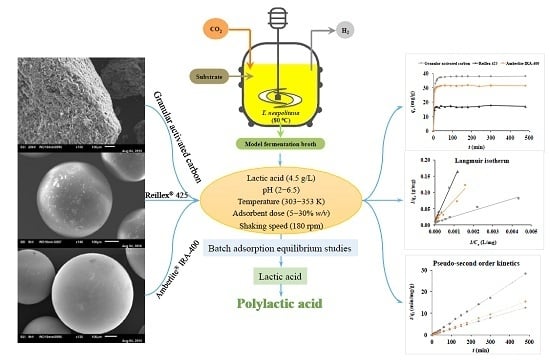
Adsorption Behaviour of Lactic Acid on Granular Activated Carbon and Anionic Resins: Thermodynamics, Isotherms and Kinetic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbents
2.2. Batch Adsorption Tests
2.3. Experimental Design
2.4. Analytical Methods
2.5. Lactic Acid Adsorption Isotherms
2.6. Lactic Acid Adsorption Kinetics
3. Results
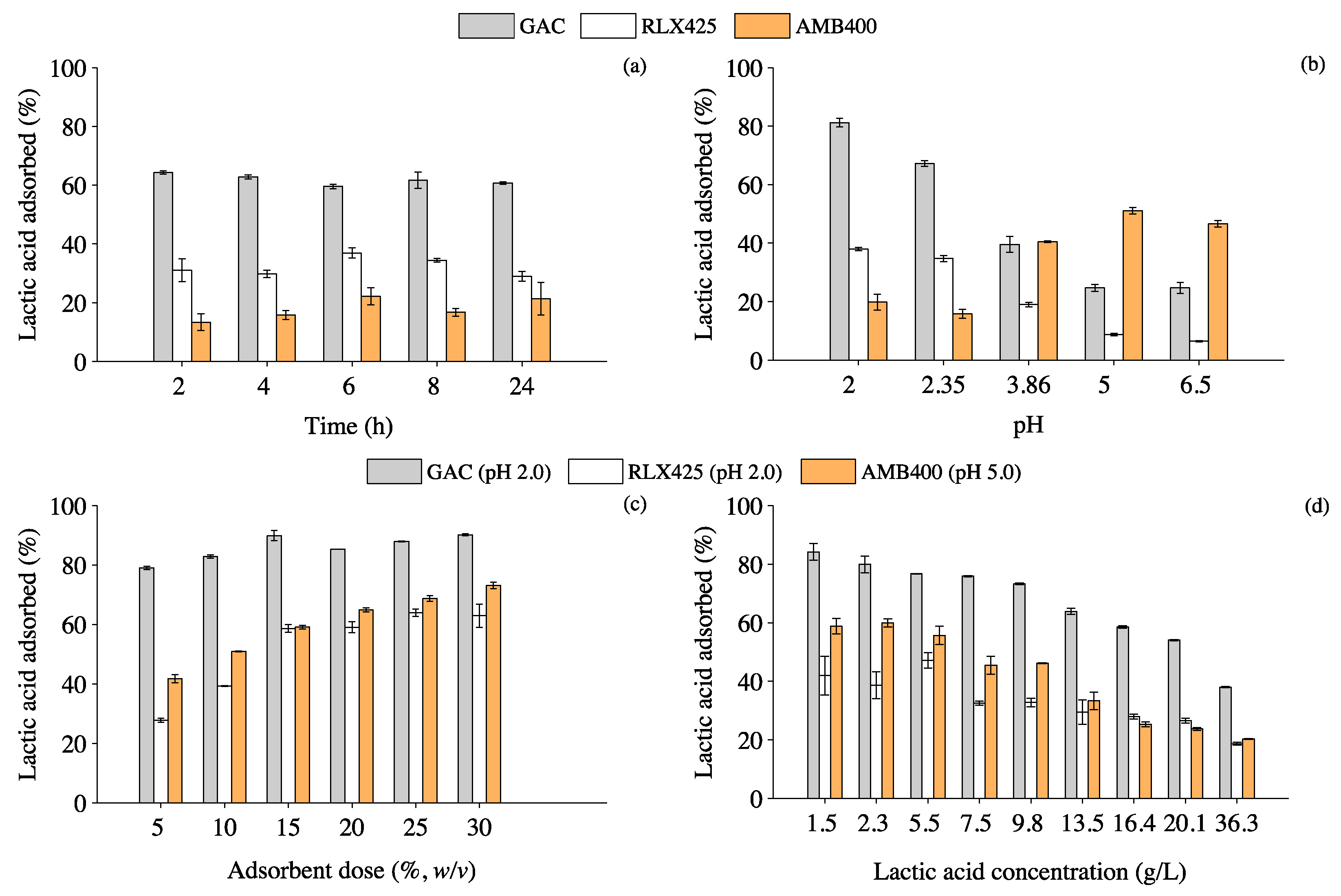
3.1. Effect of Contact Time, pH, Adsorbent Dose and Lactic Acid Concentration on Lactic Acid Adsorption
3.2. Thermodynamic Analysis and Effect of Temperature on Lactic acid Adsorption
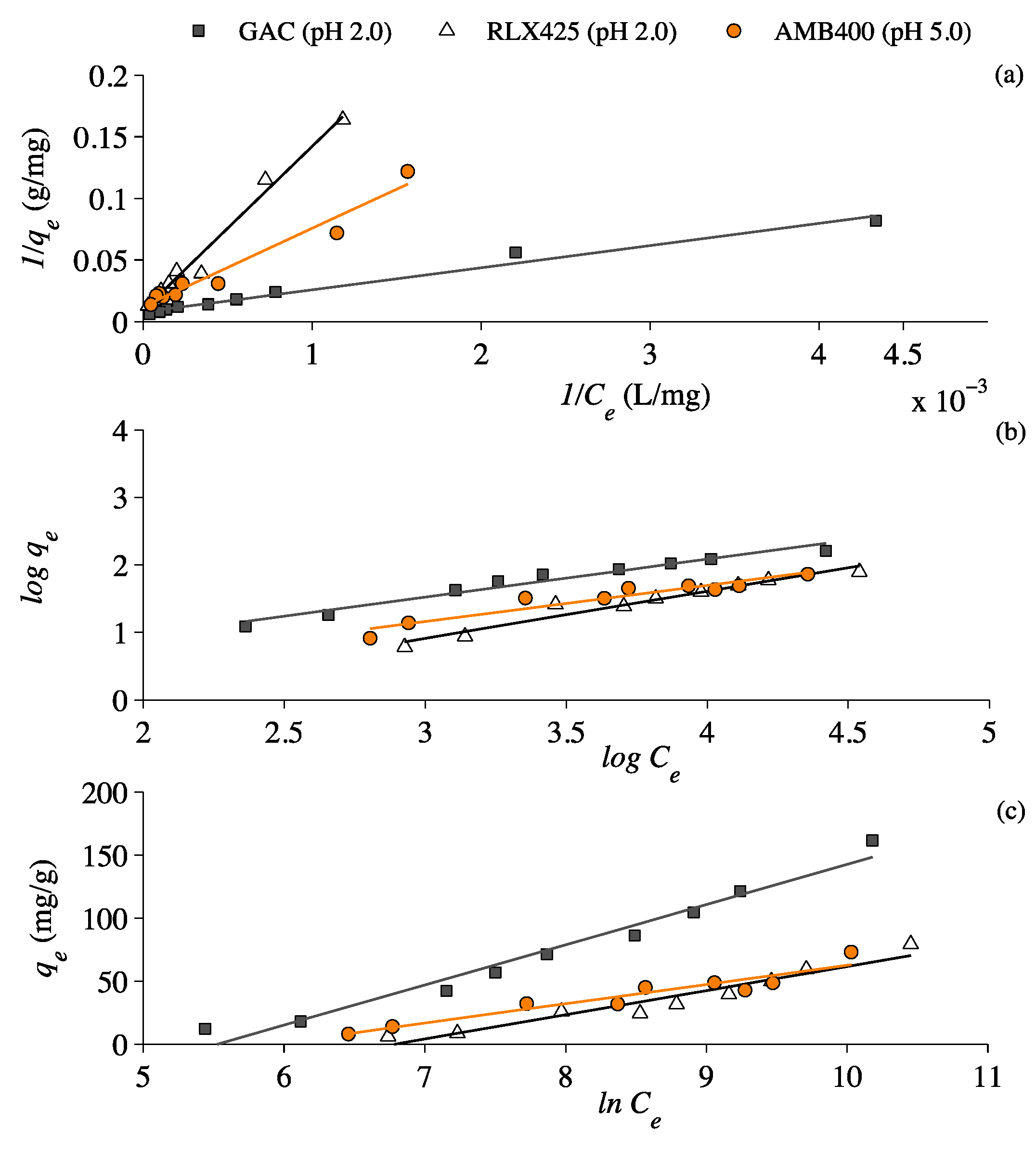
3.3. Lactic Acid Adsorption Isotherms
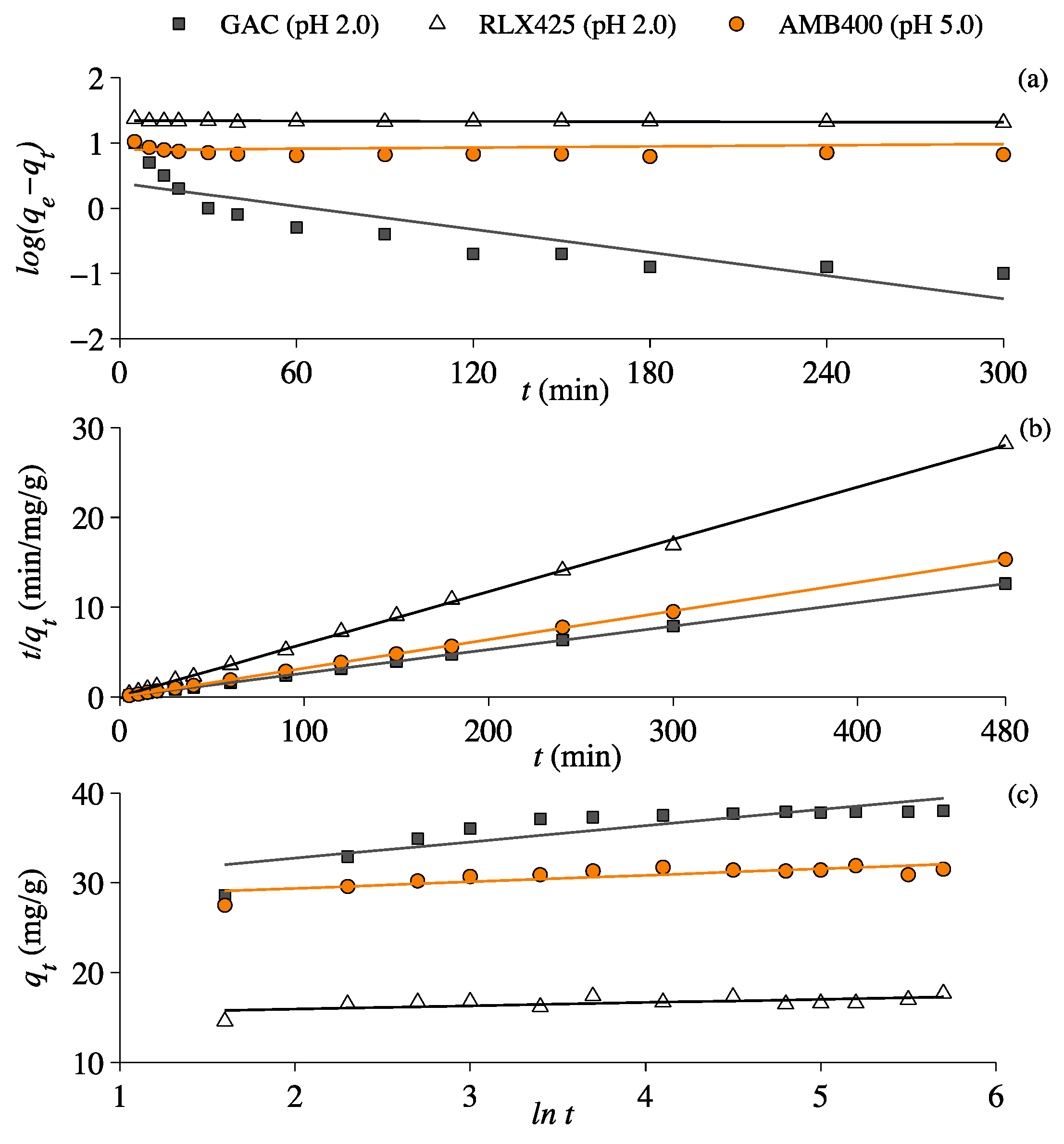
3.4. Lactic Acid Adsorption Kinetics
4. Discussion
4.1. Influence of Operating Parameters on the Adsorption of Lactic Acid onto GAC and Anionic Resins
4.1.1. Effect of pH and Adsorbent Dose on the Lactic Acid Adsorption Mechanisms
4.1.2. Effect of Temperature on Lactic Acid Adsorption
4.2. Thermodynamics Analysis of Lactic Acid Adsorption
4.3. Lactic Acid Adsorption Isotherms
4.4. Prospectives of Lactic Acid Adsorption
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hamad, K.; Kaseem, M.; Deri, F. Melt rheology of poly(lactic acid)/low density polyethylene polymer blends. Adv. Chem. Eng. Sci. 2011, 1, 208–214. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Rheological and mechanical properties of poly(lactic acid)/polystyrene polymer blend. Polym. Bull. 2010, 65, 509–519. [Google Scholar] [CrossRef]
- Bayazit, S.S.; Inci, I.; Uslu, H. Adsorption of lactic acid from model fermentation broth onto activated carbon and Amberlite IRA-67. J. Chem. Eng. Data 2011, 56, 1751–1754. [Google Scholar] [CrossRef]
