1. Introduction
Sulphur in coal is classified into inorganic and organic sulphur. The inorganic part of sulphur includes disulphides and sulphates and is embedded in the coal while the organic part of sulphur is chemically bonded to the organic matrix of the coal. The different classes of sulphur compounds in coal can be quantitatively determined by standard methods [
1]. Sulphate sulphur occurs in the form of calcium, iron and barium sulphates, but the amount is usually negligible. Almost all of the inorganic sulphur is present as the cubic pyrite or orthorhombic marcasite, whereas the organic sulphur may occur in different forms of functional groups like disulphides, aliphatic or aromatic thiols, aliphatic, aromatic or mixed sulphides and disulphides, heterocyclic compounds, thiophenes or condensed thiophenic structures [
2,
3,
4]. Stable sulphur compounds can withstand severe decomposition reaction conditions [
5,
6], which makes it difficult to correlate analytical results of the coal products with an evaluation of the organic sulphur functional group distribution in the parent coal. The two main techniques for the quantification of organic sulphur groups are instrumental quantification and chemical quantification.
In the instrumental quantification the techniques that have proved most successful are all types of X-ray spectroscopy [
4]. X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy can provide information on the chemical state of elements in solids and different functional groups but have certain limitations. Oxidation, pyrolysis and reduction are three chemical methods for the quantitative determination of organic sulphur functional groups [
7]. LaCount et al. [
8] investigated the controlled-atmosphere programmed-temperature oxidation (CAPTO) of coal. Calkins [
9] proposed that the flash pyrolysis products from coal could be used to give an insight into the organic sulphur structures with some limitations. All the sulphur groups in coal could be converted to hydrogen sulphide by reducing solvents [
10]. Each reduction reaction has a given activation energy and frequency factor which are different for different sulphur groups [
11,
12]. Majchrowicz [
13,
14] and Garcia [
15] in their research also used a similar TPR procedure to characterise organic sulphur in Belgian and US coals.
There is one type of coal high organic sulphur content which has been extensively studied throughout Europe and the US, namely Mequinenza lignite, which can be used for comparison purposes. Using Mequinenza lignite, different researchers [
16,
17,
18,
19,
20,
21] used different techniques to study various organic sulphur groups.
Table 1 shows the results of these studies.
It can be seen that there is a wide variation in the thiophenic and non-thiophenic contents obtained by different methods for the same coal sample. In general the trends obtained for pyrolysis and the instrumental techniques are the same but totally different to that obtained for TPR and the oxidation method. Different limitations of the various techniques have also discussed elsewhere [
4]. The coal samples have to be oxidised prior to analysis in order to distinguish between different groups. In addition to these problems the expensive experimental setup required, which includes a synchrotron in XANES and other X-ray equipment, limits the use of these techniques for routine analysis. It can be concluded that none of the investigations carried out so far can clearly explain the complex nature of coal matrix and each of the identification methods has its own limitations and more research is needed to get comparable results using a simple technique which is more amenable to routine analysis.
Simple and smaller molecules of organic sulphur present in coal break down producing hydrogen sulphide as a product gas quite easily with catalysed reducing agents under an increase in temperature. The behaviour of complex thiophenes towards the reducing conditions however limits the normal TPR analytical technique up to the identification of simple thiophenes, and thus the complex thiophenic part of organic sulphur is calculated by difference from the total organic sulphur content. This means that complex thiophenes are calculated and not determined. Another technique which has similarities to TPR is the so-called “Temperature Programmed Reduction” method [
22]. The contribution of pyrite was also studied by partial float/sink removal and by HNO
3 extraction. However Meyers [
23] explained that dilute warm nitric acid treatment of coal has been found to remove some organic sulphur by oxidation. Thus with a coal containing pyritic sulphur it is not possible to determine the exact organic sulphur species. In the case of a TPR analysis the pyrite interference is negligible, but complex thiophenes have to be calculated by difference.
In view of the limitations of both the techniques an attempt has been made to modify the TPR method by combining both the techniques one after the other during an experimental run for the characterisation of all the sulphur species in coal. The resulting method has been named Temperature Programmed Identification (TPI).
2. Experimental
2.1. Principle of Temperature Programmed Reduction
Several researchers [
10,
24] have described the fundamental theory of the TPR method. Mathematical equations for the rate of evolution of a gas from a sample as a function of the temperature during pyrolysis experiments with a linear temperature-time program can be found in [
16,
25,
26] listed in
Table 2. According to these equations, if it assumed that each group can be reduced to H
2S by a first order reaction. Then considering the reduction of the aliphatic sulphide, we have:
The index 1 is used to denote the sulphidic functional group; R–S–R′. If it is also assumed that the rate constant k
1, depends on temperature, then, according to the Arrhenius equation:
where A
1 is the frequency factor and E
a1 the activation energy for the reduction. Substituting the value of k
1 from Equation (2) into Equation (1):
The rate constant of the reduction becomes larger at higher temperatures which means that the rate of depletion of sulphide becomes larger at higher temperatures. The rate of evolution of H
2S from a sample with a fixed amount of the sulphide with increasing temperature will increase initially due to this increase in temperature, but since the concentration of the sulphur is depleted in the process, due to the evolution of H
2S, the rate will then fall. The mathematical equation which describes the rate of evolution of gas as a function of the temperature of the sample, where the temperature is increased linearly with time, postulates that, the temperature of the sample is increased according to the equation given below:
where T
0 is the initial temperature (K),
α is the rate of heating, K/min, and t is the time, (min.).
Then the volume of H
2S, V
i, that evolves when the i-th group is reduced, follows the equation:
where T is sample temperature (K) and V
i∞ denotes the total volume of H
2S that will evolve as a result of complete reduction of the i-th group. The temperature at which the rate of evolution of H
2S from the i-th group reaches its maximum is denoted by T
mi, and is unique function of E
ai and A
i and the rate of heating,
α:
Equation (6) means that if the sample is heated at the constant rate α, the apex of the H2S peak from the i-th group which is reduced has different parameters, and . When a mixture which contains several functional groups is reduced, the behaviour of each group is similar, except that each peak will appear at different Tm.
