Influence of Copper Particles on Breakdown Voltage and Frequency-Dependent Dielectric Property of Vegetable Insulating Oil
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of Oil Samples
2.3. Measurements
3. Experimental Results
3.1. Breakdown Voltage of Vegetable Insulating Oil Contaminated with Copper Particle
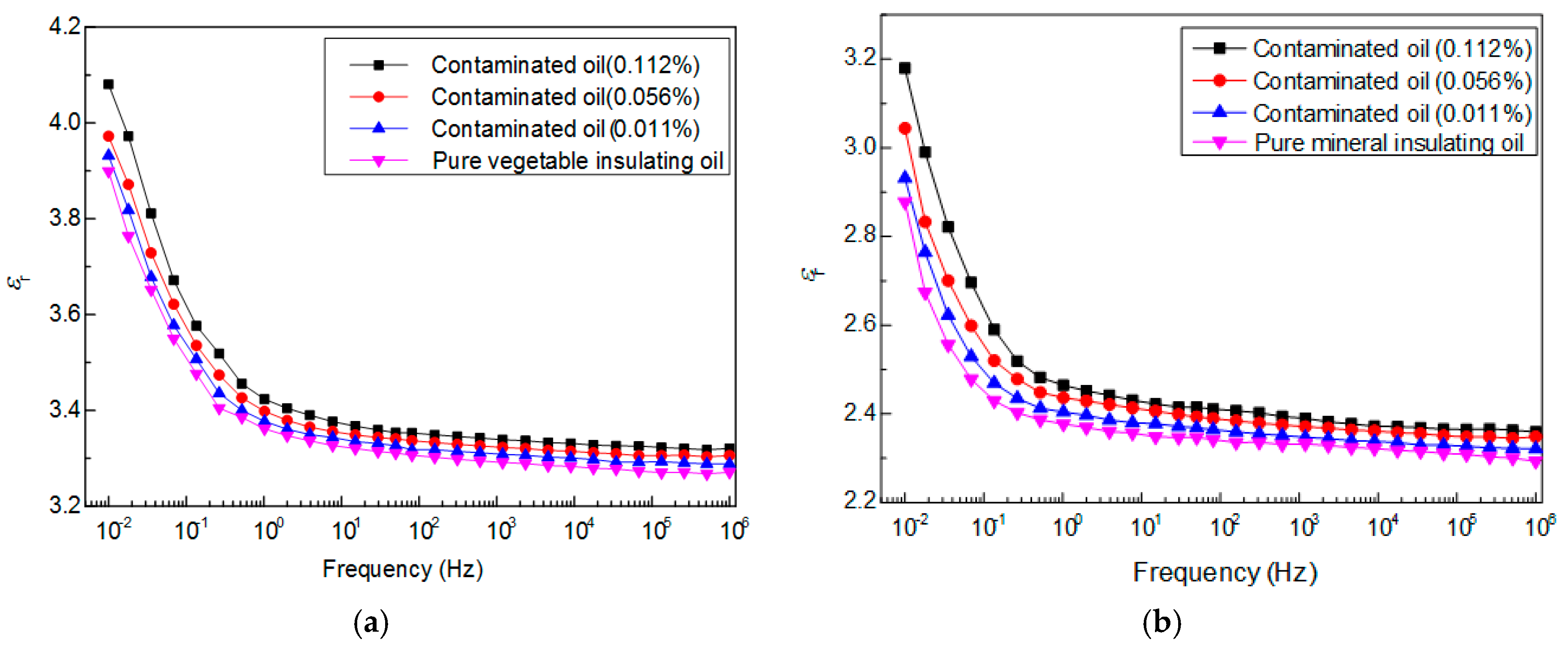
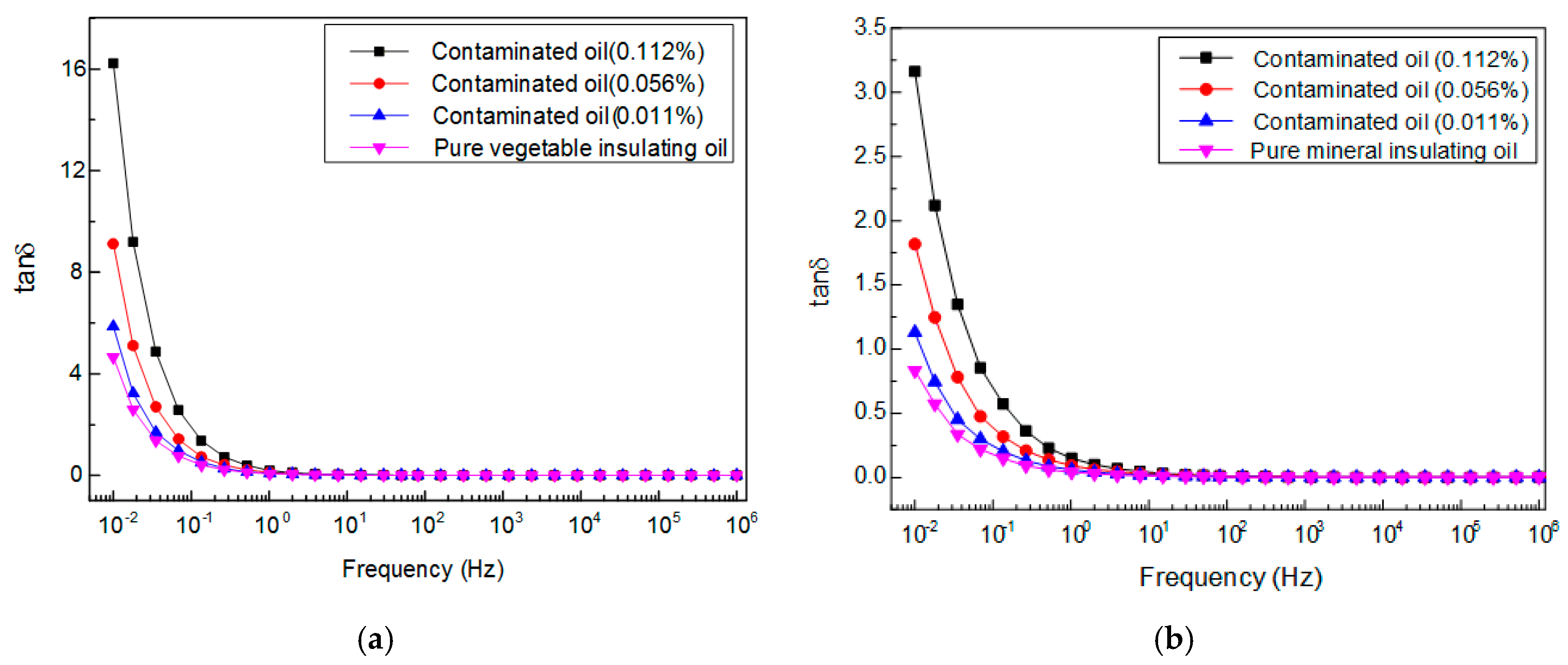
3.2. Frequency-Dependent Dielectric Property of Vegetable Insulating Oil Contaminated with Copper Particle
4. Analysis and Discussion
4.1. Influence of Copper Particles on AC Breakdown Voltage of Vegetable Oil
4.2. Influence of Copper Particles on Frequency-Dependent Dielectric Property of Vegetable Oil
4.2.1. Volume Resistivity
4.2.2. Relative Permittivity
4.2.3. Dissipation Factor
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bandara, K.; Ekanayake, C.; Saha, T.K. Modelling the dielectric response measurements of transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1283–1291. [Google Scholar] [CrossRef]
- Hopkinson, P.; Dix, L.; McShane, C.P.; Moore, H.R.; Moore, S.; Murphy, J.; Prevost, T.; Beaster, B. Progress report on natural esters for distribution and power transformers. In Proceedings of the 2009 IEEE Power & Energy Society General Meeting, Calgary, AB, Canada, 26–30 July 2009; pp. 1–3. [Google Scholar]
- Nagashree, A.N.; Champa, V.; Sumangala, B.V.; Nagabhushana, G.R. Suitability of natural vegetable seed oil as liquid dielectric coolant in an insulation system. In Proceedings of the 2015 International Conference on Emerging Research in Electronics, Computer Science and Technology (ICERECT), Mandya, India, 17–19 December 2015; pp. 429–434. [Google Scholar]
- Rozga, P.; Stanek, M. Comparative analysis of lightning breakdown voltage of natural ester liquids of different viscosities supported by light emission measurement. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 991–999. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Grzybowski, S.; Liu, Y. Characteristics of moisture diffusion in vegetable oil-paper insulation. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 157. [Google Scholar] [CrossRef]
- Aubin, J. Effect of Particles on Transformer Dielectric Strength; Cigré: Paris, France, 2000. [Google Scholar]
- Insulating Liquids—Methods for Counting and Sizing Particles. Available online: https://shop.standards.ie/preview/is/en/2008/i.s.en60970-2008.pdf?sku=1129729 (accessed on 5 July 2017).