- Joglekar, H.G.; Rahman, I.; Babu, S.; Kulkarni, B.D.; Joshi, A. Comparative assessment of downstream processing options for lactic acid. Sep. Purif. Technol. 2006, 52, 1–17. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; Gonzalez, J.M.D.; Converti, A.; Oliveira, R.P.S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Dipasquale, L.; D’Ippolito, G.; Fontana, A. Capnophilic lactic fermentation and hydrogen synthesis by Thermotoga neapolitana: An unexpected deviation from the dark fermentation model. Int. J. Hydrogen Energy 2014, 39, 4857–4862. [Google Scholar] [CrossRef]
- Pradhan, N.; Dipasquale, L.; D’Ippolito, G.; Fontana, A.; Panico, A.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Model development and experimental validation of capnophilic lactic fermentation and hydrogen synthesis by Thermotoga neapolitana. Water Res. 2016, 99, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Dipasquale, L.; D’Ippolito, G.; Fontana, A.; Panico, A.; Lens, P.N.L.; Pirozzi, F.; Esposito, G. Kinetic modeling of fermentative hydrogen production by Thermotoga neapolitana. Int. J. Hydrogen Energy 2016, 41, 4931–4940. [Google Scholar] [CrossRef]
- Pradhan, N.; Dipasquale, L.; D’Ippolito, G.; Panico, A.; Lens, P.N.L.; Esposito, G.; Fontana, A. Hydrogen production by the thermophilic bacterium Thermotoga neapolitana. Int. J. Mol. Sci. 2015, 16, 12578–12600. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.P.; Coelho, L.F.; Sass, D.C.; Contiero, J. L-(+)Lactic acid production by Lactobacillus rhamnosus B103 from dairy industry waste. Braz. J. Microbiol. 2016, 47, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Akase, S.; Nakanishi, R.; Kaneko, Y.; Harashima, S. Overexpression of ESBP6 improves lactic acid resistance and production in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2016, 122, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Wasewar, K.L. Separation of lactic acid: Recent advances. Chem. Biochem. Eng. Q. 2005, 19, 159–172. [Google Scholar]
- Dethe, M.J.; Marathe, K.V.; Gaikar, V.G. Adsorption of lactic acid on weak base polymeric resins. Sep. Sci. Technol. 2006, 41, 2947–2971. [Google Scholar] [CrossRef]
