1. Introduction
According to the Intergovernmental Panel on Climate Change, the unprecedented rate of global warming in the past few centuries is due to anthropogenic greenhouse gas emissions, and these emissions need to be curtailed to prevent a further increase in temperature [
1]. While developments in solar and wind power generation are occurring, there is still a need for baseload power on the electric grid, which cannot be attained using only renewable technologies. Thus, there will be a need for baseload plants, which in the United States are mainly based on fossil fuel technology. A proposed solution for utilizing baseload fossil fuel plants under a carbon-constrained scenario is carbon capture and sequestration. Examples of technologies are categorized into post-combustion, pre-combustion, and oxy-combustion capture [
2,
3].
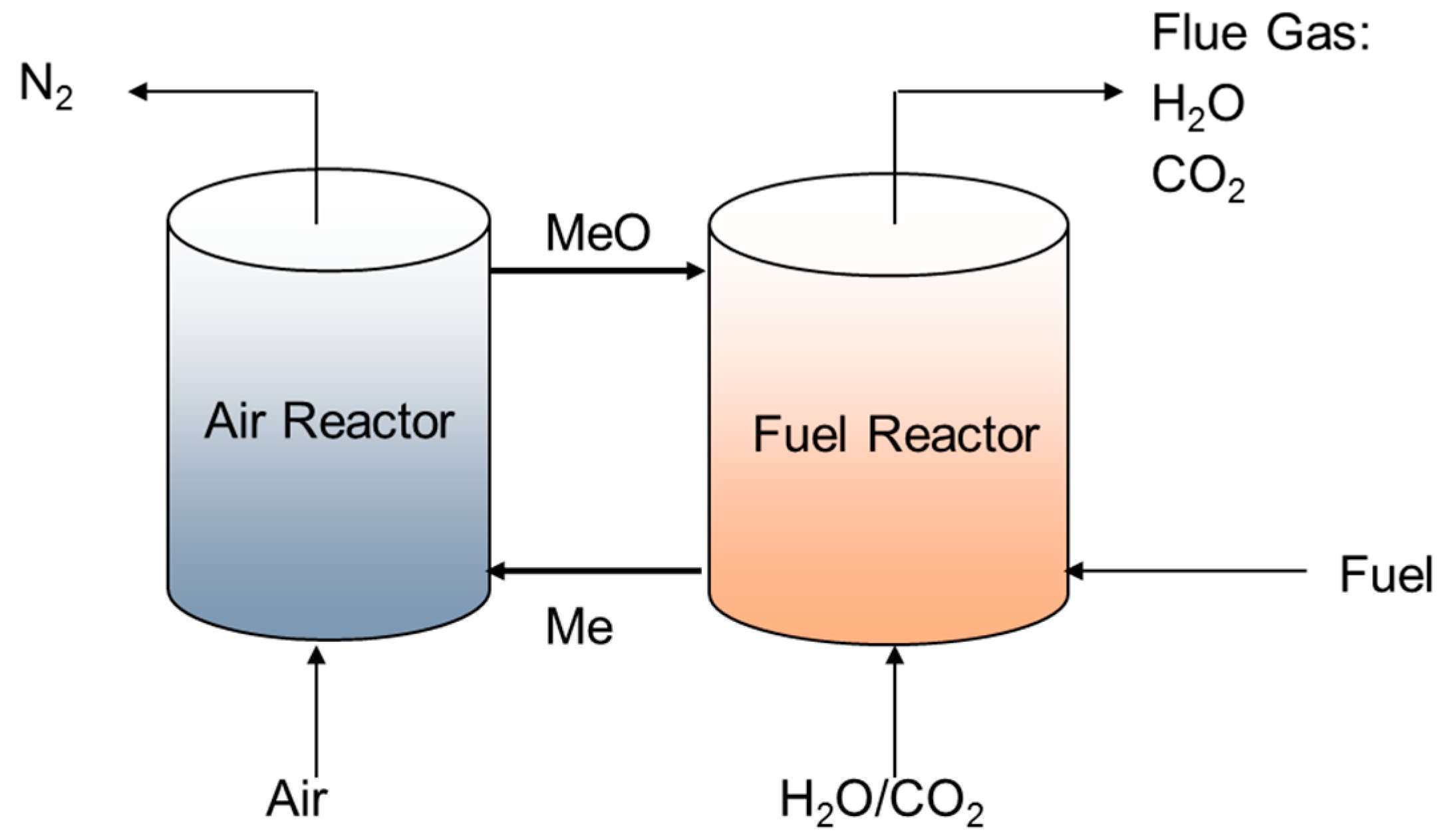
One example of an oxy-combustion technology being pursued is chemical looping combustion, as shown in
Figure 1. Chemical looping combustion splits the conventional combustion reaction into two steps, thereby resulting in a separate stream of carbon dioxide flue gas that is free of nitrogen. Instead of directly burning the fuel in the presence of air, oxidation of the fossil fuel occurs using a metal oxide oxygen carrier in what is known as a fuel reactor. If the reaction is complete, the resulting flue gas is composed entirely of carbon dioxide and steam. The resulting reduced metal oxide is sent to a second reactor, called the air reactor, which oxidizes the reduced metal oxide using air. The oxidation of the reduced metal oxide is exothermic, and the energy from this reaction can be recuperated to produce electricity as in a traditional steam cycle.
Originally, chemical looping combustion was designed as a more efficient combustion power plant, proposed by Ishida et al. [
4]. The original design incorporated reduction and oxidation of metal oxides at high pressure so that gas turbines could be used, in the form of a combined cycle type power plant. However, in recent years, research has focused more on atmospheric pressure systems because of the operational challenges of solids circulation at high pressure, as CO
2 separation has a minimal energy penalty even at atmospheric pressure. In addition to using the chemical looping scheme for combustion, the scheme has been proposed as a method to produce syngas [
5], hydrogen [
6], maleic anhydride [
7], methanol [
8], and sulfuric acid [
9].
The design of the fuel reactor is critical for chemical looping combustion, as it must convert the fuel completely to CO
2 and steam with a reasonable residence time. The simplest configuration uses a fixed bed which is cycled between oxidation states, such as that described by Kimball et al. [
10]. The advantage of cycling fixed beds is the simplicity of its design, as there is no need for hot solids transport, minimizing the mechanical attrition of the oxygen carrier. However, the transient nature of the reaction with the oxygen carrier requires that there be multiple fixed beds cycled in a proper sequence to get a continuous stream of CO
2 of consistent quality. Another fuel reactor design incorporates a fluidized bed, either in the form of a dense phase bubbling fluidized bed or a circulating fluidized bed [
11], which allows for continuous production of CO
2, and the design mimics that of circulating fluidized bed combustion of coal. A disadvantage of bubbling fluidized beds and circulating fluidized beds are that reactant fuel tends to bypass contact with the solids through bubbles. The countercurrent moving bed fuel reactor attempts to overcome this by causing the bed to be more like a plug flow reactor [
12]. Detailed reviews of the various fuel reactor and system designs have been published elsewhere [
13].
One of the main technical challenges to commercializing chemical looping combustion is the cost of the makeup oxygen carrier, since it is expected that the carrier will undergo chemical and mechanical stress, subsequently degrading during the process [
14,
15]. Thus, it is proposed to use low-cost oxygen carriers over manufactured oxygen carriers tailored for high conversion of fuel. An example of a low-cost carrier is hematite ore, which is also high in mechanical strength. The challenge with using hematite ore is its low reactivity with fossil fuels, such as natural gas. One proposed solution is to inject steam into the natural gas feed to improve the conversion. This also has the effect of suppressing carbon deposition on the particle, preventing carbon carryover into the air reactor and subsequent loss of carbon capture.
Compared to the direct reduction of carbon monoxide or hydrogen, the reduction of iron oxide is complicated, with many potential reactions occurring on the surface of the carrier and perhaps in the gas phase. The following list of reactions is possible with hematite ore as the oxygen carrier. The reaction of iron-(III) to iron-(II,III) with methane, hydrogen, and carbon monoxide is
The reaction of iron-(II,III) to iron-(II) with natural gas, hydrogen, and carbon monoxide is
In addition to the reducing gases reacting with lattice oxygen in the hematite ore, the following gaseous phase reactions can occur, such as reforming methane with steam or CO
2:
and the water-gas shift reaction
As surface oxygen becomes depleted on the particle, methane cracking can occur, causing the formation of solid carbon on the surface and gaseous hydrogen. Additionally, if carbon monoxide is present, the Boudouard reaction can occur. Thus, the reactions for carbon deposition on the particle are:
In addition, steam helps to gasify the deposited carbon.
Iron oxide has been extensively studied with methane, isothermally in thermogravimetric analyzers [
16,
17,
18,
19,
20,
21,
22,
23,
24], in fixed bed reactors [
25,
26,
27,
28,
29], and in fluidized bed reactors [
30,
31,
32,
33,
34]. Cabello et al. [
19] studied the kinetics of methane reduction for an iron-oxide/alumina oxygen carrier in a thermogravimetric analyzer. They identified two steps in the reduction process, with the first step being the phase-boundary (reaction) controlled near the beginning of reduction, and the second step being limiting gas diffusion, through the iron–aluminum spinel shell that forms as reduction of the outer layer proceeds. Using the grain model combined with the unreactive shrinking core model for the individual grains, they found that the reduction reaction order with methane was 0.25 with an activation energy of 66 kJ/mol. Similarly, Nasr and Plucknett [
20] studied a raw hematite material (around 98% Fe
2O
3) in a thermogravimetric analyzer (TGA) and found a similar reaction mechanism. However, they used a crystal phase-change model (i.e., Avrami-Erofe’ev) to capture the reaction kinetics throughout the entire reduction due to the phase change of rhombohedral hematite changing to cubic magnetite during reduction. The activation energy determined from experiments was higher (around 215 kJ/mol) than for the tailored and highly reactive carrier studied by Cabello et al., most likely due to the lower reactivity of raw hematite. Luo et al. [
21] studied a raw hematite ore (around 82% Fe
2O
3) in addition to two tailored oxygen carriers. The reaction of methane with raw hematite was determined to be phase-boundary controlled only. Ksepko et al. [
24] studied a Sinai ore and found that the reduction reaction with methane had an effective activation energy around 35.3 kJ/mol, which is lower than reported for other ores; this was probably due to the presence of manganese oxides, which can produce an oxygen uncoupling effect. They also indicated that the reduction data fit to a Jander’s diffusion model, indicating that the slow step is the diffusion of iron ions through the outer shell. Monazam et al. [
17] also studied a raw hematite, determining that the reaction rate was both phase-boundary and nucleation rate controlled, in a parallel fashion, with the rate exponent for methane concentration being 0.636 for the controlled reaction rate and around one for the controlled nucleation. An ilmenite ore has been tested with carbon monoxide as a reducing gas at up to eight bar of partial pressure (51 bar total pressure) by Ridha et al. [
35]. They found that increasing the carbon monoxide partial pressure had no effect on the morphology and that calcination caused the formation of rutile and ferric pseudobrookite with a simultaneous decrease in ilmenite [
35].
While TGA experiments are important for carrier screening and fundamental mechanism deduction, the conditions are not realistic for determining operational feasibility, such as in a fluidized bed, where there may be mass transfer limitations between the bubble and emulsion that limit the conversion of reactant gases. Breault et al. [
34] studied the reduction of a raw hematite ore in a 5 cm fluidized bed using methane to determine the effect of cycle time on pretreated and untreated hematite ore. Their work determined that heating the carrier to a higher temperature in air than the temperature for reduction–oxidation reactions allowed for the carrier grain structure to stabilize, therefore enabling a consistent surface area and conversion results over all the redox cycles. Siriwardane et al. [
30] also studied a raw hematite ore and found similar conversions for unmodified hematite, but found that the addition of magnesium oxide increased the methane conversion by around 20 percentage points.
On the other hand, there seems to be a dearth of literature regarding the reaction kinetics of steam with oxygen carriers in fluidized beds. This paper attempts to bridge that gap by adding steam as part of the reactant gas to observe the effect on conversion during the reduction reaction. Thus, the purpose of the work is to fill the data gap in the literature on the chemical looping combustion of iron oxygen carriers with methane in the presence of steam and provide useful data for further modeling, such as CFD simulations. A 5 cm small fluidized bed reactor, used in previous studies such as Breault et al. [
34] and Siriwardane et al. [
30], was used for reducing a raw hematite oxygen carrier over multiple cycles and under different conditions. A partial trial matrix consisting of the effects of reaction temperature, methane and steam concentrations was developed to explore the design space. An ordinary least squares analysis was performed to ascertain general (qualitative) trends of the methane conversion and oxygen carrier conversion on the changing independent parameters.
2. Experimental Methods
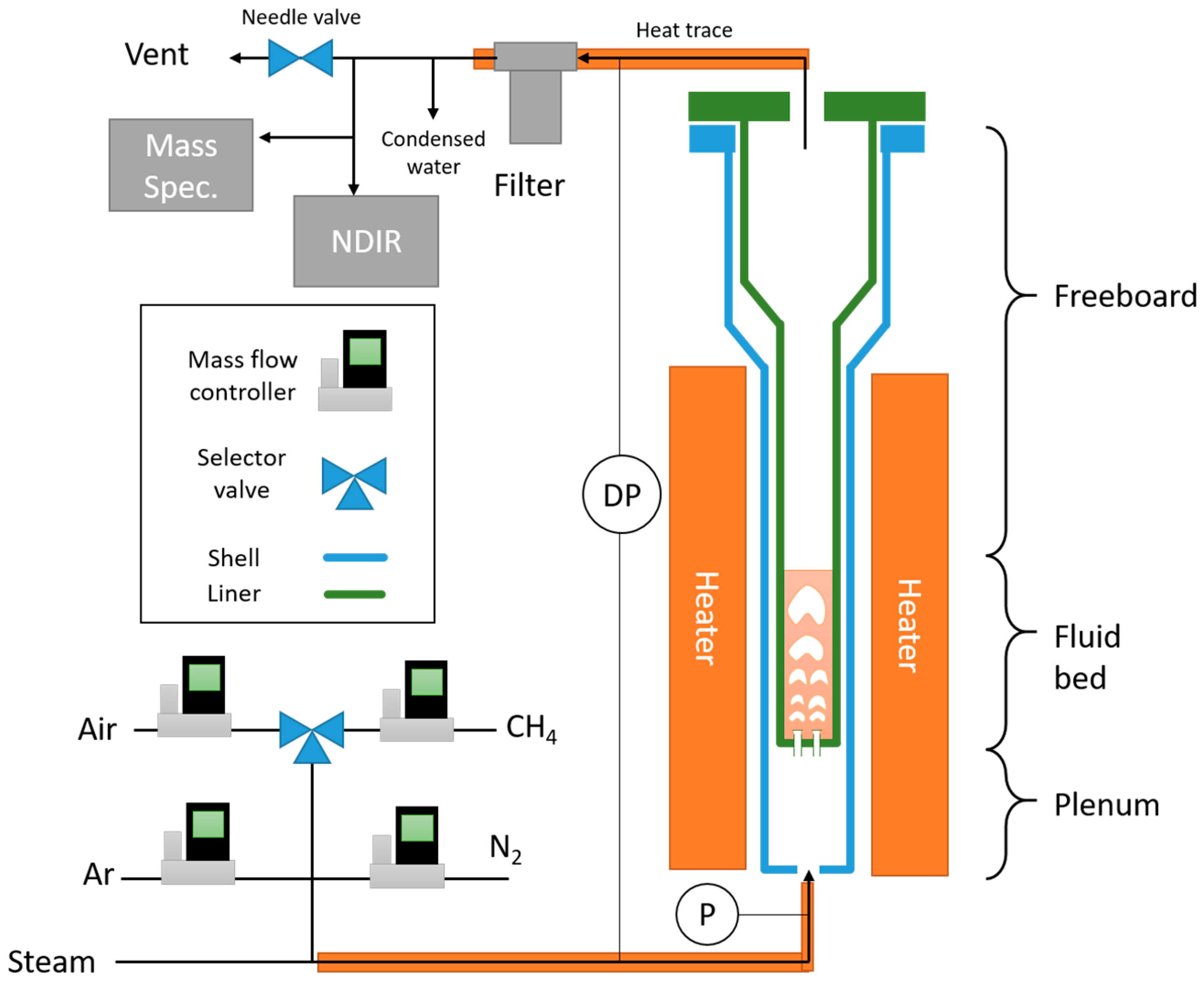
Reduction and oxidation of the hematite ore was performed in a small fluidized bed as shown in
Figure 2. The setup consists of a Hastelloy tube 5 cm in diameter, with four bubble caps at the bottom. Each bubble cap consists of a modified bolt with three holes drilled underneath the head of the bolt; the holes have a drilled diameter of approximately 1.19 mm (3/64 inches). The reactor consists of three sections: the plenum, where the gases enter through the bubblecaps, the fluidized bed, which is the raw hematite material, and the freeboard region, which expands at a region higher up to minimize the loss of oxygen carrier. The reactor consists of an outer shell which contains the plenum and an interchangeable liner which holds the bed material. The reactant gases are premixed upstream and introduced into the plenum, namely nitrogen, methane, argon, and air, which are controlled using mass flow controllers from Alicat Scientific. The maximum flows for nitrogen, methane, and argon are 20 standard liters per minute (SLPM) with accurate control down to 0.1 SLPM, and the maximum flow for air is 100 SLPM with accurate control down to 0.5 SLPM. The steam comes from a vaporizer system from Brooks Instruments. The natural gas consisted of a tank of ultra-high purity (UHP) methane (99.999%). The water, nitrogen and air were provided in house. Data collected included reactor temperature, static plenum pressure, bed differential pressure, gaseous flow rates in, and gas analysis data (mass spectrometer, non-disperse infrared). Gas composition was measured using a mass spectrometer from Thermofisher Scientific (Prima BT) and non-disperse infrared analyzers from Siemens (Ultramat 23). Gases exit from the reactor and enter a filter, where fines are collected. The outlet contains a large needle valve that allows the system to have enough back pressure for the sample to flow to the mass spectrometer.
A raw hematite ore was procured from Cliffs Natural Resources, which was taken from Wabush mine in Newfoundland, Canada. The hydrodynamic and chemical properties of the particle are shown in
Table 1. The particle size distribution, shown in
Figure 3, was measured using the image analysis sensor QICPIC from Sympatec.
Figure 3 shows that 50% of the particles were greater than 238 μm. The material was also not completely round, with an average sphericity of 0.876. The composition of the material, as shown in
Table 1 and determined using X-ray fluorescence (XRF), was mostly iron oxide, with smaller amounts of silica and manganese oxide. The minimum fluidization was determined experimentally at room temperature in a 6.35 cm diameter fluidized bed by observing the bed pressure drop curve. The particle density was measured with helium pycnometry. Additionally, before this test, the material was calcined at 950 °C in air under fluidization conditions (around 3 U
mf) for 1 h.
The purpose of the testing was to determine the effects of steam on the kinetics of natural gas conversion with hematite ore, and the test was focused on exploring the design space. The three main variables tested in the campaign were the temperature, the methane concentration, and the steam concentration. For this test, the gas velocity (and corresponding total gas inlet flow rate) were set to be constant, namely at three times the minimum fluidization velocity of 9.87 cm/s at temperature. The solids inventory was also set to be constant at 1000 g for this testing. The resulting experimental test matrix is laid out in
Table 2. Since the campaign was focused on exploring the design space, the matrix was a partial test matrix, with certain parameters selected to discover general trends over the design space using limited resources. Another reason for the incomplete test matrix was to avoid the test points where carbon deposition was expected to occur, namely at high temperature (for forward Reaction 10) and low temperature (for forward Reaction 11). To prevent making conclusions based on variables that change as time progresses through the test, the test matrix was randomized. However, for trials that included replication, the replicates were artificially selected to be at the beginning, the middle, and near the end of the test campaign to ascertain the effects of time on the variables.