- Wang, X.; Wang, Z.D. Particle effect on breakdown voltage of mineral and ester based transformer oils. In Proceedings of the Conference on Electrical Insulation and Dielectric Phenomena, Quebec, QC, Canada, 26–29 Octomber 2008; pp. 598–602. [Google Scholar]
- Wang, X.; Wang, Z.D.; Noakhes, J. Motion of conductive particles and the effect on AC breakdown strengths of esters. In Proceedings of the 2011 IEEE International Conference on Dielectric Liquids, Trondheim, Norway, 26–30 June 2011; pp. 1–4. [Google Scholar]
- Sinan, S.; Jasni, J.; Azis, N.; Kadir, A.; Abidin, M.Z.; Mohtar, M. Effect of particles on the AC breakdown voltage of palm oil and coconut oil. Appl. Mech. Mater. 2015, 793, 70–74. [Google Scholar] [CrossRef]
- Miners, K. Particles and moisture effect on dielectric strength of transformer oil using vde electrodes. IEEE Trans. Power Appar. Syst. 1982, 101, 751–756. [Google Scholar] [CrossRef]
- Mathes, K.; Atkins, J. Influence of particles on partial discharges and breakdown in oil. In Proceedings of the International Conference on Electrical Insulation, Philadelphia, PA, USA, 12–14 June 1978; pp. 226–231. [Google Scholar]
- Lu, W.; Liu, Q. Effect of cellulose particles on impulse breakdown in ester transformer liquids in uniform electric fields. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2554–2564. [Google Scholar] [CrossRef]
- Farooq, K. The effect of particulate and water contamination on the dielectric strength of insulating oils. In Proceedings of the Conference Record of the 1996 IEEE International Symposium on Electrical Insulation, Montreal, QC, Canada, 16–19 Jun 1996; pp. 728–732. [Google Scholar]
- Krins, M.; Borsi, H.; Gockenbach, E. Influence of carbon particles on the breakdown voltage of transformer oil. In Proceedings of the 12th International Conference on Conduction and Breakdown in Dielectric Liquids, Roma, Italy, 15–19 July 1996; pp. 296–299. [Google Scholar]
- Dascalescu, L.; Mihailescu, M.; Tobazeon, R. Modeling of conductive particle behavior in insulating fluids affected by DC electric fields. IEEE Trans. Ind. Appl. 1998, 34, 66–74. [Google Scholar] [CrossRef]
- Krins, M.; Borsi, H.; Gockenbach, E. Impact of carbon particles on the electrical strength of different solid/liquid interfaces in a non-uniform field. In Proceedings of the Conference Record of the 1998 IEEE International Symposium on Electrical Insulation, Arlington, VA, USA, 7–10 June 1998; pp. 623–626. [Google Scholar]
- Panov, V.A.; Kulikov, Y.M.; Son, E.E.; Tyuftyaev, A.S.; Gadzhiev, M.K.; Akimov, P.L. Electrical breakdown voltage of transformer oil with gas bubbles. High Temp. 2014, 52, 770–773. [Google Scholar] [CrossRef]
- Mahmud, S. Influence of Contamination on the Electrical Performance of Power Transformer Oil; University of Southampton: Southampton, UK, 2015. [Google Scholar]
- Mahmud, S.; Chen, G.; Golosnoy, I.; Wilson, G.; Jarman, P. Experimental studies of influence of DC and AC electric fields on bridging in contaminated transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 152–160. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Zou, P.; Grzybowski, S.; Zahn, M. Preparation of a vegetable oil-based nanofluid and investigation of its breakdown and dielectric properties. IEEE Electr. Insul. Mag. 2012, 28, 43–50. [Google Scholar] [CrossRef]
- Peppas, G.D.; Bakandritsos, A.; Charalampakos, V.P.; Pyrgioti, E.C.; Tucek, J.; Zboril, R.; Gonos, I.F. Ultrastable natural ester-based nanofluids for high voltage insulation applications. ACS Appl. Mater. Interf. 2016, 8, 25202–25209. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhong, Y.; Zhou, J.; Li, X.; Zhou, Y. Effect of semiconductive nanoparticles on insulating performances of transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 42–45. [Google Scholar]
- N’cho, J.; Fofana, I.; Hadjadj, Y.; Beroual, A. Review of physicochemical-based diagnostic techniques for assessing insulation condition in aged transformers. Energies 2016, 9, 367. [Google Scholar] [CrossRef]
- Carraz, F.; Rain, P.; Tobazeon, R. Particle initiated breakdown in a quasi-uniform field in transformer oil. IEEE Trans. Dielectr. Electr. Insul. 1995, 2, 1052–1063. [Google Scholar] [CrossRef]
- Mahmud, S.; Chen, G.; Golosnoy, I.; Wilson, G.; Jarman, P. Experimental studies of influence of different electrodes on bridging in contaminated transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2433–2441. [Google Scholar] [CrossRef]
- Miao, J.; Dong, M.; Ren, M.; Wu, X.; Shen, L.; Wang, H. Effect of nanoparticle polarization on relative permittivity of transformer oil-based nanofluids. J. Appl. Phys. 2013, 113, 204103. [Google Scholar] [CrossRef]
- Choi, C.; Yoo, H.; Oh, J. Preparation and heat transfer properties of nanoparticle-in-transformer oil dispersions as advanced energy-efficient coolants. Curr. Appl. Phys. 2008, 8, 710–712. [Google Scholar] [CrossRef]
- Taha-Tijerina, J.; Narayanan, T.N.; Gao, G.; Rohde, M.; Tsentalovich, D.A.; Pasquali, M.; Ajayan, P.M. Electrically insulating thermal nano-oils using 2D fillers. ACS Nano 2012, 6, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- IEC/IEEE Guide for the Statistical Analysis of Electrical Insulation Breakdown Data (Adoption of IEEE Std 930-2004); IEEE: Piscataway, NJ, USA, 2007; pp. 1–53.
