Comparison of Biodiesel Obtained from Virgin Cooking Oil and Waste Cooking Oil Using Supercritical and Catalytic Transesterification
Abstract
:1. Introduction
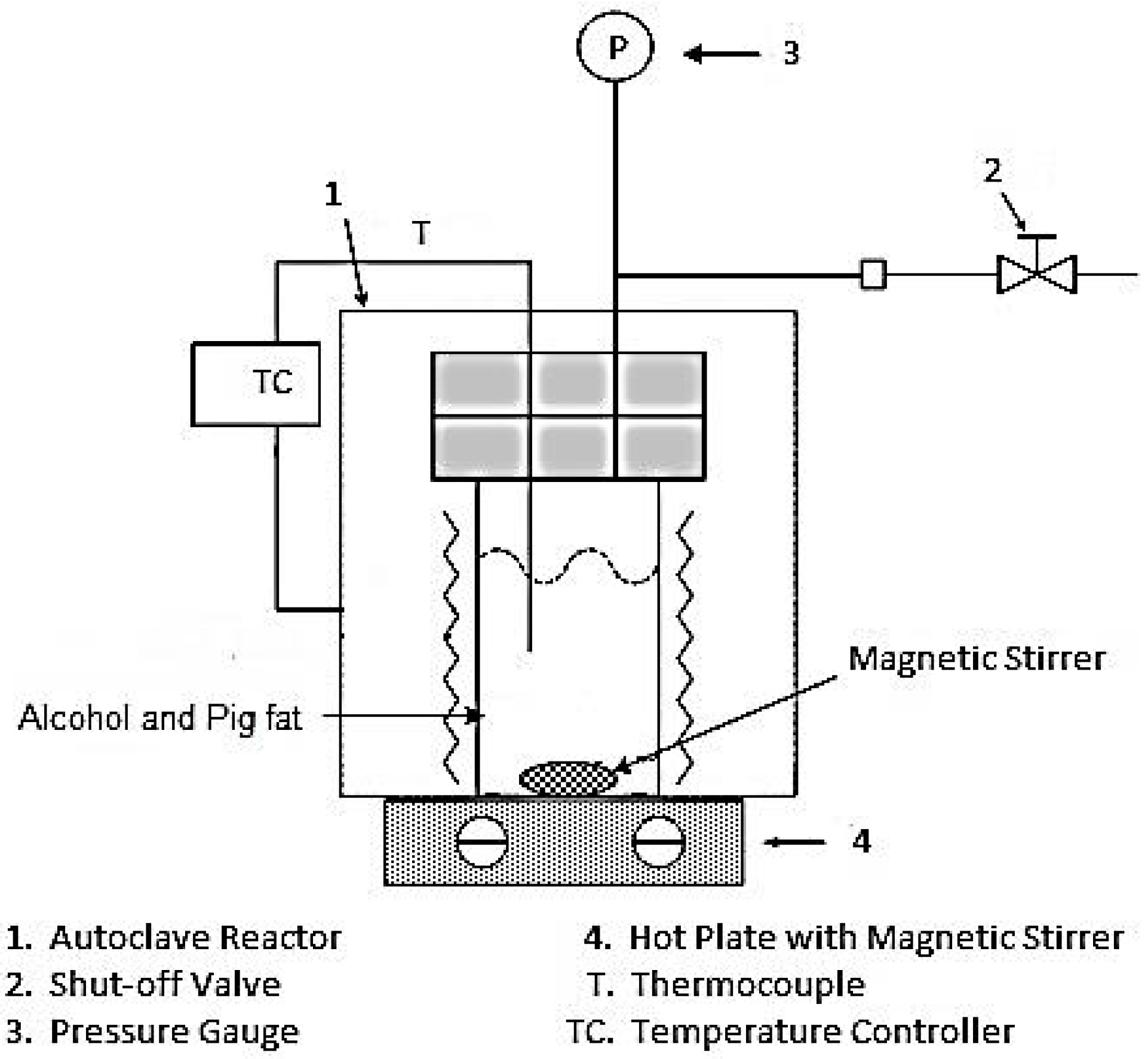
2. Experimental Section
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Jayed, M.; Masjuki, H.; Saidur, R.; Kalam, M.; Jahirul, M.I. Environmental aspects and challenges of oilseed produced biodiesel in Southeast Asia. Renew. Sustain. Energy Rev. 2009, 13, 2452–2462. [Google Scholar] [CrossRef]
- Murugesan, A.; Umarani, C.; Subramanian, R.; Nedunchezhian, N. Bio-diesel as an alternative fuel for diesel engines—A review. Renew. Sustain. Energy Rev. 2009, 13, 653–662. [Google Scholar] [CrossRef]
- Macor, A.; Pavanello, P. Performance and emissions of biodiesel in a boiler for residential heating. Energy 2009, 34, 2025–2032. [Google Scholar] [CrossRef]
- Roy, M.M.; Wang, W.; Bujold, J. Biodiesel production and comparison of emissions of a DI diesel engine fueled by biodiesel–diesel and canola oil–diesel blends at high idling operations. Appl. Energy 2013, 106, 198–208. [Google Scholar] [CrossRef]
- Roy, M.M.; Calder, J.; Wang, W.; Mangad, A.; Diniz, F.C.M. Emission analysis of a modern Tier 4 DI diesel engine fueled by biodiesel-diesel blends with a cold flow improver (Wintron Synergy) at multiple idling conditions. Appl. Energy 2016, 179, 45–54. [Google Scholar] [CrossRef]
- Roy, M.M.; Calder, J.; Wang, W.; Mangad, A.; Diniz, F.C.M. Cold start idle emissions from a modern Tier-4 turbo-charged diesel engine fueled with diesel-biodiesel, diesel-biodiesel-ethanol, and diesel-biodiesel-diethyl ether blends. Appl. Energy 2016, 180, 52–65. [Google Scholar] [CrossRef]
- He, B. Advances in emission characteristics of diesel engines using different biodiesel fuels. Renew. Sustain. Energy Rev. 2016, 60, 570–586. [Google Scholar] [CrossRef]
- Nalgundwar, A.; Paul, B.; Sharma, S.K. Comparison of performance and emissions characteristics of DI CI engine fueled with dual biodiesel blends of palm and jatropha. Fuel 2016, 173, 172–179. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; Rodriguez-Fernandez, J. Effect of biodiesel fuels on diesel engine emissions. Prog. Energy Combust. Sci. 2008, 34, 198–223. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Kim, M.S.; Choi, N.J. Application of Canola Oil Biodiesel/Diesel Blends in a Common Rail Diesel Engine. Appl. Sci. 2016, 7, 34. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, M.; McLean, D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [PubMed]
- Marchetti, J.; Miguel, V.; Errazu, A. Possible methods for biodiesel production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Primata, M.; Seo, Y.C.; Chu, Y.H. Effect of alkali catalyst on biodiesel production in South Korea from mixtures of fresh soybean oil and waste cooking oil. J. Mater. Cycles Waste Manag. 2013, 15, 223–228. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.; Liu, D.; Zeng, J. Comparative study on lipase-catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J. Mol. Catal. B Enzym. 2004, 30, 125–129. [Google Scholar] [CrossRef]
- Oda, M.; Kaieda, M.; Hama, S.; Yamaji, H.; Kondo, A.; Izumoto, E.; Fukuda, H. Facilitatory effect of immobilized lipase-producing Rhizopus oryzae cells on acyl migration in biodiesel-fuel production. Biochem. Eng. J. 2005, 23, 45–51. [Google Scholar] [CrossRef]
- Noureddini, H.; Gao, X.; Philkana, R. Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresour. Technol. 2005, 96, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Yucel, Y.; Tekeli, Y. Biodiesel production from canola oil using immobilized lipase. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2011, 27, 337–346. [Google Scholar]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Shah, M.; Poudel, J.; Kwak, H.; Oh, S.C. Kinetic analysis of transesterification of waste pig fat in supercritical alcohols. Process Saf. Environ. Prot. 2015, 98, 239–244. [Google Scholar] [CrossRef]
- Poudel, J.; Shah, M.; Karki, S.; Oh, S.C. Qualitative Analysis of Transesterification of Waste Pig Fat in Supercritical Alcohols. Energies 2017, 10, 265. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel production from high FFA rubber seed oil. Fuel 2005, 84, 335–340. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Optimization of pretreatment reaction for methyl ester production from chicken fat. Fuel 2010, 89, 4035–4039. [Google Scholar] [CrossRef]
- Farag, H.; El-Maghraby, A.; Taha, N.A. Optimization of factors affecting esterification of mixed oil with high percentage of free fatty acid. Fuel Process. Technol. 2011, 92, 507–510. [Google Scholar] [CrossRef]
- Sanjel, N.; Gu, J.H.; Oh, S.C. Transesterification Kinetics of Waste Vegetable Oil in Supercritical Alcohols. Energies 2014, 7, 2095–2106. [Google Scholar] [CrossRef]
- Meher, L.; Sagar, D.V.; Naik, S. Technical aspects of biodiesel production by transesterification—a review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Felizardo, P.; Correia, M.J.N.; Raposo, I.; Mendes, J.F.; Berkemeier, R.; Bordado, J.M. Production of biodiesel from waste frying oils. Waste Manag. 2006, 26, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, A.S.; Sarma, A. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous catalysts for biodiesel production. