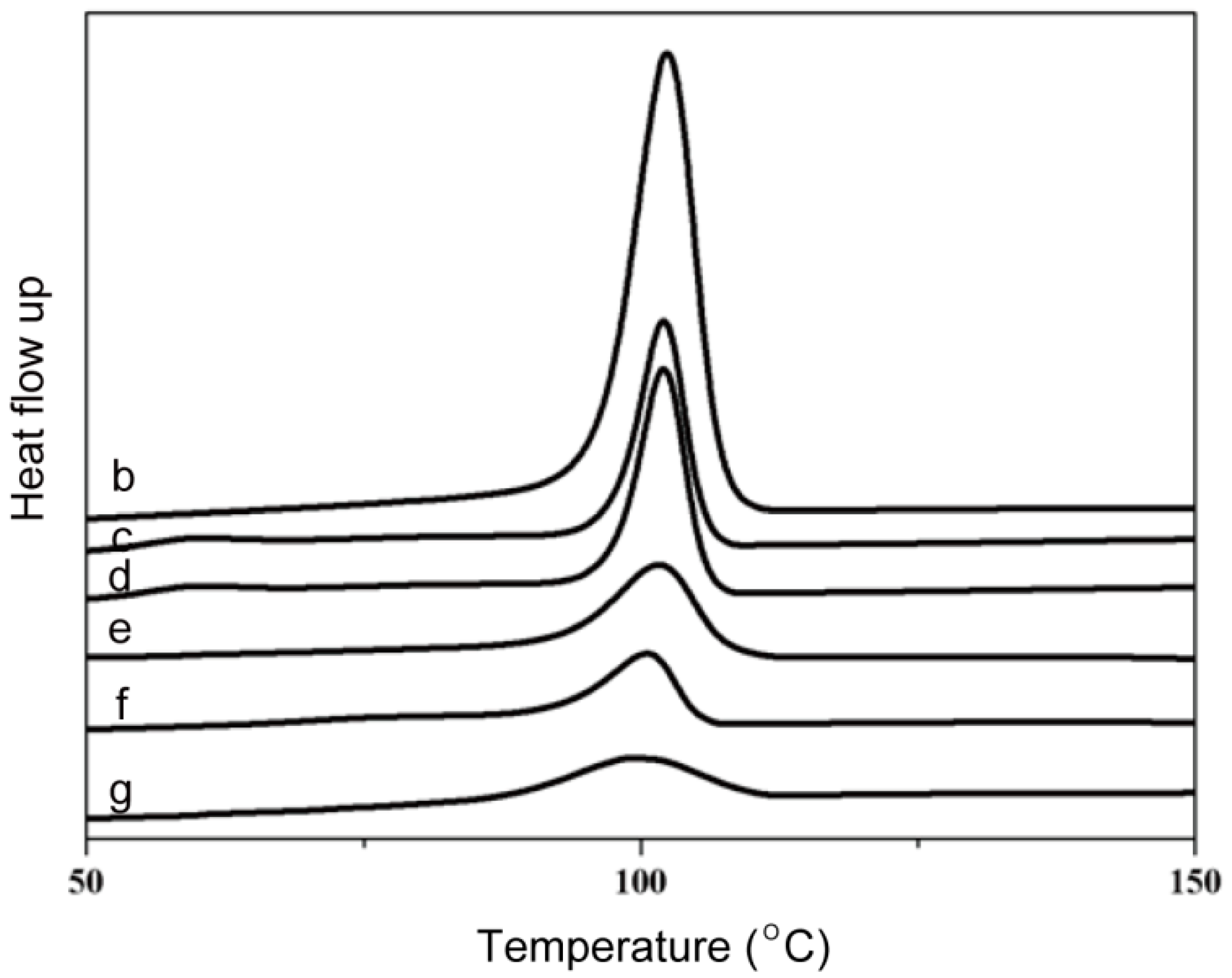
2.1. Morphology/Structure and Differential Scanning Calorimeter Analysis
2.2. Mechanical Properties and Porosity/Electrolyte Uptake
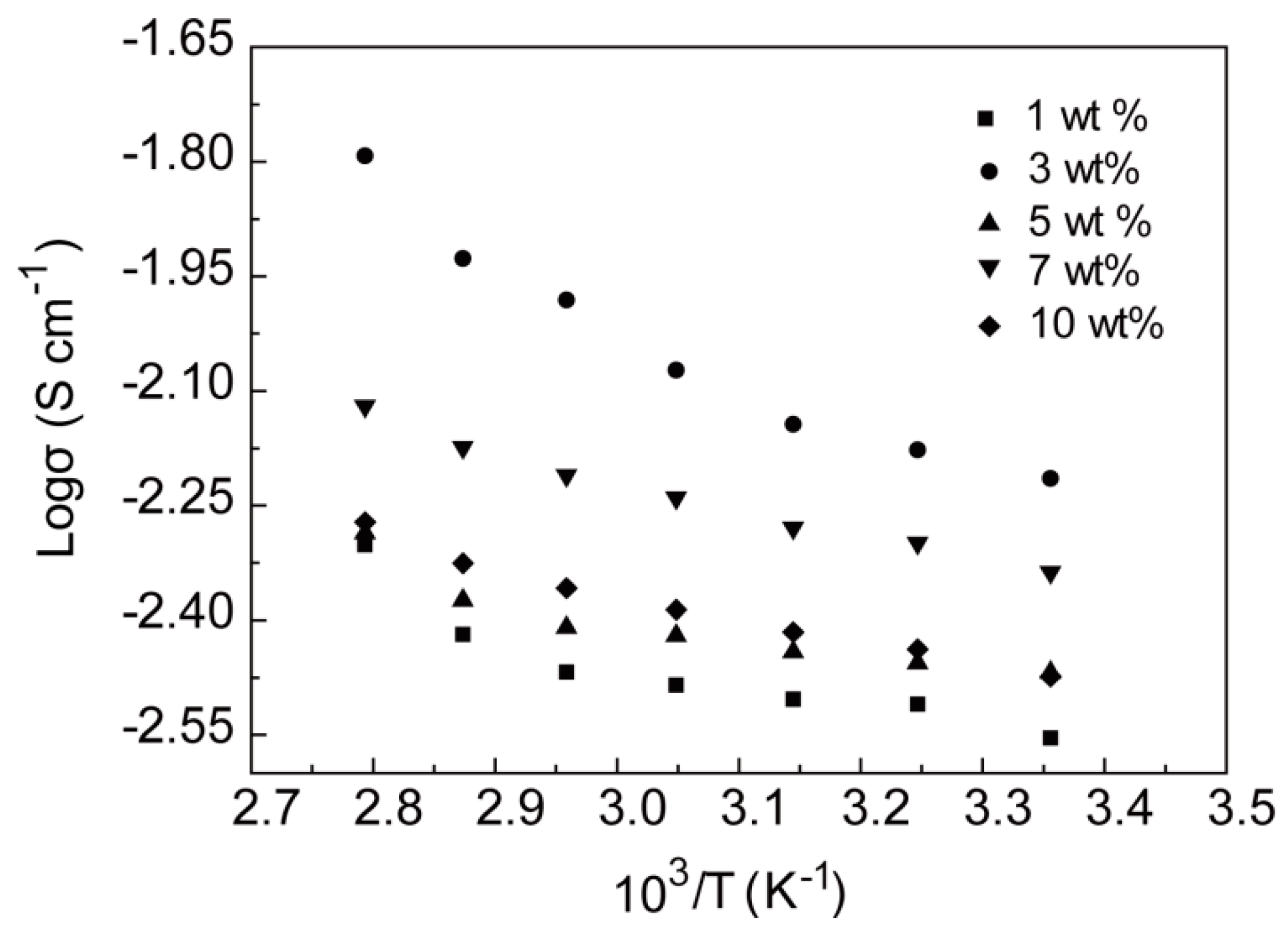
2.3. Ionic Conductivity
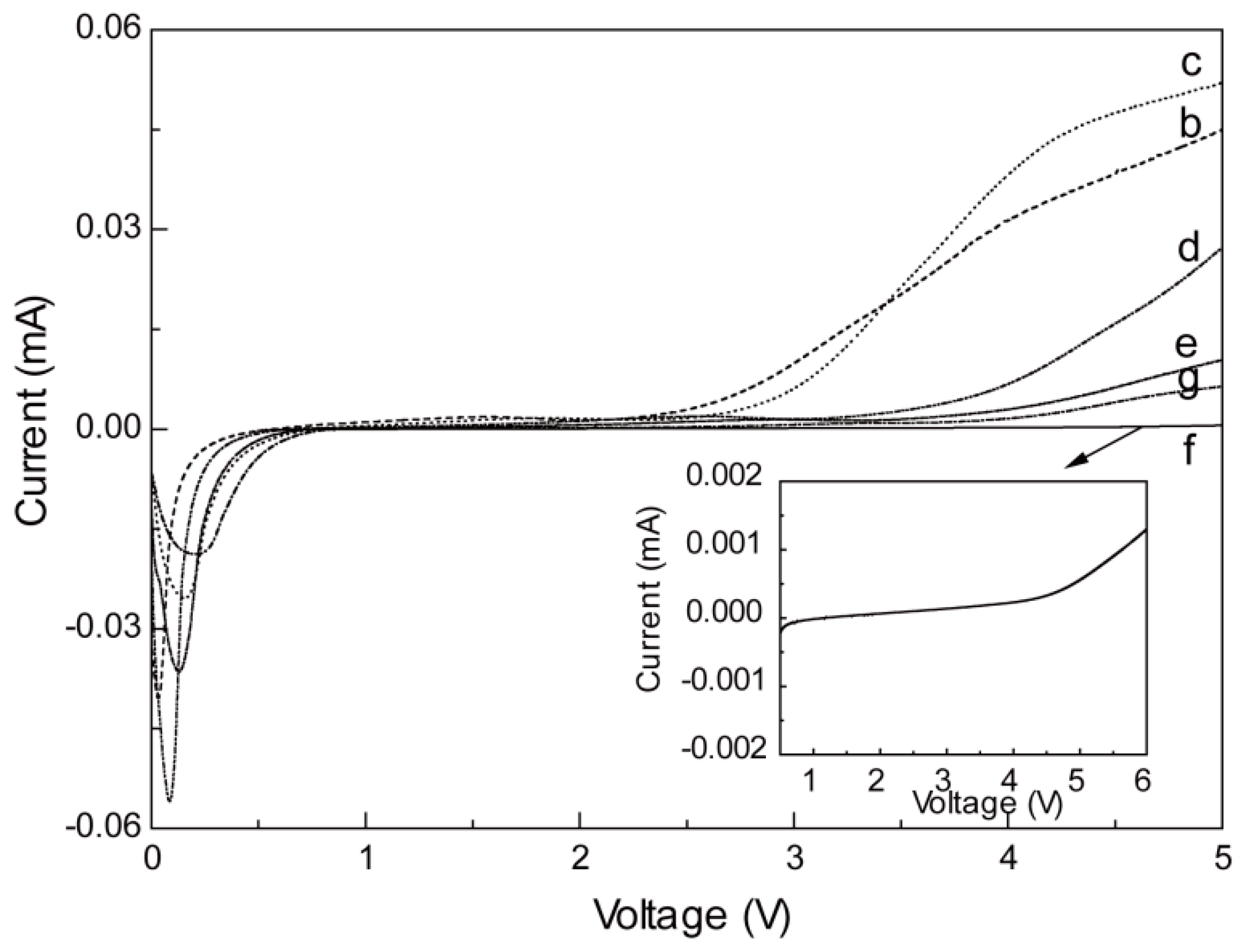
2.4. Electrochemical Activity and Stability
3. Materials and Methods
Membrane Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Pasta, M.; Wessells, C.D.; Huggins, R.A.; Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 2012, 3, 1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, H.S. A reversible long-life lithium-air battery in ambient air. Nat. Commun. 2013, 4, 1817. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Lyu, Y.-C.; Xiao, R.-J.; Yu, X.-Q.; Yin, Y.-X.; Yang, X.-Q.; Li, H.; Gu, L.; Guo, Y.-G. A highly reversible, low strain Mg-ion insertion anode material for rechargeable Mg-ion batteries. NPG Asia Mater. 2014, 6, e120. [Google Scholar] [CrossRef]
- Wu, N.; Yin, Y.-X.; Guo, Y.-G. Improving the electrochemical properties of the red P anode in Na-ion batteries via the spaceconfinement of carbon nanopores. J. Mater. Chem. A 2015, 3, 24221–24225. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, R.J.; Yang, S.Q.; Ma, H.; Liang, J.; Chen, J. Rechargeable Mg Batteries with Graphene-like MoS2 Cathode and Ultrasmall Mg Nanoparticle Anode. Adv. Mater. 2011, 23, 640–643. [Google Scholar] [CrossRef] [PubMed]