The test campaign consisted of cycling reducing and oxidizing gases on a constant inventory of material over a period of cycles, with different reduction feed gas conditions for each cycle to ascertain parametric effects. The reaction cycle consisted of a reduction cycle, with premixed methane, nitrogen, argon, and sometimes steam. The reduction cycle was then followed by a nitrogen purge of 15 L of reactor volume. After the nitrogen purge, oxidation occurred, whereby a mixture of nitrogen and air was added such that the oxygen concentration was around 5 vol %; this value was chosen to ensure that the temperature of the material did not go above the calcining temperature of 950 °C. Previous work showed that the calcining temperature affects the kinetics of reduction [
34]. Finally, a second nitrogen purge of 15 L was implemented after the oxidation cycle to clear out the remaining oxygen. The length of the reduction cycle was set to be 300 s (occasionally longer due to operator error). The oxidation time was 15 min after oxygen breakthrough occurred, namely when the oxygen reads a steady 5 vol % for 15 min. Due to the design of the unit, the pressure was operated slightly above one atmosphere, between 2.6 and 5.4 psig measured in the plenum. Additionally, the flow to the mass spectrometer was measured using the rapid multi-sampler. If the flow was below 50 cm
3/min, the data were thrown out, since there were significant delays in the concentration measurements.
The performance of the carrier was ascertained by analyzing parameters such as the species molar flow rates out (), methane conversion (, ), and the integrated molar oxygen carrier conversion ().
The known flow of argon into the system was used for calculating the molar flow rates of each gaseous species. The total flow of product gas on a dry basis was calculated by dividing the known flow rate of argon into the system by the argon tracer measurement from the mass spectrometer:
The corresponding product species
i were then calculated using the product of the total flow and the measurement from the mass spectrometer:
Three conversion parameters were deduced from the gas analysis for each experiment. The natural gas conversion was calculated in two ways. The first natural gas conversion value was based on the disappearance of natural gas, namely:
This value gives a general conversion value, where the methane can be converted to one of the gaseous species or to solid carbon. The second natural gas conversion value was based on the conversion of natural gas to carbon dioxide, or:
The second conversion value helps to determine the effectiveness of the hematite for carbon capture, since the production and isolation of pure CO
2 is the main goal of chemical looping combustion. The amount of oxygen consumed can be deduced via measurement of the product gases that contain oxygen, namely carbon dioxide, carbon monoxide, and steam. However, since the water drops out before it reaches the mass spectrometer and the continuous IR analyzers, the oxygen consumption needed to be deduced from an assumed hydrogen balance. Starting with the mole balance of oxygen, the amount of oxygen stripped from the hematite is:
Since the steam out is not directly measured, an element balance on hydrogen will be used to ascertain this value indirectly. The hydrogen entering the system from both the steam and the natural gas should be equal to the hydrogen, the natural gas, and the unknown steam leaving the system:
The oxygen consumption rate is then:
If the total oxygen in the bed of oxygen carrier is:
Then the rate of oxygen carrier conversion is:
This can be integrated from the start time to a selected end time for each trial to ascertain the oxygen carrier conversion:
The solids residence time in a 50 kW
th unit using the same raw hematite material was around 200 s [
36]. Thus, 200 s was chosen to be a representative value for integration. Additionally, the mass of the bed
was 1000 g and the iron oxide content by weight (
) was 86.6% from
Table 2.
To check that the results were valid, and to monitor for coking on the hematite, the gaseous carbon balance was calculated by:
If the measurement system is perfectly accurate, the carbon balance
should be unity. Tests with steam showed a carbon element closure of 97.78 ± 4.01%, unless carbon deposition was occurring on the carrier, which was predominant when steam was not added to the system. Tests without steam showed a carbon balance closure of 72.90 ± 6.53%. The quantity of carbon deposited on the oxygen carrier was determined by integrating the flowrate of CO
2 exiting the reactor during the oxidation cycle:
where
is the time of oxidation. The carbon dioxide flow rate was determined from Equations (13) and (14), but the flow rate of argon into the reactor for Equation (13) was assumed to be 0.9 vol % of the air flow rate in, according to the natural composition of argon in atmospheric air.
3. Results
A summary of the overall results is tabulated in
Table 3. The table shows the target bed temperature, feed natural gas mole percent and steam mole percent, but the actual values deviate slightly from the target, and these values are shown next to the target columns. During the experiment, the temperatures were around 10–20 °C higher due to the offset of the heater controller. For simplicity, most of the plots presented later in this paper use the actual values unless otherwise noted.