- Yi, S.S.; Lu, Y.C.; Luo, G.S. Separation and concentration of lactic acid by electro-electrodialysis. Sep. Purif. Technol. 2008, 60, 308–314. [Google Scholar] [CrossRef]
- Takatsuji, W.; Nakauchi, M.; Yoshida, H. Removal of salt and organic acids from solution used to season salted Japanese apricots (ume) by electrodialysis, precipitation and adsorption. J. Biosci. Bioeng. 1999, 88, 348–351. [Google Scholar] [CrossRef]
- Evangelista, R.L.; Mangold, A.J.; Nikolov, Z.L. Recovery of lactic acid by sorption: Resin evaluation. Appl. Biochem. Biotechnol. 1994, 45–46, 131–144. [Google Scholar] [CrossRef]
- Moldes, A.B.; Alonso, J.L.; Parajo, J.C. Recovery of lactic acid from simultaneous saccharification and fermentation media using anion exchange resins. Bioprocess Biosyst. Eng. 2003, 25, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, A.; Bonk, F.; Oyanedel, J.R.B.; Schmidt, J.E. Recovery of carboxylic acids produced during dark fermentation of food waste by adsorption on Amberlite IRA-67 and activated carbon. Bioresour. Technol. 2016, 217, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.Y.; Fu, X.Y.; Lee, S.M.; Yu, J.; Liu, J.W.; Wei, D.W.; Koo, Y.M. Purification of L-(+)lactic acid from fermentation broth with paper sludge as a cellulosic feedstock using weak anion exchanger Amberlite IRA-92. Biochem. Eng. J. 2004, 18, 89–96. [Google Scholar] [CrossRef]
- Cao, X.; Yun, H.S.; Koo, Y.M. Recovery of L-(+)lactic acid by anion exchange resin Amberlite IRA-400. Biochem. Eng. J. 2002, 11, 189–196. [Google Scholar] [CrossRef]
- Antonio, G.R.; Vaccari, G.; Dosi, E.; Trilli, A.; Rossi, M.; Matteuzzi, D. Enhanced production of L-(+)lactic acid in chemostat by Lactobacillus casei DSM 20011 using ion-exchange resins and cross-flow filtration in a fully automated pilot plant controlled via NIR. Biotechnol. Bioeng. 2000, 67, 147–156. [Google Scholar]