- NuLi, Y.; Yang, J.; Wang, J.; Li, Y. Electrochemical Intercalation of Mg2+ in Magnesium Manganese Silicate and Its Application as High-Energy Rechargeable Magnesium Battery Cathode. J. Phys. Chem. C. 2009, 113, 12594–12597. [Google Scholar] [CrossRef]
- Levi, E.; Gofer, Y.; Aurbach, D. On the Way to Rechargeable Mg Batteries: The Challenge of New Cathode Materials. Chem. Mater. 2010, 22, 860–868. [Google Scholar] [CrossRef]
- Chen, X.Z.; Li, H. Thermodynamic analysis on energy densities of batteries. Energy Environ. Sci. 2011, 4, 2614–2624. [Google Scholar]
- Tao, Z.L.; Xu, L.N.; Gou, X.L.; Chen, J.; Yuan, H.T. TiS2 nanotubes as the cathode materials of Mg-ion batteries. Chem. Commun. 2004, 18, 2080–2081. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Yang, Z.Z.; Yao, H.R.; Yin, Y.X.; Gu, L.; Guo, Y.G. Improving the electrochemical performance of Li4Ti5O12 electrode in a rechargeable Mg battery by lithium-magnesium co-intercalation. Angew. Chem. Int. Ed. 2015, 54, 5757–5761. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.; You, J.; Park, M.-S.; Hossain, M.S.A.; Yamauchi, Y.; Kim, J.H. Conductive polymers for next-generation energy storage systems: Recent progress and new functions. Mater. Horiz. 2016, 3, 517–535. [Google Scholar] [CrossRef]