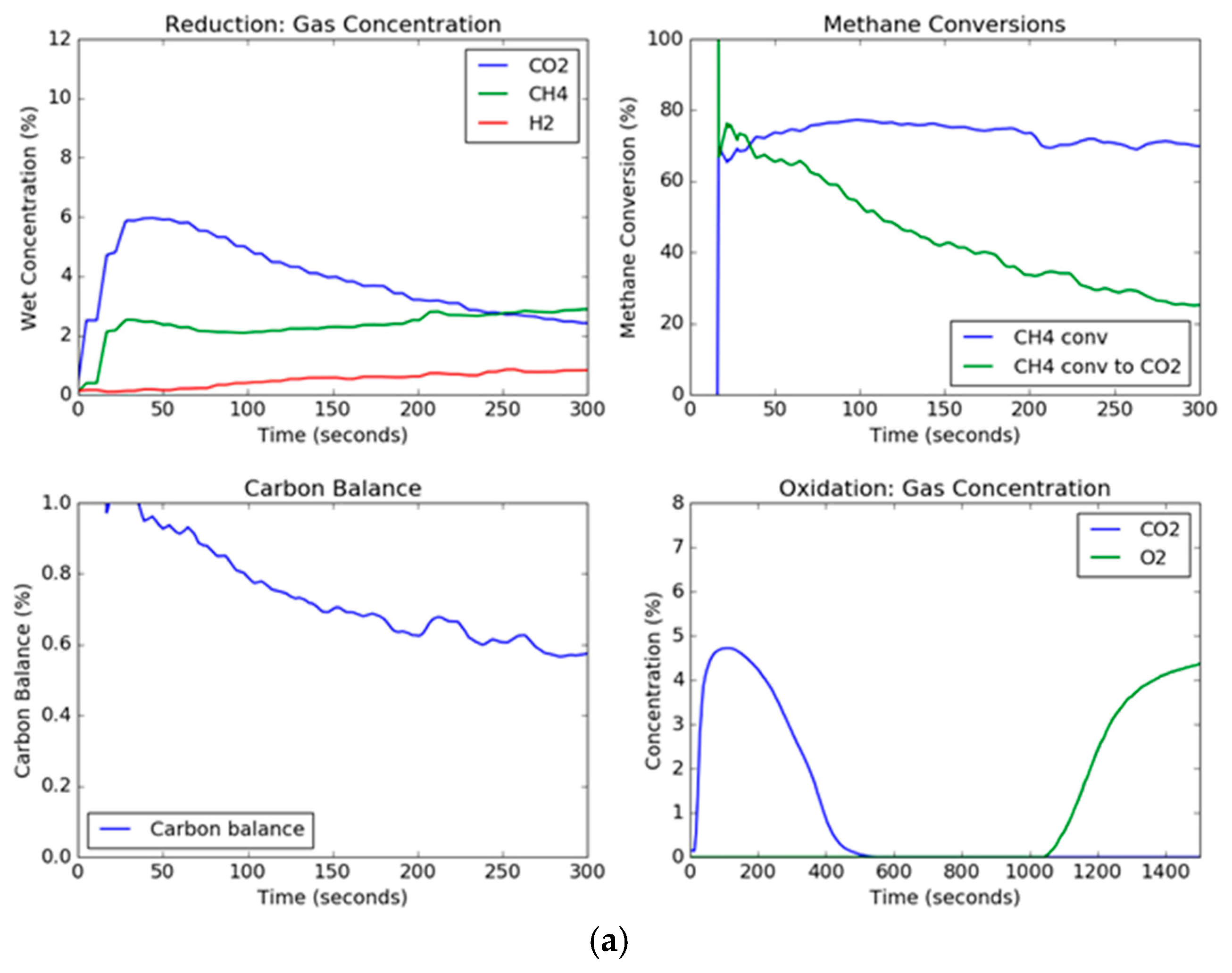
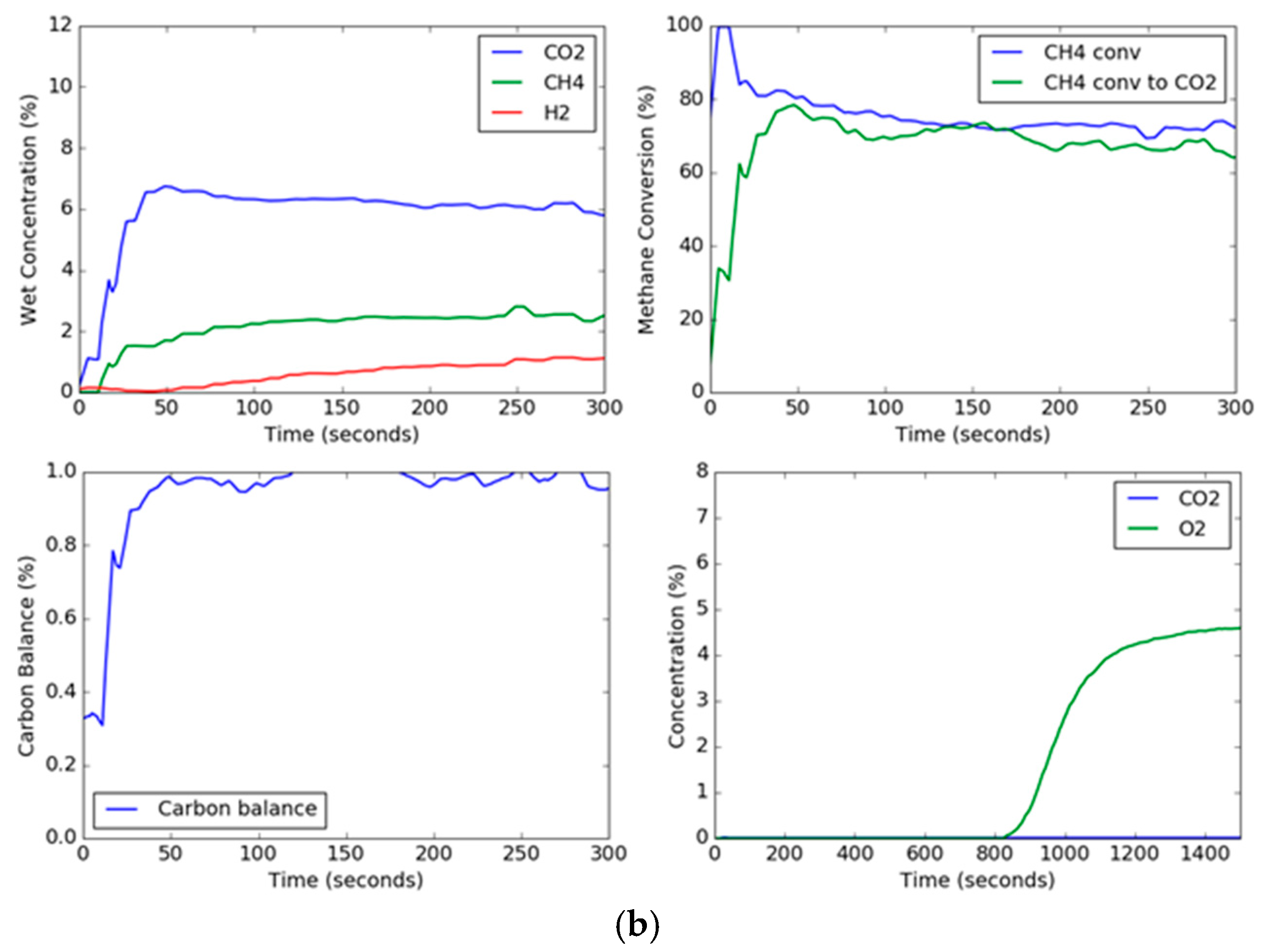
Since solids are not being continuously added and taken away as in a circulating system, the transient concentration and conversion curves are important for observing the changes in the oxygen carrier as a function of time. The transient curves for the extreme conditions of steam, methane, and temperature are shown in
Figure 4. The effect of the presence of steam in the feed gas mixed with methane can be noted. For these two trials, the set point temperature was 900 °C and the set point methane concentration in the feed gas was 10 vol %. However, the steam set point was 0 vol % and 30 vol % for
Figure 4a,b, respectively. Thus, the conditions were the same except for the presence of steam in the feed gas. The CO
2 concentration in the trial without steam started out high and began to drop as the carbon began to deposit onto the hematite. On the other hand, the CO
2 concentration for the steam trial held relatively constant, although the CO
2 dropped slowly due to the formation of lower oxidation states of iron oxide (Fe
3O
4, FeO), which have slower kinetics with methane and reaction products (CO, H
2) [
37,
38]. Additionally, the overall methane conversion was similar for both trials, ending up around 70%, but for the trial without steam, the methane conversion to carbon dioxide deviated significantly after a period of around 30 s. The effect of steam can also be seen in the carbon balance, which hovered around one hundred percent for the steam trial, however for the steam-free trial, while it started out with a gaseous carbon balance of one hundred percent (slightly above due to instrument error), the carbon balance decreased drastically as the carbon deposits on the hematite dropped below 60% near the end of the reduction period. This indicated that the remaining percentage of methane (up to around 40%) being fed to the fluidized bed was depositing on the oxygen carrier. This transient effect of the deposition of carbon is a result of the gradual formation of wüstite and elemental iron as the reaction progresses, where the deeply reduced iron helps to catalyze the formation of carbon depositions on the particle, and oxygen transported out of the grain lattice has a much slower reaction [
28,
29].