- Gao, M.T.; Shimamura, T.; Ishida, N.; Takahashi, H. pH-uncontrolled lactic acid fermentation with activated carbon as an adsorbent. Enzym. Microb. Technol. 2011, 48, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.R.; Amarasiriwardena, G.S.; Prather, K.L.J. Predicting the adsorption of second generation biofuels by polymeric resins with applications for in situ product recovery (ISPR). Bioresour. Technol. 2010, 101, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Nam, H.G.; Lee, K.B.; Mun, S. Adsorption behaviors of sugars and sulfuric acid on activated porous carbon. J. Ind. Eng. Chem. 2016, 34, 21–26. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, F.; Zhang, T.; Zhang, J.; Jia, S.; Yu, C.; Jiang, K.; Gao, N. The role of lactic acid adsorption by ion exchange chromatography. PLoS ONE 2010, 5, e13948. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, F.; Wu, W.; Bu, R.; Li, W.; Yang, F. Reactive orange 5 removal from aqueous solution using hydroxyl ammonium ionic liquids/layered double hydroxides intercalation composites. Chem. Eng. J. 2016, 285, 198–206. [Google Scholar] [CrossRef]
- Rich, W.; Johnson, E.; Lois, L.; Kabra, P.; Stafford, B.; Marton, L. Determination of organic acids in biological fluids by ion chromatography: Plasma lactate and pyruvate and urinary vanillylmandelic acid. Clin. Chem. 1980, 26, 1492–1498. [Google Scholar] [PubMed]
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S.; Martins, A.C.; Silva, T.L.; Junior, O.O.S.; Visentainer, J.V.; Almeida, V.C. NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2016, 288, 778–788. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Webber, T.W.; Chakkravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Chen, X. Modeling of experimental adsorption isotherm data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef]
- Uslu, H. Adsorption equilibria of formic acid by weakly basic adsorbent Amberlite IRA-67: Equilibrium, kinetics, thermodynamic. Chem. Eng. J. 2009, 155, 320–325. [Google Scholar] [CrossRef]
- Shi, Y.; Kong, X.; Zhang, C.; Chen, Y.; Hua, Y. Adsorption of soy isoflavones by activated carbon: Kinetics, thermodynamics and influence of soy oligosaccharides. Chem. Eng. J. 2013, 215–216, 113–121. [Google Scholar] [CrossRef]