2.2. Experimental Equipment
The experimental apparatus consisted of a tube furnace and a gas chromatograph. The coal samples were prepared and placed in the tube furnace in a ceramic boat. The tube furnace was heated at a specified rate. The downstream end of the tube furnace was connected to a Perkin Elmer-gas chromatograph, by a stainless steel tube of 3 mm diameter. The hydrogen sulphide gas produced by the reduction of the organic sulphur functional groups present in the coal sample was carried to the chromatograph by a continuous flow of nitrogen through the tube furnace. This nitrogen-H2S mixture was automatically injected into the chromatograph by a computer programmed pneumatic gas sampling valve.
2.3. Sample Preparation and Operating Conditions
The coal samples were ground (<51 µm) to reduce the effect of mass transfer. The ground samples were pre-extracted with 5%
v/
v HCl to remove carbonates as soluble chlorides [
27,
28]. The relatively fine particle size assisted reagent penetration and minimised the retention of evolved H
2S. Acid washing dissolved the alkaline minerals which could react with evolved H
2S, and regenerated any thiols bound as calcium thiolates. Coal (40–500 mg, according to the organic sulphur content) was impregnated with pyrogallol, the principle reducing agent. Pyrogallol (250 mg) was added as a 50%
w/
v solution in methanol. The solvent was allowed to evaporate. The dried coal was then slurried with tetralin (1,2,3,4-tetrahydronaphthalene). Six to eight drops or 200 mg of tetralin was used. 9,10-Dihydrophenanthrene (125 mg) and resorcinol (100 mg) were also added to this slurry. Finally 1 to 2 mg of sulphidised cobalt-molybdenum catalyst (Harshaw 0402) was added to enhance the rate of reduction of the sulphur groups. After preparation the samples were left overnight in the ceramic boat for better impregnation of the reducing chemicals. The tube furnace was heated at a predetermined rate (typically 2–5 °C/min) from room temperature to 600 °C with a nitrogen flow of 20 mL/min. A Chromosil 330 column was selected as it is ideally suited for all types of Sulphur-containing gases [
29]. The concentrations of mercaptans, RSH and thiophenes can accurately be determined in the finely ground (<51 µm) coals [
27,