- Martin, D.; Martin, D.; Wang, Z.; Wang, Z. Statistical analysis of the ac breakdown voltages of ester based transformer oils. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1044–1050. [Google Scholar] [CrossRef]
- Kuffel, J.; Kuffel, P. High Voltage Engineering Fundamentals; Newnes: Oxford, UK, 2000. [Google Scholar]
- Chadband, W.G. From bubbles to breakdown, or vice-versa. In Proceedings of the IEEE 11th International Conference on Conduction and Breakdown in Dielectric Liquids, Baden-Dattwil, Switzerland, 19–23 July 1993; pp. 184–193. [Google Scholar]
- Robinson, D.A.; Friedman, S.P. Electrical conductivity and dielectric permittivity of sphere packings: Measurements and modelling of cubic lattices, randomly packed monosize spheres and multi-size mixtures. Phys. A Stat. Mech. Appl. 2005, 358, 447–465. [Google Scholar] [CrossRef]
- Zhu, S.; Zareifard, M.; Chen, C.; Marcotte, M.; Grabowski, S. Electrical conductivity of particle–fluid mixtures in ohmic heating: Measurement and simulation. Food Res. Int. 2010, 43, 1666–1672. [Google Scholar] [CrossRef]
- Maxwell, J. A Treatise on Electricity and Magnetism; Clarendon Press: Oxford, UK, 1873. [Google Scholar]
- Takada, T.; Hayase, Y.; Tanaka, Y.; Okamoto, T. Space charge trapping in electrical potential well caused by permanent and induced dipoles for LDPE/MgO nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 152–160. [Google Scholar] [CrossRef]
- Akmal, A.S.; Borsi, H.; Gockenbach, E.; Wasserberg, V.; Mohseni, H. Dielectric behavior of insulating liquids at very low frequency. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 532–538. [Google Scholar] [CrossRef]
- Mu-zhen, S. Dielectric Physics; University of Technology Press: Guangzhou, China, 2000. [Google Scholar]
- Richert, R.; Agapov, A.; Sokolov, A.P. Appearance of a Debye process at the conductivity relaxation frequency of a viscous liquid. J. Chem. Phys. 2011, 134, 104508. [Google Scholar] [CrossRef] [PubMed]
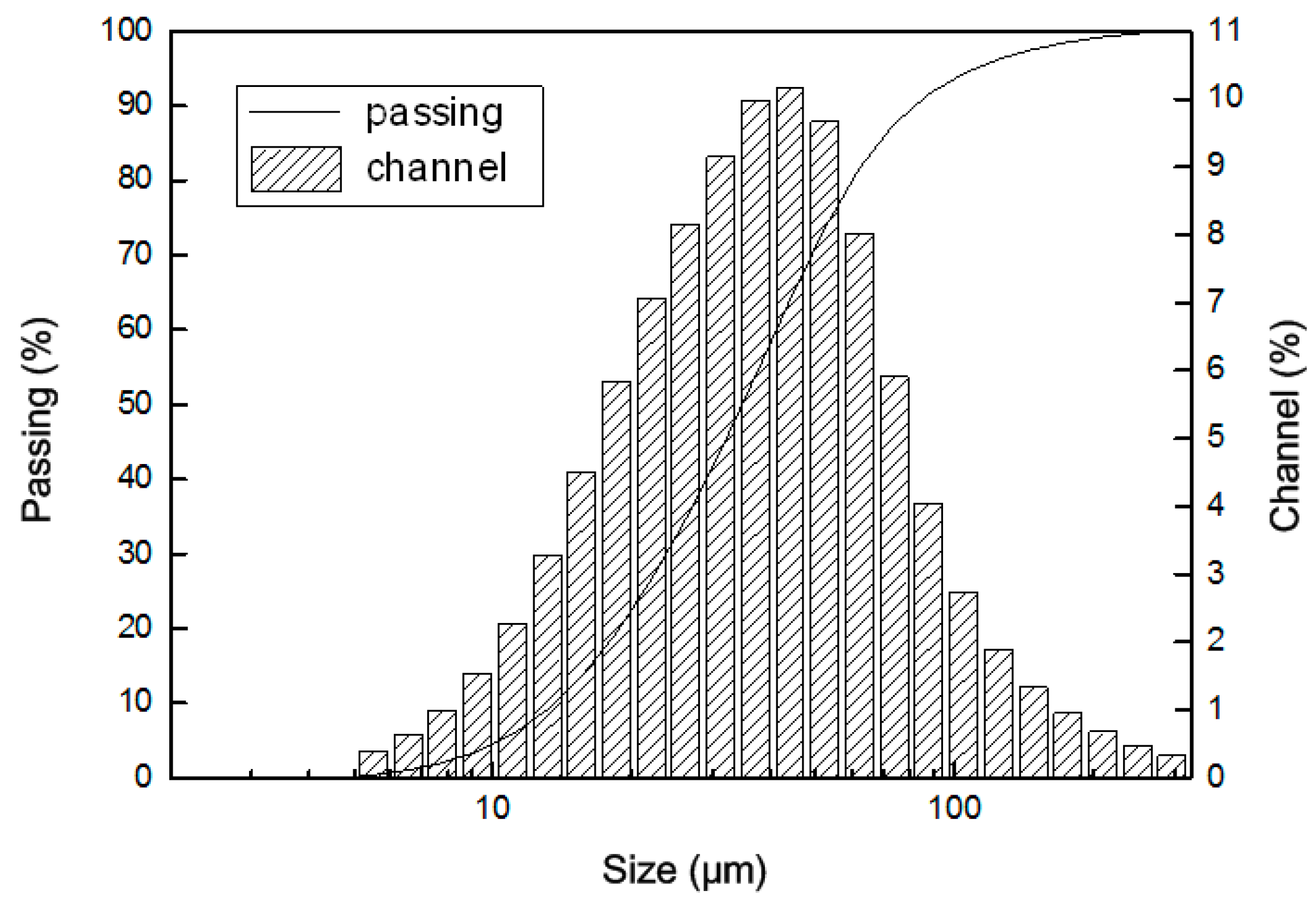
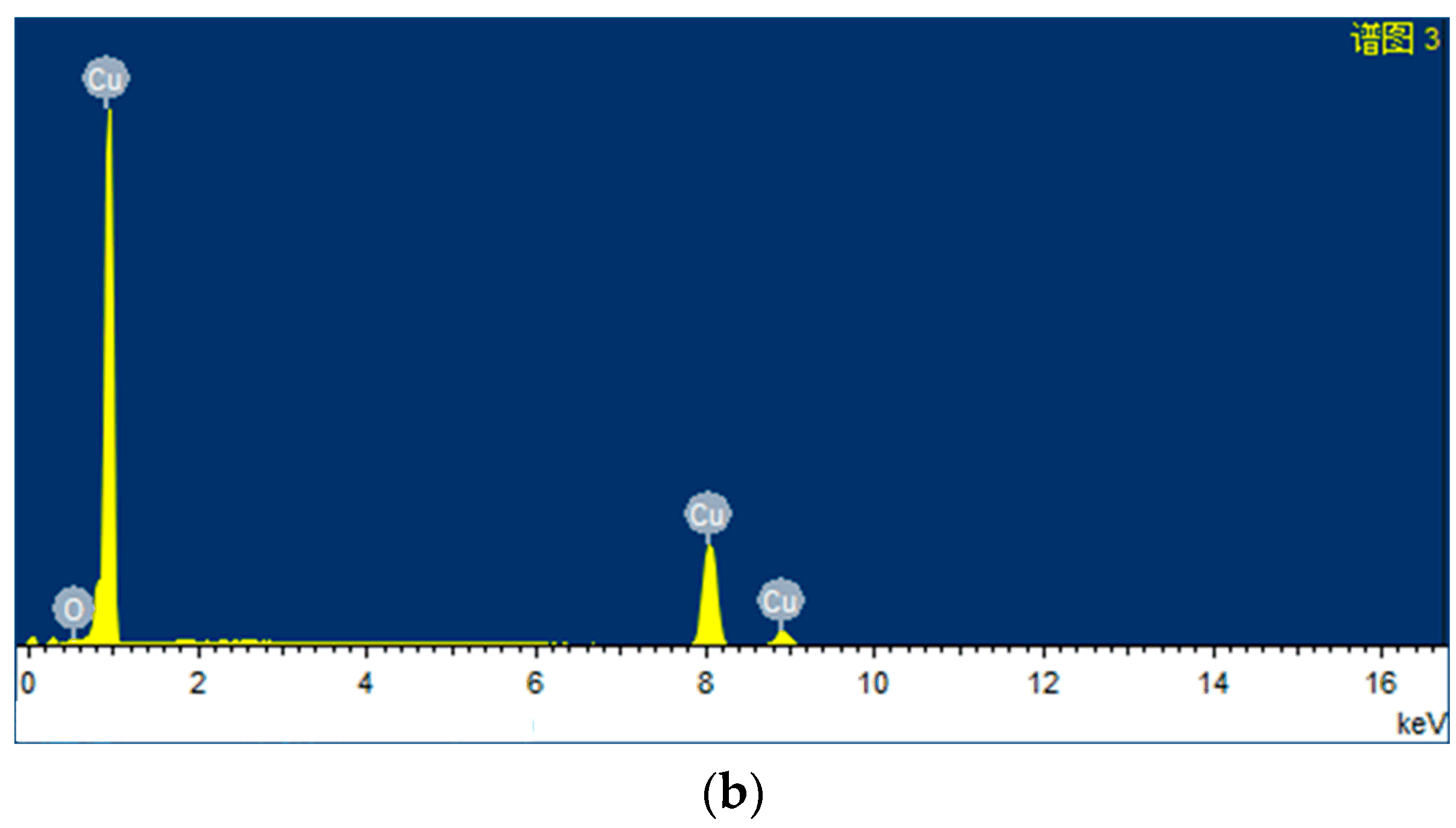



| Parameter | Value | |
|---|---|---|
| Vegetable Insulating Oil | Mineral Insulating Oil | |
| Appearance | Light yellow | Transparent |
| Density (g·cm−3, 20 °C) | 0.90 | <0.895 |
| Kinematic viscosity (mm2·s−1, 40 °C) | 43.0 | ≤13.0 |
| Pour point (°C) | −18 | ≤−22 |
| Flash point (°C) | 325 | ≥135 |
| Acid value (mg KOH·g−1) | 0.03 | ≤0.01 |
| Interfacial tension (mN·m−1) | 30 | ≥40 |
| Dissipation factor (90 °C) | 2.0% | ≤0.1% |
| Relative permittivity (90 °C) | 2.9 | 2.2 |
| Volume resistivity (Ω·m) | 2 × 1010/90 °C | 7 × 1011/25 °C |
| Particle Size (μm) | Fitting Expression | R2 | ||
|---|---|---|---|---|
| Vegetable Oil | Mineal Oil | Vegetable Oil | Mineal Oil | |
| 3 | U = −3.70 lgN + 63.82 | U = −3.73 lgN + 52.46 | 0.9976 | 0.9878 |
| 10 | U = −3.95 lgN + 59.25 | U = −4.14 lgN + 49.08 | 0.9879 | 0.9959 |
| 37 | U = −4.26 lgN + 56.33 | U = −4.52 lgN + 43.09 | 0.9819 | 0.9900 |
| 60 | U = −5.05 lgN + 55.24 | U = −5.35 lgN + 42.18 | 0.9698 | 0.9931 |
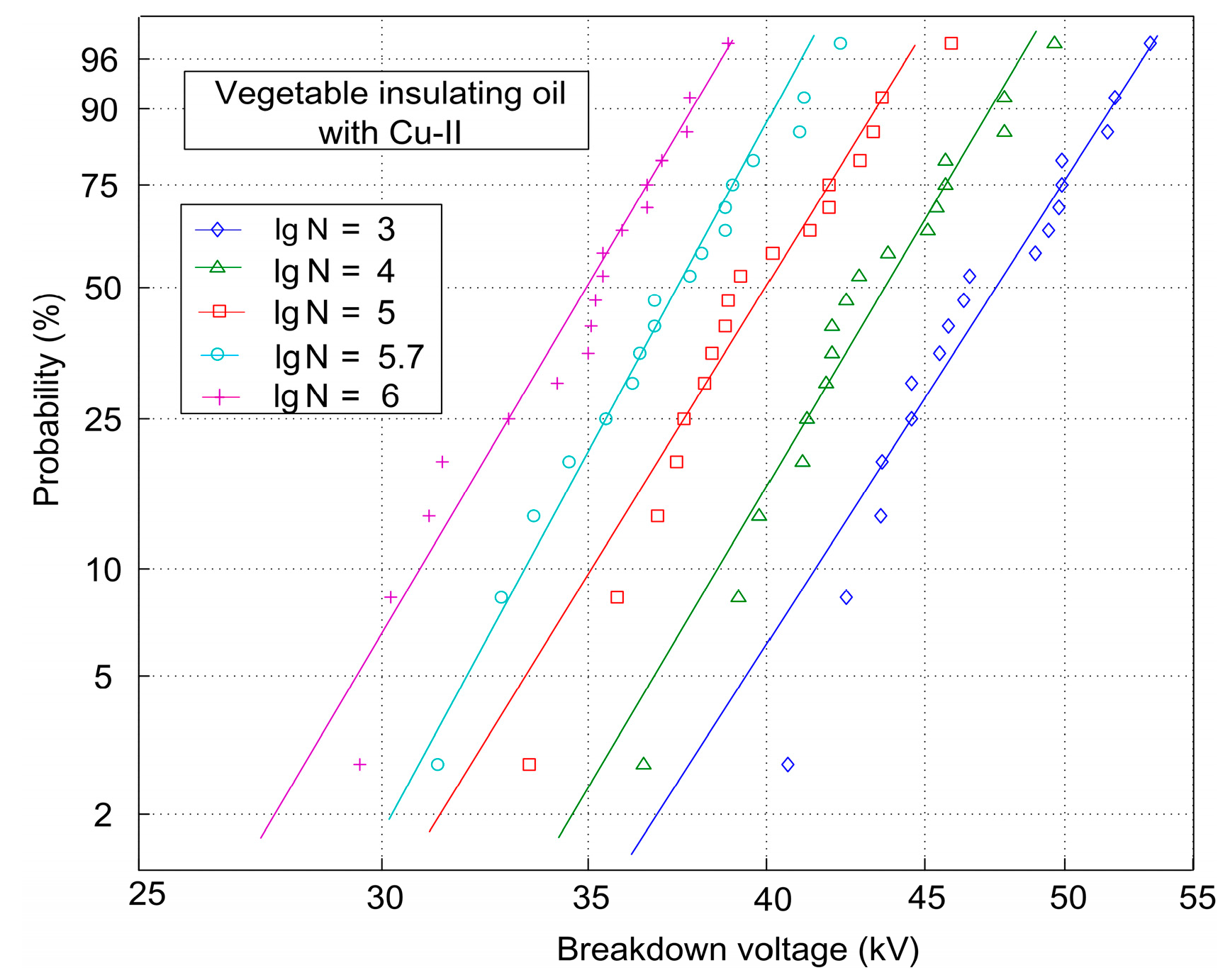