- Shin, W.-K.; Cho, J.; Kannan, A.; Lee, Y.-S.; Kim, D.-W. Cross-Linked Composite Gel Polymer Electrolyte using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries. Sci. Rep. 2016, 6, 26332. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Pandey, G.P.; Agrawal, R.C.; Hashmi, S.A. Performance studies on composite gel polymer electrolytes for rechargeable magnesium battery application. J..Phys. Chem. Solids 2011, 72, 1408–1413. [Google Scholar] [CrossRef]
- Pandey, G.P.; Agrawal, R.C.; Hashmi, S.A. Magnesium ion-conducting gel polymer electrolytes dispersed with nanosized magnesium oxide. J. Power Sources 2009, 190, 563–572. [Google Scholar] [CrossRef]
- Kumar, G.G.; Munichandraiah, N. Solid-state rechargeable magnesium cell with poly (vinylidenefluoride)-magnesium triflate gel polymer electrolyte. J. Power Sources 2001, 102, 46–54. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Yakushiji, S.; Ishikawa, M. Rechargeable magnesium batteries with polymeric gel electrolytes containing magnesium salts. Electrochim. Acta 2003, 48, 2317–2322. [Google Scholar] [CrossRef]
- Oh, J.S.; Ko, J.M.; Kim, D.W. Preparation and characterization of gel polymer electrolytes for solid state magnesium batteries. Electrochim. Acta 2004, 50, 903–906. [Google Scholar] [CrossRef]