- Thang, V.H.; Novalin, S. Green biorefinery: Separation of lactic acid from grass silage juice by chromatography using neutral polymeric resin. Bioresour. Technol. 2008, 99, 4368–4379. [Google Scholar] [CrossRef] [PubMed]
- Quintero, J.; Acosta, A.; Mejıa, C.; Rios, R.; Torres, M. Purification of lactic acid obtained from a fermentative process of cassava syrup using ion exchange resins. Rev. Fac. Ing.-Univ. Antioq. 2016, 65, 139–151. [Google Scholar]
- Bishai, M.; De, S.; Adhikari, B.; Banerjee, R. A platform technology of recovery of lactic acid from a fermentation broth of novel substrate Zizyphus oenophlia. 3 Biotech 2015, 5, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Sodsai, W.; Sookkumnerd, T. Modeling of lactic acid adsorption isotherm by anion exchange resin Amberlite IRA-96. KMITL Sci. Technol. J. 2013, 13, 82–86. [Google Scholar]
- Zhou, J.; Wu, J.; Liu, Y.; Zou, F.; Wu, J.; Li, K.; Chen, Y. Modeling of breakthrough curves of single and quaternary mixtures of ethanol, glucose, glycerol and acetic acid adsorption onto a microporous hyper-cross-linked resin. Bioresour. Technol. 2013, 143, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Rampai, T.; Thitiprasert, S.; Boonkong, W.; Kodama, K.; Tolieng, V.; Thongchul, N. Improved lactic acid productivity by simultaneous recovery during fermentation using resin exchanger. Asia-Pac. J. Sci. Technol. 2016, 21, 193–199. [Google Scholar]
- Aljundi, I.H.; Belovich, J.M.; Talu, O. Adsorption of lactic acid from fermentation broth and aqueous solutions on zeolite molecular sieves. Chem. Eng. Sci. 2005, 60, 5004–5009. [Google Scholar] [CrossRef]
- Ye, C.; Wang, X.; Wang, H.; Wang, Z. Effects of counter anions on the adsorption properties of 4-methylimidazolium-modified silica materials. J. Taiwan Inst. Chem. Eng. 2014, 45, 2868–2877. [Google Scholar] [CrossRef]