| Logarithm of Particle Number (lgN) | AC Breakdown Voltage (kV) | ||
|---|---|---|---|
| P = 63.2% (Scale Parameter) | P = 50% | P = 5% | |
| 3 | 48.82 | 45.58 | 38.71 |
| 4 | 44.84 | 43.73 | 36.21 |
| 5 | 41.23 | 40.01 | 32.83 |
| 5.7 | 38.76 | 37.46 | 31.61 |
| 6 | 35.95 | 35.02 | 28.96 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, F.; Li, J.; Ran, H.; Huang, D. Influence of Copper Particles on Breakdown Voltage and Frequency-Dependent Dielectric Property of Vegetable Insulating Oil. Energies 2017, 10, 938. https://doi.org/10.3390/en10070938
Zhang J, Wang F, Li J, Ran H, Huang D. Influence of Copper Particles on Breakdown Voltage and Frequency-Dependent Dielectric Property of Vegetable Insulating Oil. Energies. 2017; 10(7):938. https://doi.org/10.3390/en10070938
Chicago/Turabian StyleZhang, Jing, Feipeng Wang, Jian Li, Hehuan Ran, and Dali Huang. 2017. "Influence of Copper Particles on Breakdown Voltage and Frequency-Dependent Dielectric Property of Vegetable Insulating Oil" Energies 10, no. 7: 938. https://doi.org/10.3390/en10070938