From the transient curves, the deposited carbon can be measured during the oxidation cycle. When air is added to the fluidized bed, the carbon deposited on the particle reacts with the air to produce CO
2. As can be seen, the trial without steam produces a high concentration peak of carbon dioxide, while the reduction trial with steam produces no measurable carbon dioxide, as shown in
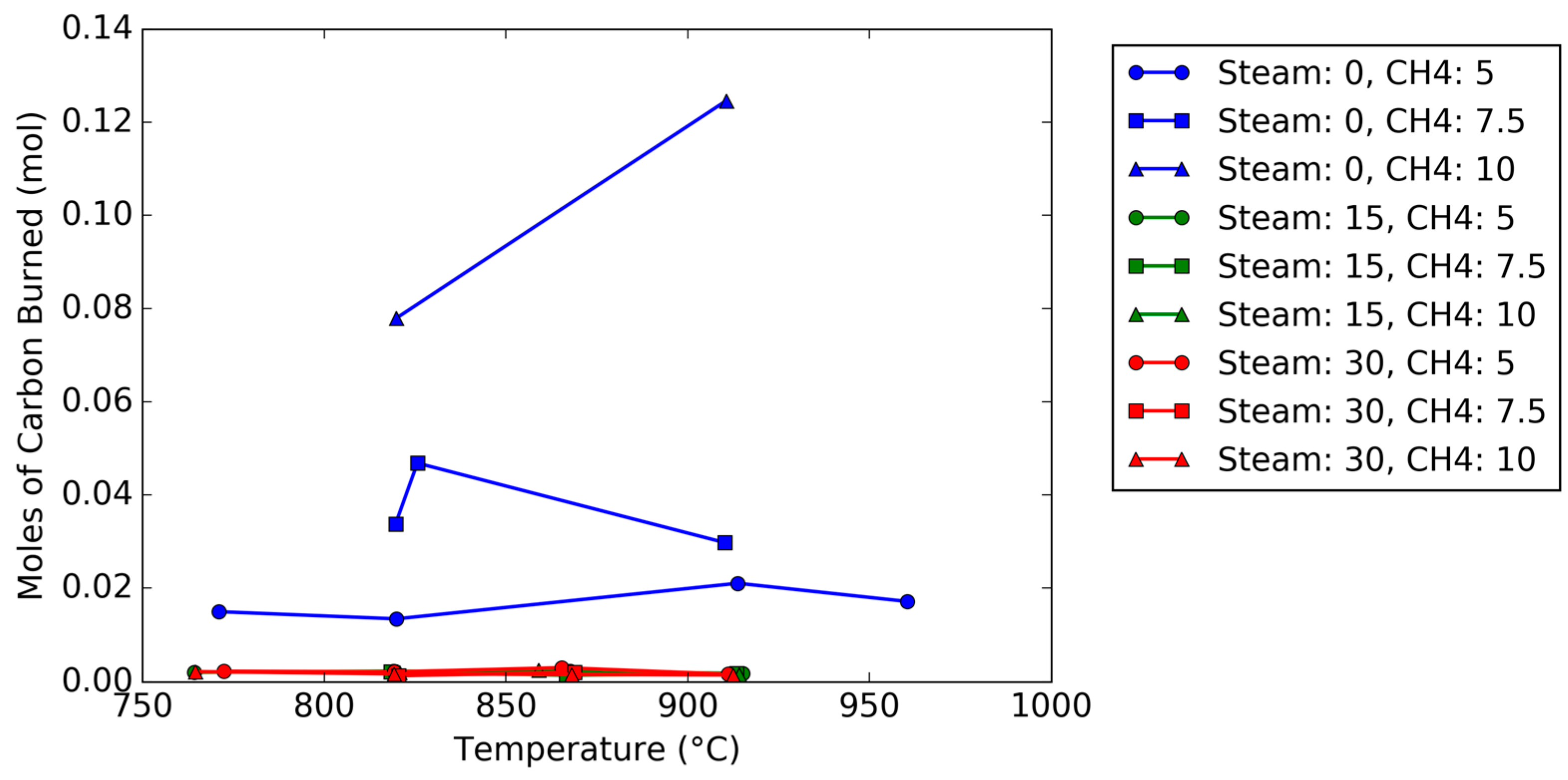
Figure 4. The oxidation breakthrough—which occurred when the oxygen concentration reached 5%—for the steam trial occurred later than the trial without steam since (1) the reduction steps were longer, and (2) the rate of oxygen carrier conversion was higher with steam than without steam. Using the known argon composition from the known flow rate of air into the reactor as a tracer to calculate the molar flow rates (Equations (1), (2) and (12)), the total moles of carbon burned off the carrier can be measured by integration during the oxidation cycle; a plot of the total carbon deposited as a function of temperature, steam concentration, and methane concentration is shown in
Figure 5. The plot shows that for steam feed concentrations of 15 and 30 vol %, the moles of carbon are negligible (less than 3 millimoles), regardless of steam concentration. Meanwhile, for trials without steam, the amount of carbon increased as the methane concentration feed increased from 5 vol % to 10 vol %.