- Farhadpour, F.A.; Bono, A. Sorptive separation of ethanol-water mixtures with a bi- dispersed hydrophobic molecular sieve, silicalite: Determination of the controlling mass transfer mechanism. Chem. Eng. Process. 1996, 35, 141–155. [Google Scholar] [CrossRef]
- Lian, L.; Guo, L.; Guo, C. Adsorption of Congo red from aqueous solutions onto Ca-bentonite. J. Hazard. Mater. 2009, 161, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kolodynska, D. Adsorption characteristics of chitosan modified by chelating agents of a new generation. Chem. Eng. J. 2012, 179, 33–43. [Google Scholar] [CrossRef]
- Da Silva, A.H.; Miranda, A.E. Adsorption/desorption of organic acids onto different adsorbents for their recovery from fermentation broths. J. Chem. Eng. Data 2013, 58, 1454–1463. [Google Scholar] [CrossRef]

| Isotherms | Non-Linear Form | Linear Form | Plot | Equation |
|---|---|---|---|---|
| Langmuir | (I) | |||
| Freundlich | (II) | |||
| Temkin | (III) |
| Kinetic Model | Differential Form | Linear Form | Plot | Equation |
|---|---|---|---|---|
| Pseudo-First Order | (A) | |||
| Pseudo-Second Order | (B) | |||
| Elovich | (C) |
| Adsorbents | GAC | RLX425 | AMB400 |
|---|---|---|---|
| Temperature (K) | ΔG0 (kJ/mol) | ΔG0 (kJ/mol) | ΔG0 (kJ/mol) |
| 303.15 | −10.9 | −4.5 | −5.5 |
| 313.15 | −10.7 | −4.0 | −5.8 |
| 323.15 | −10.5 | −3.5 | −6.0 |
| 333.15 | −10.4 | −3.0 | −6.3 |
| 343.15 | −10.2 | −2.4 | −6.5 |
| 353.15 | −9.9 | −1.9 | −6.8 |
| Isotherms | Coefficients | Units | GAC | RLX425 | AMB400 |
|---|---|---|---|---|---|
| Langmuir | qm | mg/g | 126.6 | 108.7 | 63.5 |
| KL | L/mg | 4.4 × 10−4 | 6.9 × 10−5 | 1.9 × 10−4 | |
| R2 | - | 0.98 | 0.98 | 0.96 | |
| Freundlich | n | - | 1.8 | 1.4 | 1.9 |
| KF | mg/g (L/g)n | 1.2 | 3.3 | 1.6 | |
| R2 | - | 0.96 | 0.95 | 0.90 | |
| Temkin | AT | L/g | 251.7 | 887.6 | 371.3 |
| KT | - | 31.9 | 19.2 | 15.3 | |
| R2 | - | 0.97 | 0.93 | 0.91 |
| Kinetics | Coefficients/Constants | Units | GAC | RLX425 | AMB400 |
|---|---|---|---|---|---|
| Pseudo-Second Order | qe | mg/g | 38.2 | 17.2 | 31.2 |