- Wu, X.-L.; Li, Y.-H.; Wu, N.; Xin, S.; Kim, J.-H.; Yan, Y.; Lee, J.-S.; Guo, Y.-G. Enhanced working temperature of PEO-based polymer electrolyte via porous PTFE film as an efficient heat resister. Solid State Ion. 2013, 245–246, 1–7. [Google Scholar] [CrossRef]
- Wu, N.; Cao, Q.; Wang, X.Y.; Li, X.Y.; Deng, H.Y. A novel high-performance gel polymer electrolyte membrane basing on electrospinning technique for lithium rechargeable batteries. J. Power Sources 2011, 196, 8638–8643. [Google Scholar] [CrossRef]
- Jeong, G.; Kim, J.-G.; Park, M.-S. Core–shell structured silicon nanoparticles@TiO2-x/carbon mesoporous microfiber composite as a safe and high-performance lithium-ion battery anode. ACS Nano 2014, 8, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Lim, Y.-G.; Kim, J.-G.; Heo, Y.-U.; Lim, J.H.; Yamauchi, Y.; Park, M.-S.; Kim, Y.-J.; Dou, S.X.; Kim, J.H. A case study on fibrous porous SnO2 anode for robust, high-capacity lithium-ion batteries. Nano Energy 2014, 10, 53–62. [Google Scholar] [CrossRef]
- Hwang, S.M.; Kim, S.Y.; Kim, J.-G.; Kim, K.J.; Lee, J.-W.; Park, M.-S.; Kim, Y.-J.; Shahabuddin, M.; Yamauchi, Y.; Kim, J.H. Electrospun manganese–cobalt oxide hollow nanofibres synthesized via combustion reactions and their lithium storage performance. Nanoscale 2015, 7, 8351–8355. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Cao, Q.; Wang, X.Y.; Li, S.; Li, X.Y.; Deng, H.Y. In situ ceramic fillers of electrospun thermoplastic polyurethane/poly(vinylidene fluoride) based gel polymer electrolytes for Li-ion batteries. J. Power Sources 2011, 196, 9751–9756. [Google Scholar] [CrossRef]
- Kim, J.R.; Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, B.C. Electrospun PVdF-based fibrous polymer electrolytes for lithium ion polymer batteries. Electrochim. Acta 2004, 50, 69–75. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.; Wang, W.; Wei, Y.; Li, T. Studies on the Effect of Nano-Sized MgO in Magnesium-Ion Conducting Gel Polymer Electrolyte for Rechargeable Magnesium Batteries. Energies 2017, 10, 1215. https://doi.org/10.3390/en10081215
Wu N, Wang W, Wei Y, Li T. Studies on the Effect of Nano-Sized MgO in Magnesium-Ion Conducting Gel Polymer Electrolyte for Rechargeable Magnesium Batteries. Energies. 2017; 10(8):1215. https://doi.org/10.3390/en10081215
Chicago/Turabian StyleWu, Na, Wei Wang, Yu Wei, and Taohai Li. 2017. "Studies on the Effect of Nano-Sized MgO in Magnesium-Ion Conducting Gel Polymer Electrolyte for Rechargeable Magnesium Batteries" Energies 10, no. 8: 1215. https://doi.org/10.3390/en10081215