30].
The amount of H2S evolved from a particular organic sulphur functional group was determined by mathematical integration as follows;
2.4. Experimental Runs with Modification of the TPR
The temperature programmed experimental runs were performed on the different coal samples, namely: Ollerton, Harworth, Silverdale and Prince of Wales. Untreated and representative oxydesulphurised samples were run in order to check and identify the structural changes incorporated to the coal matrix due to particular oxydesulphurisation reaction. The temperature ramp rate between the range of a particular group was kept between 2–5 °C. Analysis of the coals used in this study are given in
Table 3 on an “as received basis”.
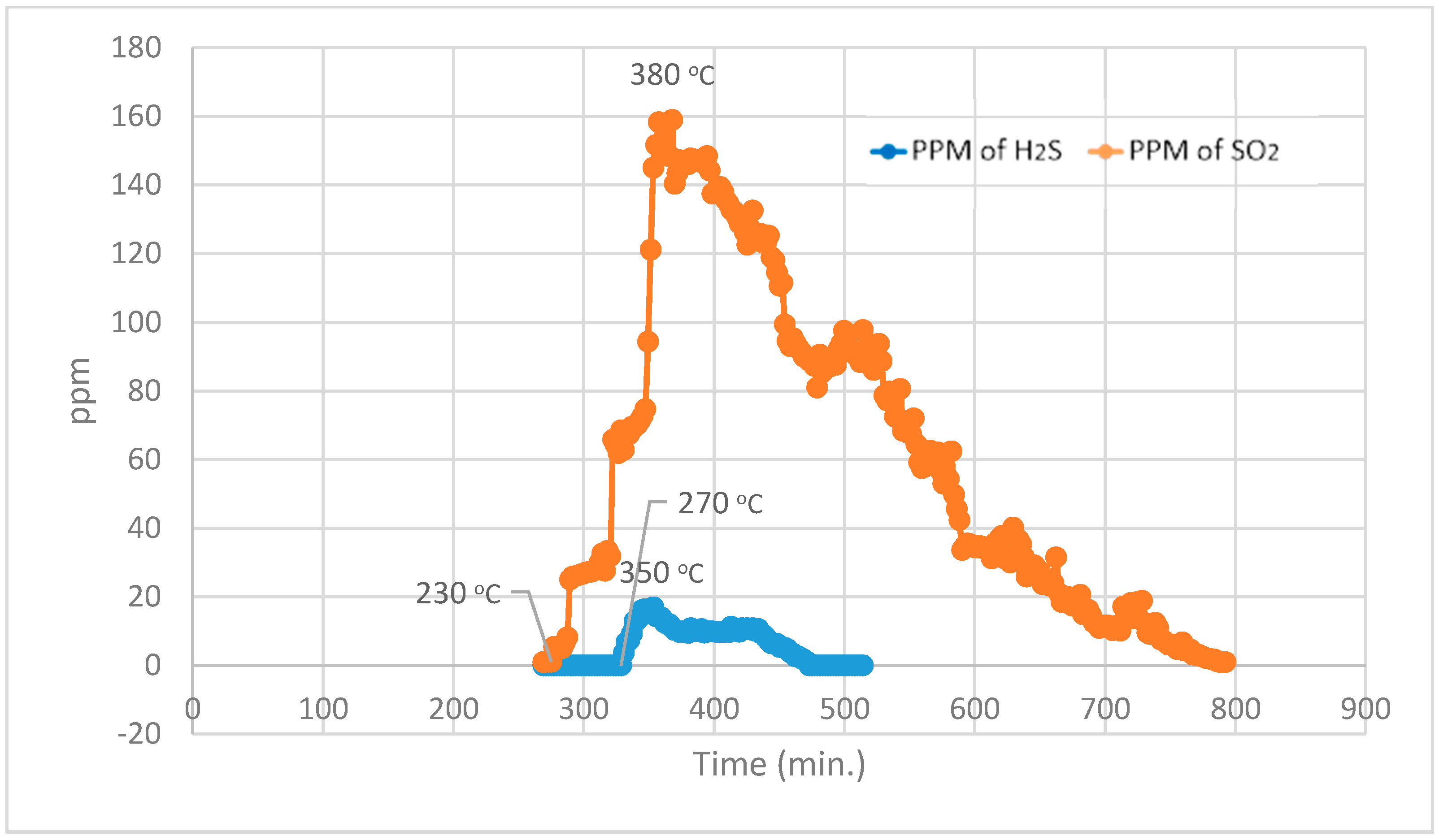
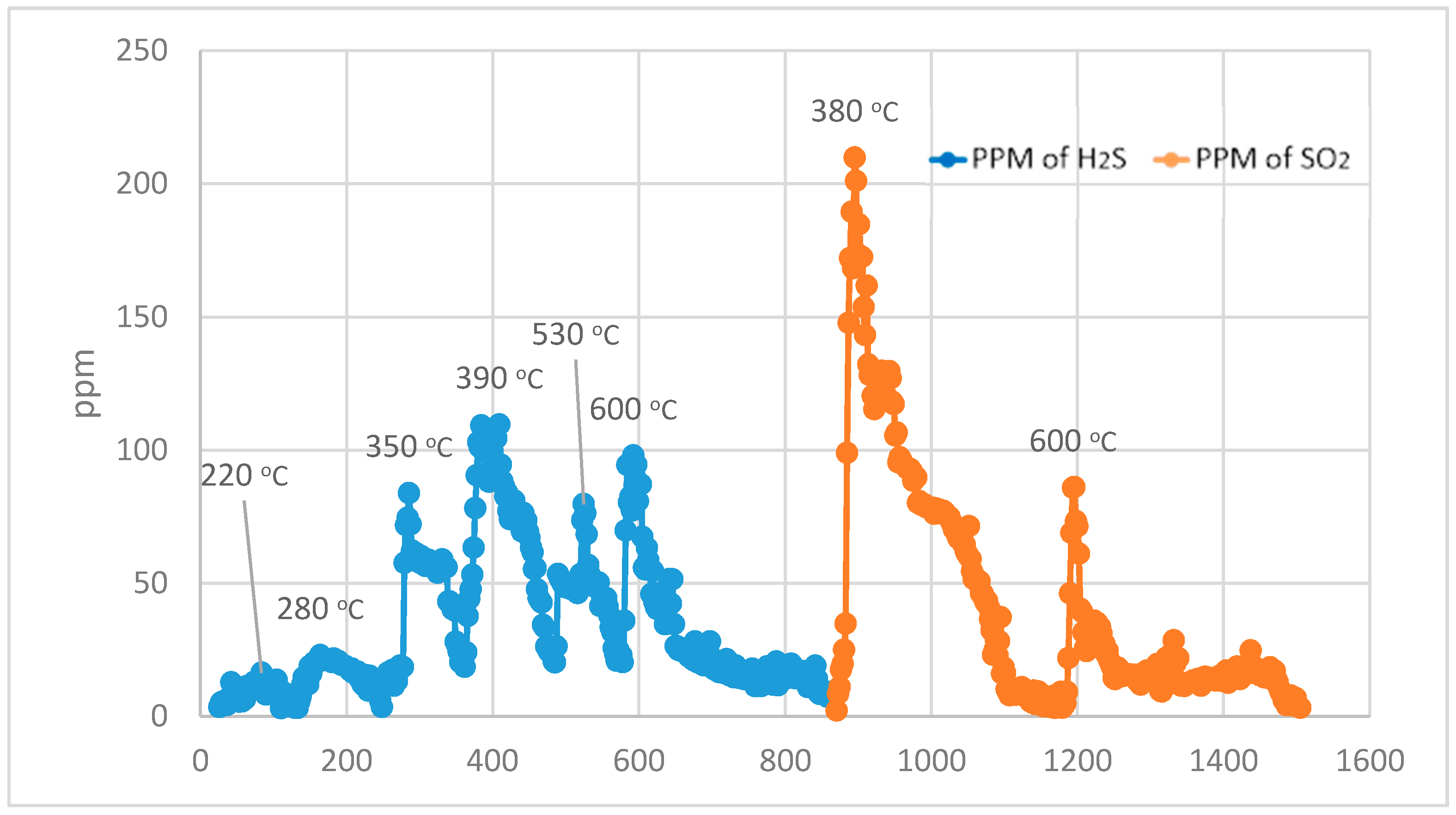
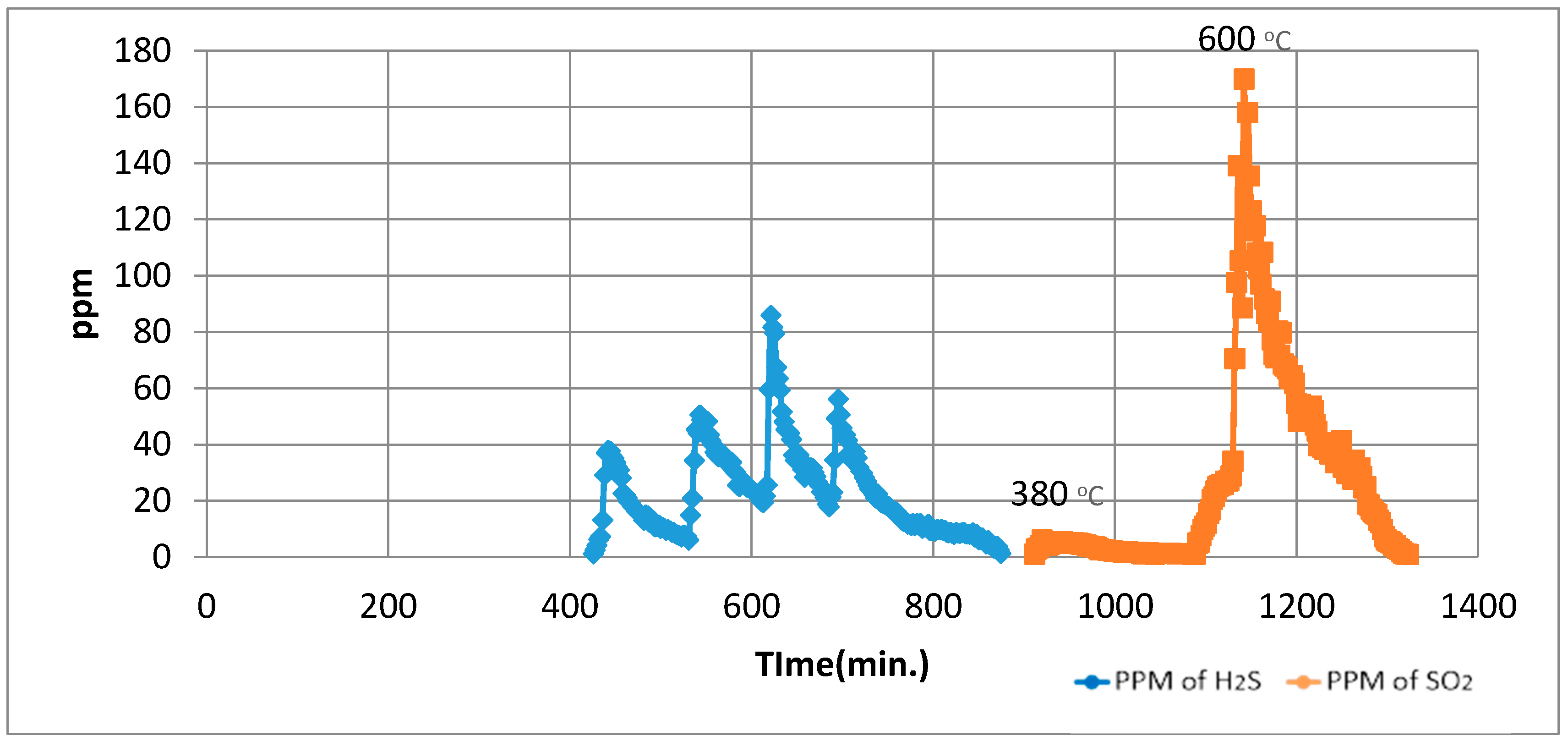
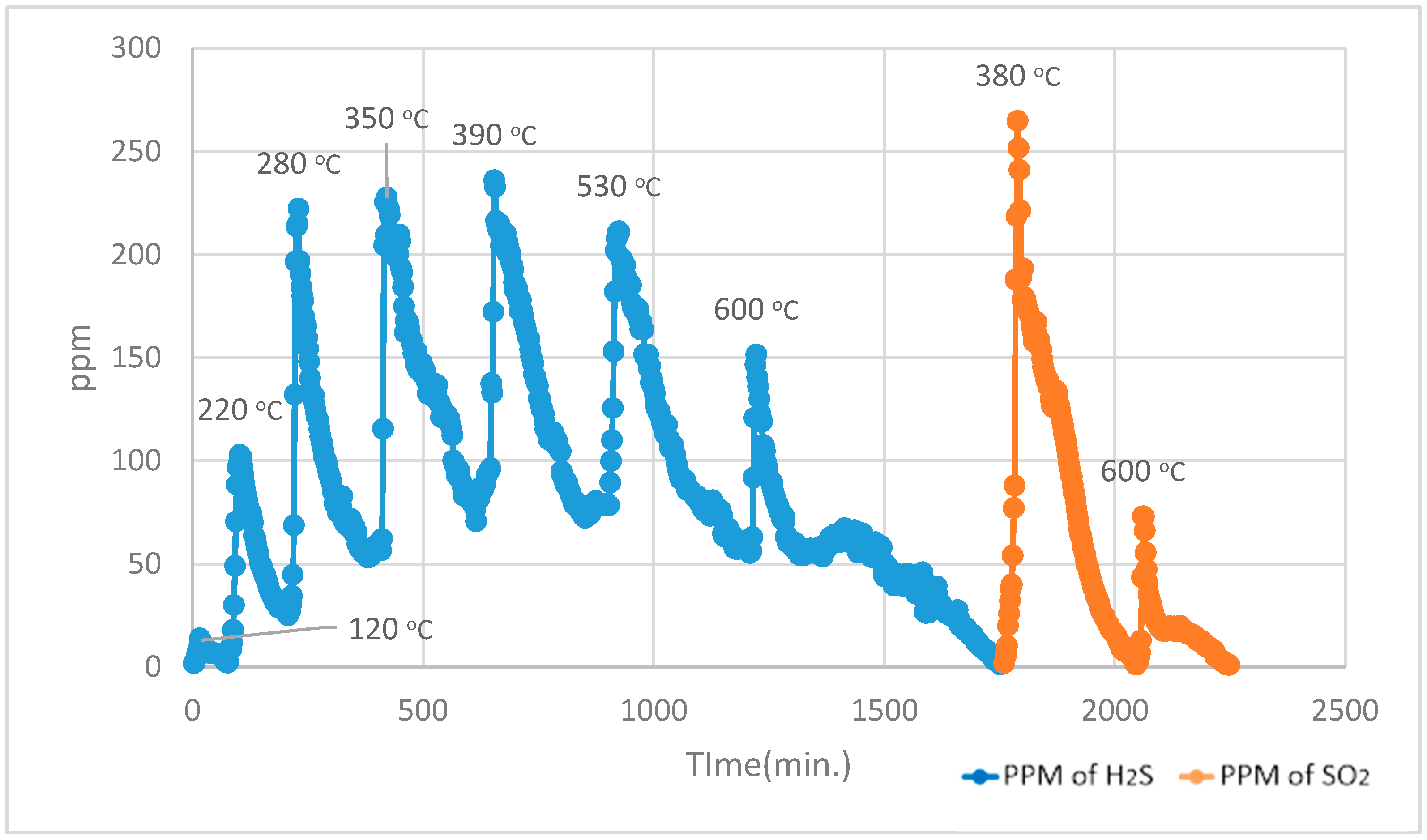
For the Temperature Programmed Identification (TPI) technique, the experimental run is split into two parts, i.e., normal Temperature Programmed Reduction (TPR) analysis and normal Temperature Programmed Oxidation (TPO) analysis in a sequence of TPO after TPR.
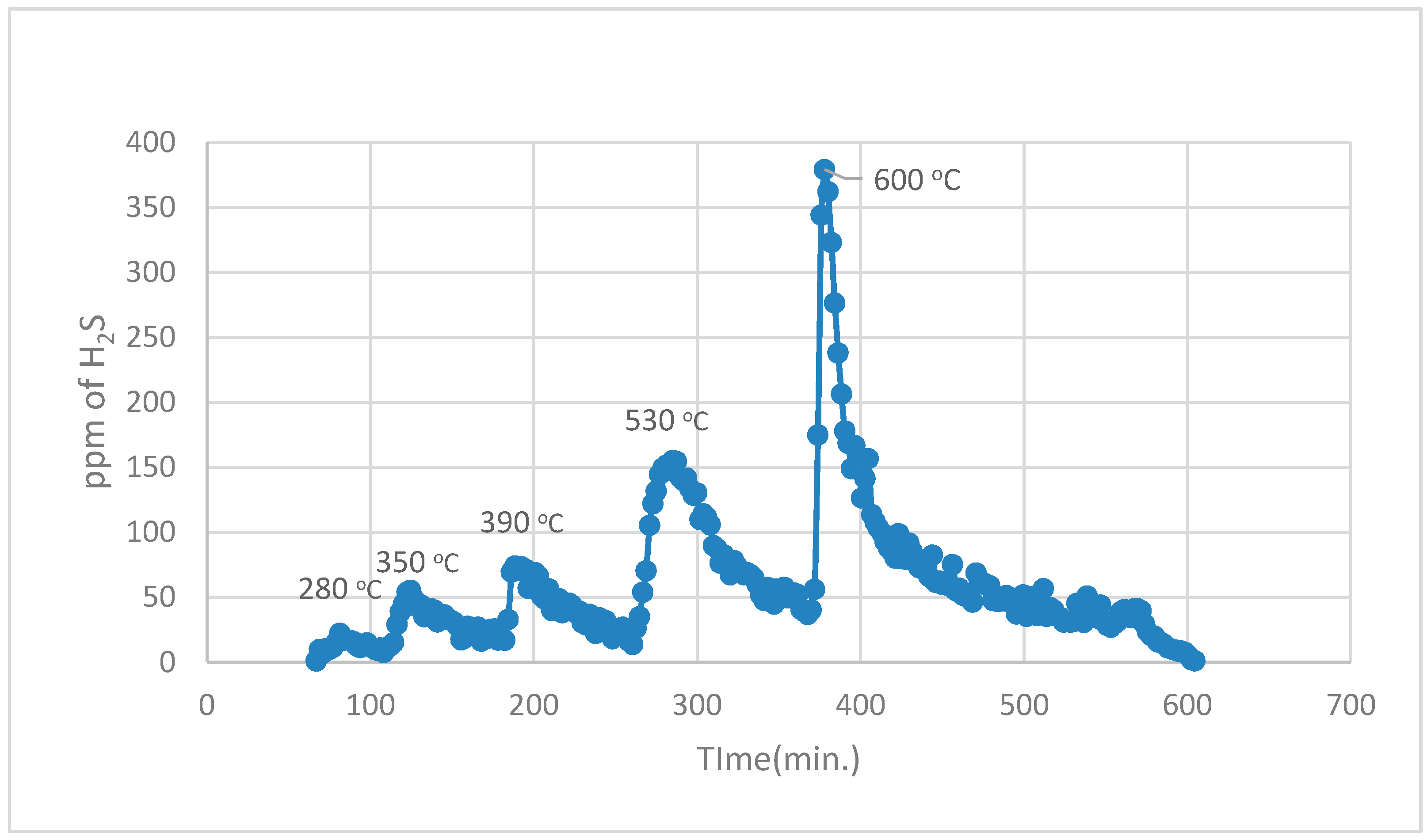
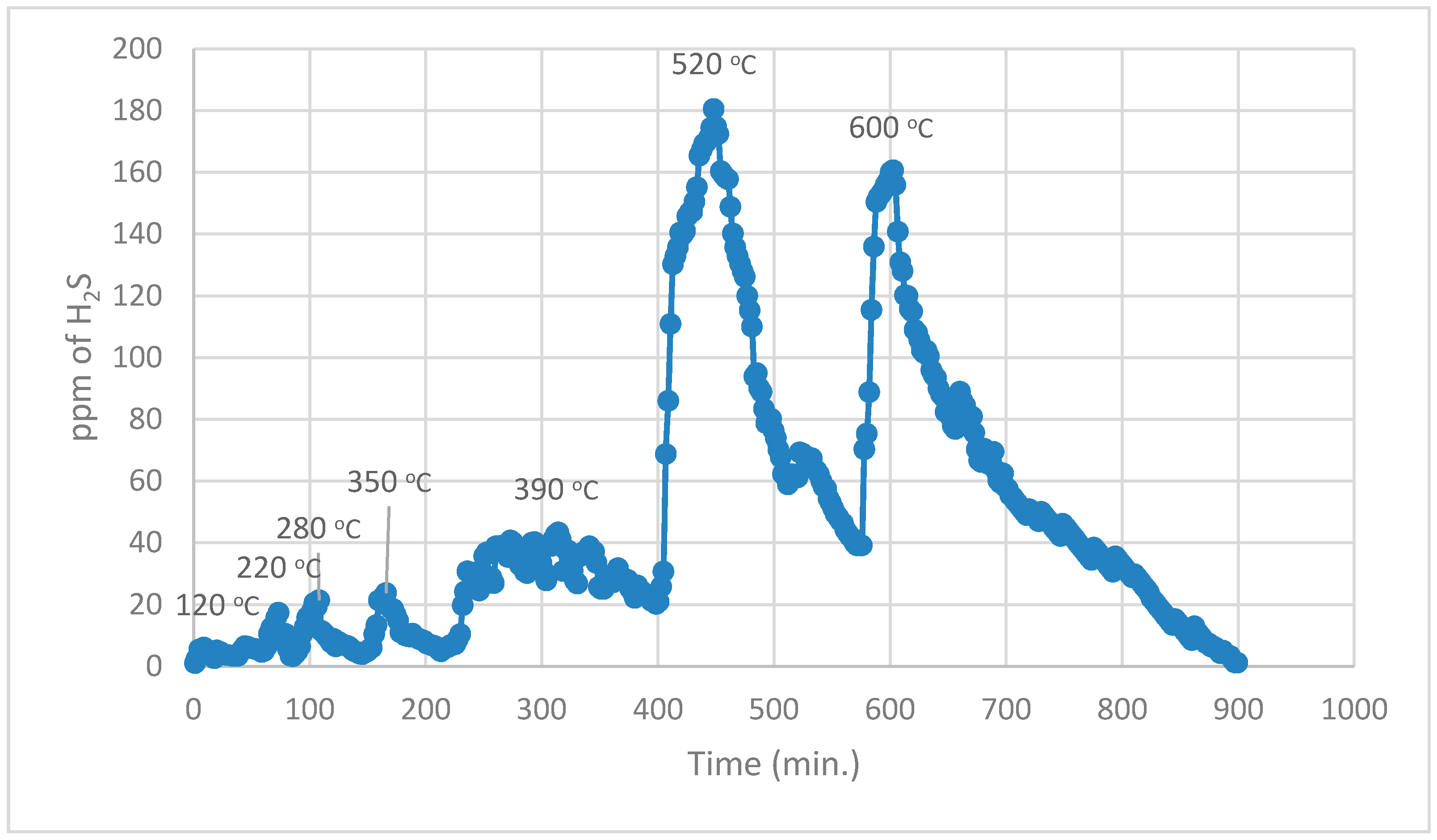
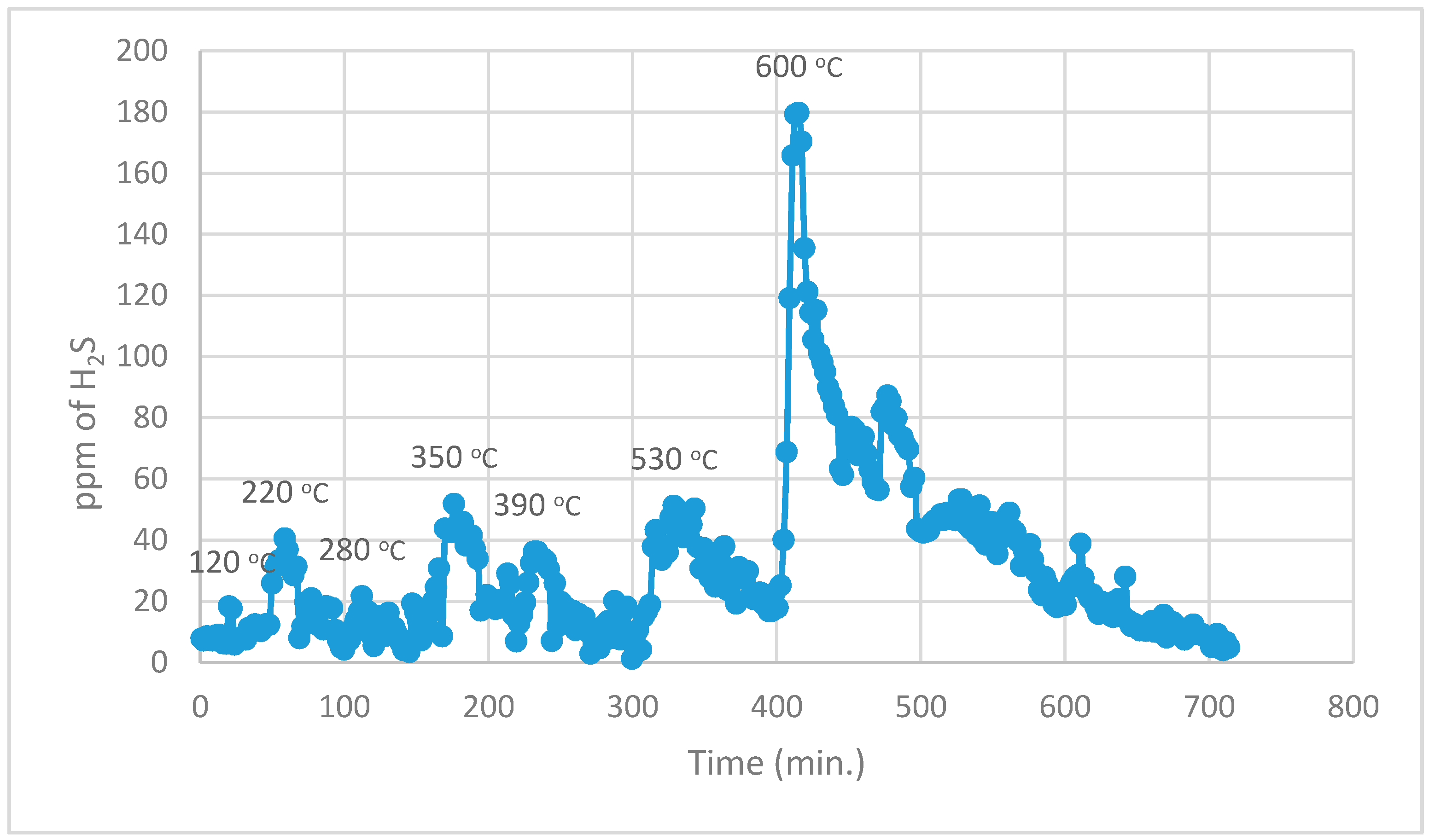
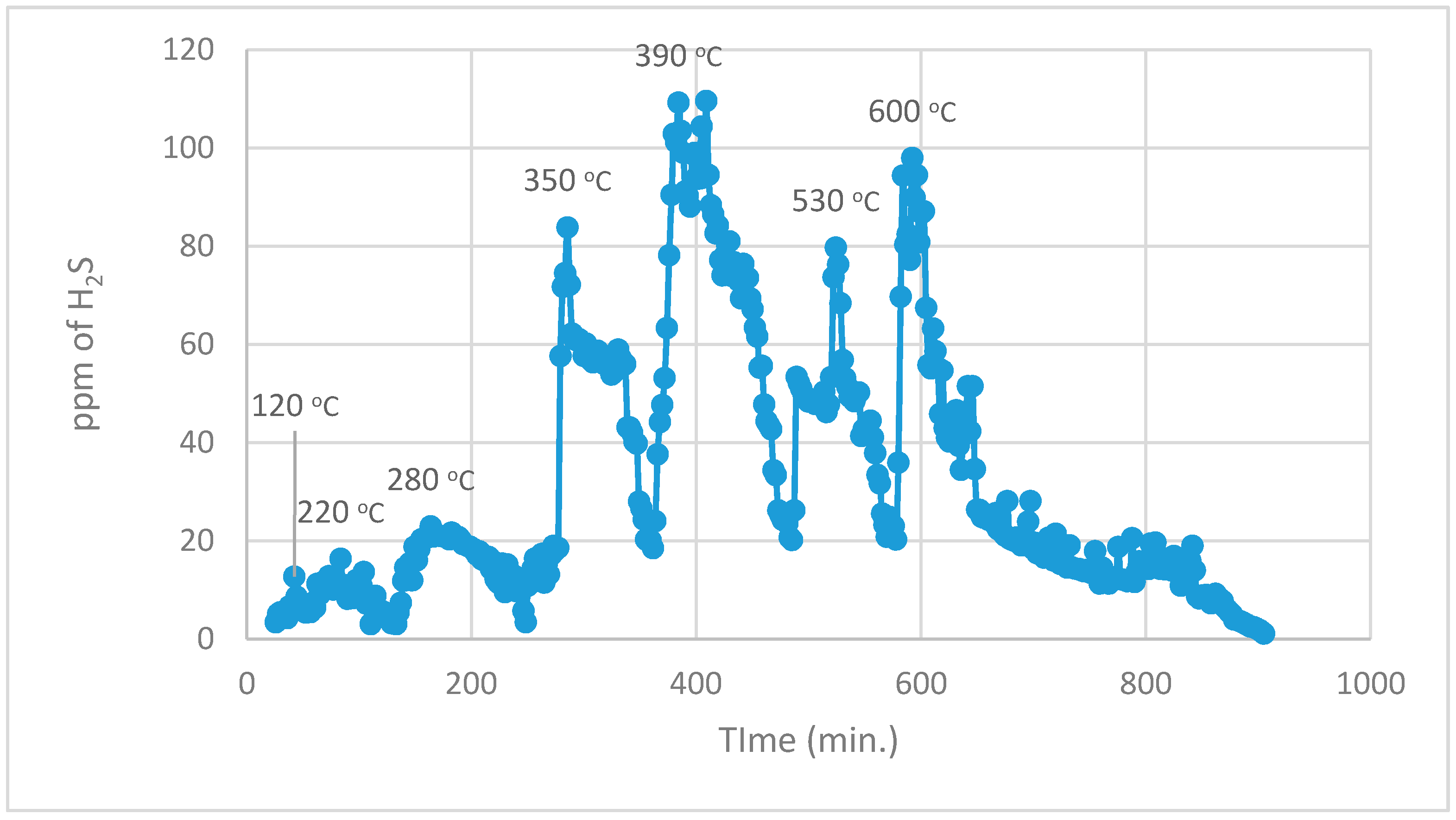
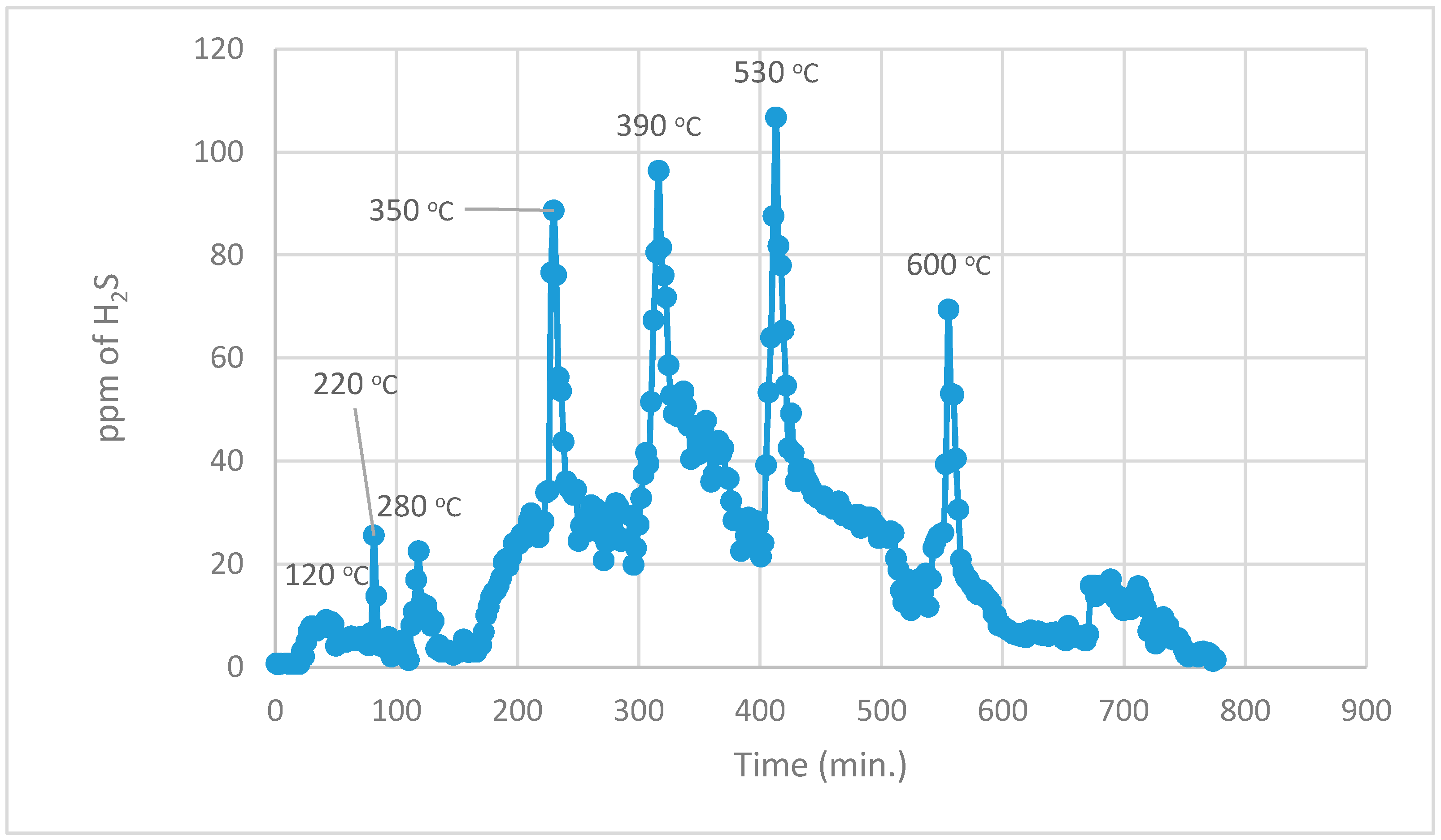
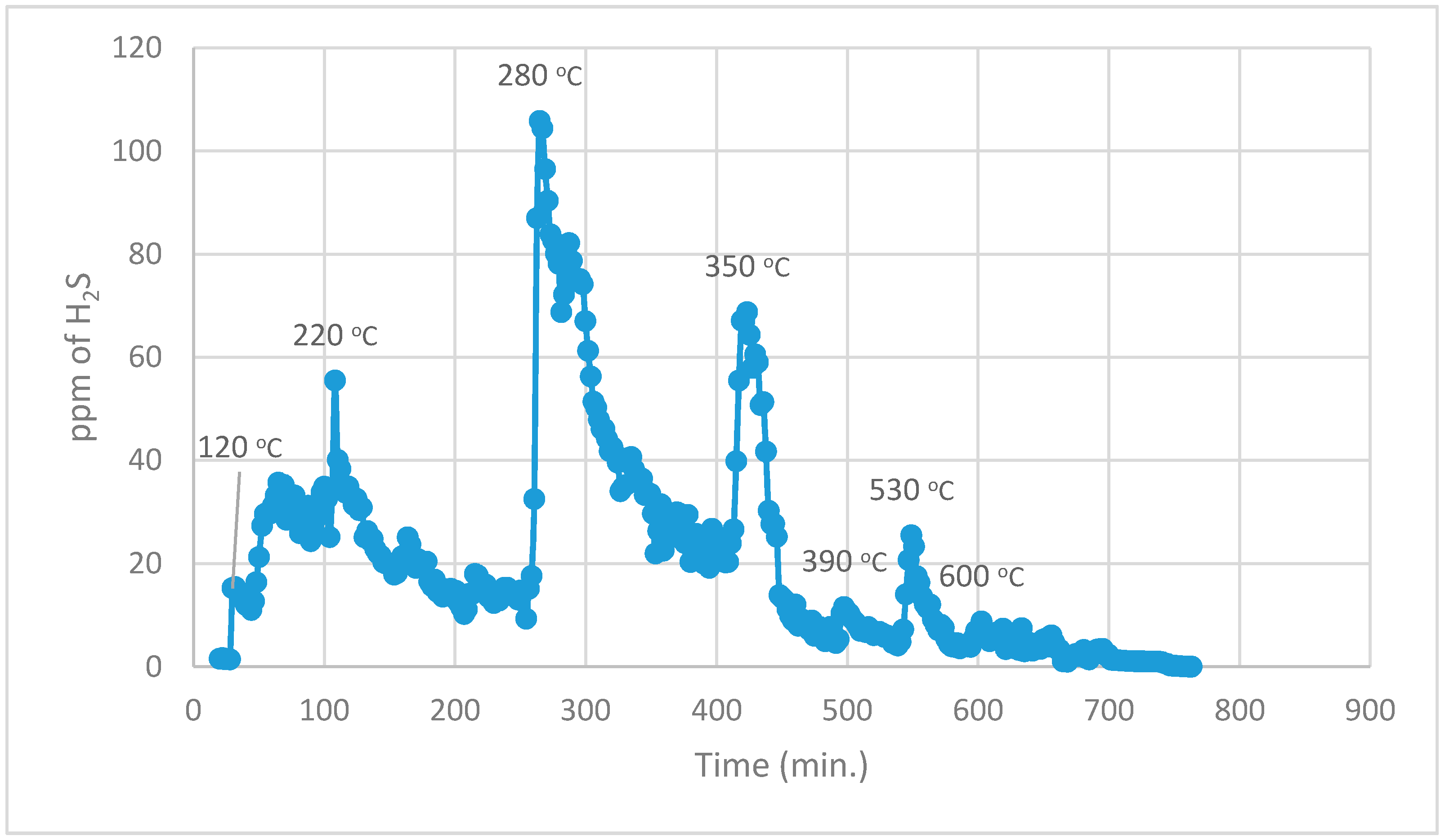
During the 1st part, using normal TPR analysis, identification of all the organosulphur species up to simple thiophenes. The simple thiophenes emit H
2S between 520–600 °C. After complete evolution of H
2S from simple thiophenes the furnace was cooled down to <200 °C. Then the 2nd part of the analytical run was started. It should be noted that at this stage there are only three main groups left behind i.e., pyrite, complex thiophenes and inorganic sulphates. The oxidation temperature ranges as found by [
16,
17,
18,
19,
20,
21,
22] for the evolution of SO
2 for these three groups are pyrite (250–380 °C), complex thiophenes (450–600 °C), and inorganic sulphates (above 650 °C), respectively.
4. Conclusions
The use of the TPI technique is quite straightforward and the mixing of different groups is negligible. The results on the basis of overall recovery are comparable with those obtained by the standard analytical techniques. The very slight difference could be due to the reason that the detection device in the case of the gas chromatograph is very sensitive. Above all the TPI technique is quite simple as it does not involve the use of very expensive and special machines like X-rays and synchrotrons, etc. Due to the non-availability of such equipment TPI can be used as a routine laboratory analysis technique for the complete characterisation of organic sulphur species in coal.
The major error observed with the technique was the less than 100% recovery of pyrite as sulphur dioxide due to the partial conversion to H2S. The other reason could be due to some conversion between functional groups. It was observed that a sample with high pyritic sulphur content the organic sulphur recovery was above 100%, whereas for a sample with lower pyritic sulphur content this was not observed.
The characterisation of various organic sulphur functional groups present in coal by the temperature programmed reduction method and later by the temperature programmed identification method confirmed that these methods can identify the organic sulphur groups in coal and that the results on the basis of total sulphur are comparable with standard analytical techniques. The analysis of the untreated and treated coal samples showed that the structural changes in the organic matrix due to a reaction can be determined.