| K2 | mg/g/min | 0.02 | 0.05 | 0.15 | |
| ho | min−1 | 31.5 | 13.6 | 142.9 | |
| R2 | - | 1 | 0.99 | 0.99 |
| Resin/Adsorbent | Initial pH | Temperature (K) | Initial Lactic Acid Concentration (g/L) | qe (mg Lactic Acid/g Adsorbent) | E (%) | References |
|---|---|---|---|---|---|---|
| GAC | 2 | 303.15 | 50 | 175 | 35 | [22] |
| 2 | 298.15 | 7 | 48 | 69 | [18] | |
| 2 | 303.15 | 22 | 121 | 54 | This study | |
| 2 | 303.15 | 4.8 | 38 | 81 | This study | |
| 2 | 353.15 | 4.7 | 35 | 76 | This study | |
| RLX425 | 2.83 | 303.15 | 5.7 | 32 | 56 | [16] |
| 2 | 303.15 | 22 | 60 | 27 | This study | |
| 2 | 303.15 | 4.8 | 17 | 35 | This study | |
| 2 | 353.15 | 4.7 | 9 | 18 | This study | |
| AMB400 | 4 | 303.15 | 100 | 350 | 35 | [39] |
| 5 | 298.15 | 88.7 | 197 | 22 | [20] | |
| 4.85 | 303.15 | 60 | 160 | 27 | [17] | |
| 5 | 298.15 | 34 | 107 | 31 | [35] | |
| 5 | 303.15 | 18 | 48 | 24 | This study | |
| 5 | 303.15 | 5.4 | 31 | 57 | This study | |
| 5 | 353.15 | 4.7 | 25 | 52 | This study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, N.; Rene, E.R.; Lens, P.N.L.; Dipasquale, L.; D’Ippolito, G.; Fontana, A.; Panico, A.; Esposito, G. Adsorption Behaviour of Lactic Acid on Granular Activated Carbon and Anionic Resins: Thermodynamics, Isotherms and Kinetic Studies. Energies 2017, 10, 665. https://doi.org/10.3390/en10050665
Pradhan N, Rene ER, Lens PNL, Dipasquale L, D’Ippolito G, Fontana A, Panico A, Esposito G. Adsorption Behaviour of Lactic Acid on Granular Activated Carbon and Anionic Resins: Thermodynamics, Isotherms and Kinetic Studies. Energies. 2017; 10(5):665. https://doi.org/10.3390/en10050665
Chicago/Turabian StylePradhan, Nirakar, Eldon R. Rene, Piet N. L. Lens, Laura Dipasquale, Giuliana D’Ippolito, Angelo Fontana, Antonio Panico, and Giovanni Esposito. 2017. "Adsorption Behaviour of Lactic Acid on Granular Activated Carbon and Anionic Resins: Thermodynamics, Isotherms and Kinetic Studies" Energies 10, no. 5: 665. https://doi.org/10.3390/en10050665