Conversions of the natural gas and the oxygen carrier as a function of steam and methane concentrations are plotted in
Figure 6, where
Figure 6a consists of the methane conversion and
Figure 6b consists of the oxygen carrier conversion. For all trials, the conversions are measured at 200 s after the feed of natural gas begins for consistency. The methane conversion is an instantaneous value of the flows (cross reference Equation (16)), while the oxygen carrier conversion is an integrated value from the time of initiation to 200 s (cross reference Equation (22)). For trials that consist of replicates, error bars are added, which represent plus or minus one standard deviation.
Figure 6a shows the effects of the addition of steam for trials with 5%, 7.5% and 10% methane fed into the feed gas. Some general trends are as follows. For constant steam concentrations for the 5% methane feed, the increase in temperature resulted in slightly higher conversions for all three steam concentrations. This temperature pattern was unfortunately not exactly repeated for the other methane concentrations, perhaps due to limits of instrument accuracy. This may be because it takes several cycles to activate the carrier, so the kinetics may change with the cycle number [
32]. However, a trend that is consistent across all three methane concentrations is the dramatic increase in conversion from 0 to 15% steam and a slight decrease in conversion from 15% to 30% steam. The dramatic increase in conversion from 0 to 15% steam concentration is in part due to (1) the steam-methane reforming reaction (Reaction 7) and the water–gas shift reaction (Reaction 9) producing more hydrogen, and (2) the suppression of carbon deposition, resulting in a greater molar flow rate of CO
2 instead of carbon on the particle.
The decrease in conversion from 15% to 30% steam concentration could be due to several effects. First, the steam methane reforming reaction creating more hydrogen and carbon monoxide via Reaction 7. Second, the water–gas shift reaction will also form more hydrogen from carbon monoxide. While the kinetics of hydrogen and carbon monoxide directly reacting with hematite are faster than with methane, the steam reforming reaction produces hydrogen and carbon monoxide exceeding what the bed of oxygen carrier can oxidize, since the oxygen from the grain lattice cannot get to the surface fast enough.
A surprising result was found in regards to the effect of steam on the oxygen utilization.
Figure 6b shows that the addition of steam caused an increase in the oxygen carrier conversion. Normally, a greater steam concentration in the feed should shift the equilibrium of Reaction 2 towards the reactant side, resulting in less reduction of the oxygen carrier. However, the direct reaction of methane and hematite was rather slow, and adding more steam shifted the steam methane reforming reaction (Reaction 7) to the product side, causing more of the methane to convert to hydrogen and carbon monoxide, thus reducing the oxygen carrier further.
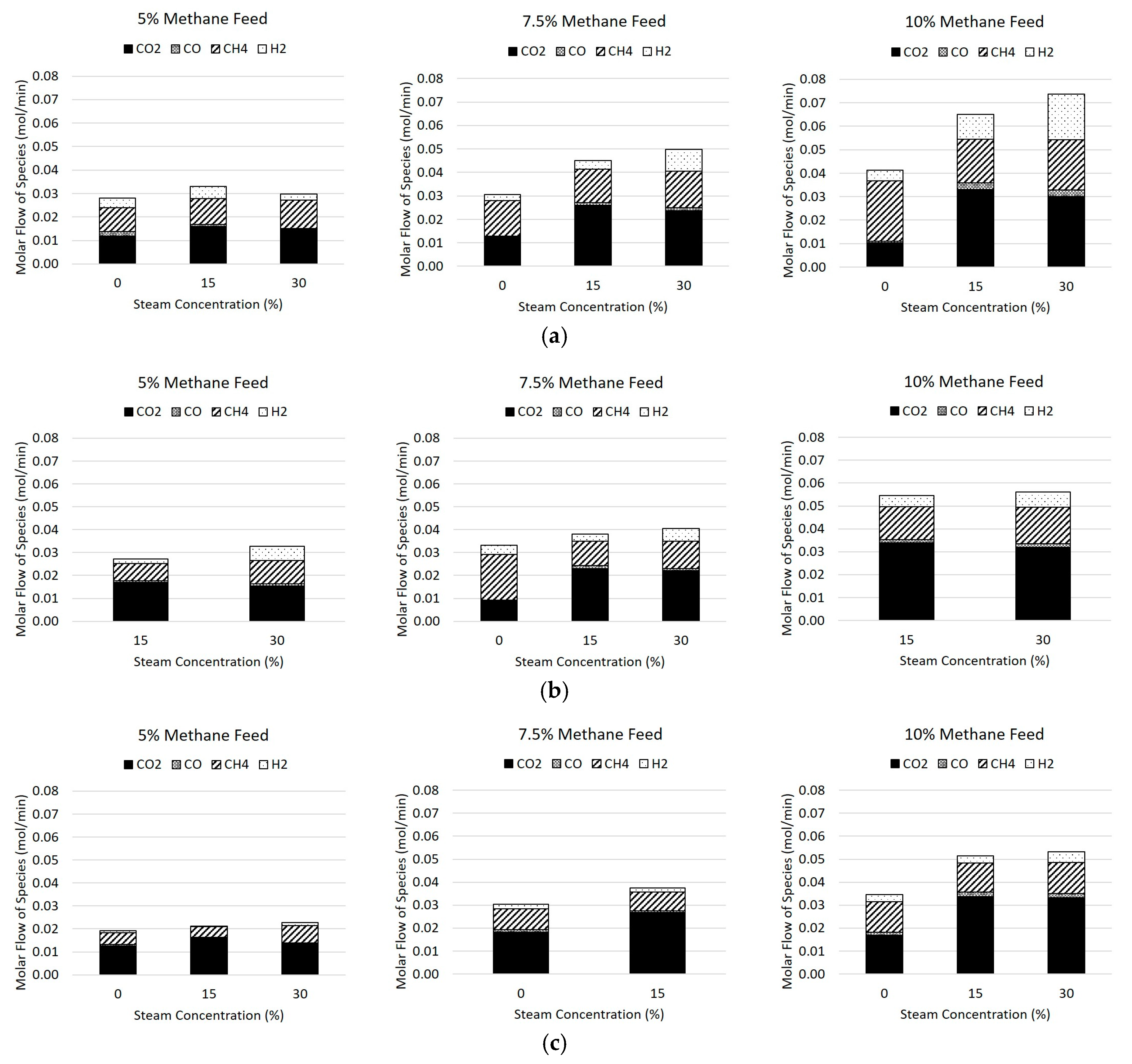
The molar flow rates of the product species in the flue gas are reported in
Table 4 and
Figure 7, where
Figure 7a shows the flows at 800 °C,
Figure 7b shows the flows at 850 °C, and
Figure 7c shows the flows at 900 °C. The inert gases of nitrogen and argon were excluded from the plot for clarity, as well as the steam produced. The two major non-condensable products were carbon dioxide, followed by methane, and the minor species are hydrogen and carbon monoxide, with hydrogen being more prevalent of the minor species due to the stoichiometry of Reactions 1, 4, and 7. It can be seen in general that the increase of methane and steam to the feed gas caused an increase of both hydrogen and carbon monoxide at 800 °C, but this effect was diminished as the temperature increased due to the fast kinetics of hydrogen with the hematite.
4. Discussion
To observe the effect of temperature, the conversion of methane and oxygen carriers were plotted as a function of temperature, as shown in
Figure 8. As can be seen, the majority of the plots show a positive correlation between temperature and conversion, with occasional exceptions. For the overall methane conversion, the values for different methane and steam feeds as a function of temperature mostly overlap due to the conversion of methane to different products (carbon deposition, production of hydrogen or carbon monoxide, or carbon dioxide). It can be noted that the CO
2 specific conversion for all trials is less than the overall methane conversion, particularly for the trials without steam. For the conversion to CO
2, adding more methane drops the conversion, but the effect is mitigated as more steam is added to the system. As seen in previous plots, increasing the steam to 15 vol % improves conversion significantly but it drops slightly as the steam concentration increases to 30 vol %. The oxygen carrier conversion is also plotted as a function of temperature, and the oxygen carrier conversion is not as strong of a function as the gas conversion, but is highly dependent on the feed gas. Like the trends ascertained from
Figure 6, the addition of more steam and methane to the feed gas causes the oxygen carrier conversion to increase.
To complete the analysis, a multivariable ordinary least squares regression was performed on the dataset to determine which variables had greater effects than others and what variables are statistically significant. The dependent variables chosen were methane conversion to carbon dioxide and the oxygen carrier conversion, and the independent variables selected for the analysis were the reactor temperature, pressure, inlet steam concentration, and inlet methane concentration. Additionally, dual interaction terms were chosen. Each of the independent variables were coded to allow for direct comparison of the magnitude of the coefficients to represent the strength of the effect on the conversion. The regression equation chosen to fit the independent variable
(where
O or
) at 200 s was:
where
are the fit regression coefficients, and the prime symbol (’) indicates that the independent variables are coded, where the lowest value is indicated by −1 and the highest value is 1. The uncoded variables can be converted to coded variables using the following equation:
where
and
of the set of independent parameter data. The resulting best-fit parameters are listed in
Table 5,
Table 6,
Table 7 and
Table 8 in addition to the goodness of fit parameters. Assuming alpha is chosen as 0.10, the primary variables that are most significant are the temperature, pressure, and steam concentration. Running the analysis with the statistically insignificant variables dropped, the resulting linear regressions for the methane conversion and the oxygen carrier conversion at 200 s are:
The coefficient of determination for Equation (27) is 81.3% and for Equation (28) is 95.5%. Equation (15) shows that the largest effect on the conversion in the test runs was the interaction between the pressure and steam concentration, followed by the effects of temperature and pressure. The positive effect of pressure and temperature on the conversion should be expected, since increasing the temperature increases the kinetic rate constant, and increasing the pressure causes the partial pressure of the species to increase. Increasing the steam concentration in the feed also causes an increase in conversion, as this should cause Reaction 7 to shift to the right, causing carbon monoxide and hydrogen to form quicker than methane cracking. Reactions 9 and 12 also cause the conversion to carbon dioxide to increase. Carbon monoxide and hydrogen react faster than methane would directly with hematite. For the oxygen carrier conversion, the parameter with the greatest effect was the concentration of the steam in the feed, followed by the methane concentration in the feed, and the interaction between the temperature and the methane and steam concentrations. Alone, neither temperature nor pressure seemed to cause a statistically significant effect on the oxygen carrier conversion. It should be noted that Equations (15) and (16) are not intended to rigorously model all the effects including hydrodynamics; rather, it is intended to provide a qualitative understanding of parameters for the analysis of data. It should also be cautioned that the detailed results may not be relevant for a reactor in a different regime than a bubbling fluid bed. However, results could still be relevant for the design of a bubbling fluidized bed fuel reactor operating near the hydrodynamic conditions presented in this work.
It should not be surprising that, for the 0 vol %–15 vol % steam trials, an increase in pressure causes the conversion to increase due to an increase in the steam partial pressure. However, for higher steam concentrations, the compound effect of pressure and steam complicates the analysis, since increasing the steam concentration causes the plenum pressure to be lower. Additionally, the steam condenses from the line before exiting through the outlet needle valve, causing there to be less pressure drop across it when higher steam concentrations are used. Thus, more controlled experiments in the future need to be performed that consider this anomaly. This is probably the reason that the pressure and steam concentration interaction parameter are of the greatest magnitude: to nullify the effect of lower system pressure when the steam concentration in the feed is highest.
The methane conversion results of the steam-free trials are lower than the results from Breault et al. [
34], which ranged from 40% to 53% at 200 s after a start of reaction at 800 °C, 10% methane concentration in the feed, and a total gas flow of 6 SLPM. The gas flow in the present study is twice as high, resulting in less conversion of the methane. The results were similar to that from Siriwardane et al. [
30] at the same temperature (800 °C), although trials from their work were performed with a larger inventory of hematite and a lower gas velocity (1.2 times the minimum fluidization velocity of the material instead of three times the minimum fluidization velocity). The results from a 50 kW
th pilot chemical looping reactor however are lower than those measured in this work, the former which were around 10%–20% methane conversion to CO
2 [
33]; however, the results from this unit are not with steam in the feed gas.
While there is a significant cost advantage to using raw minerals such as hematite ore, one of the risks associated with using an unsupported raw mineral as an oxygen carrier is sintering and defluidization of the material. Sintering of raw iron-based minerals has been observed by Schwebel et al. under fixed bed conditions during oxidation due to the high degree of reduction in fixed beds [
39]. In the present testing, sintering and defluidization were not observed, as the material was not reduced significantly and the oxidation reaction rate was tempered by decreasing the oxygen feed to 5 vol%. However, the same material exhibited minor agglomeration in a 50 kW
th unit between the bubble caps of the air reactor fluidized bed, but this did not hinder the operation of the unit, as it was able to be operated for 13 h under chemical looping conditions and two days of circulation at high temperature (800 °C–1000 °C) [
33,
36]. The same hematite ore was tested, albeit coated with MgO to improve the reactivity with natural gas, by Siriwardane et al. [
30], in a 5-cm fluidized bed and did not exhibit agglomeration or sintering. Thus, if the extent of the reduction of raw hematite is low and the temperatures are kept under control, such as in circulating units, it is expected that the mineral can be used without concern of major agglomeration.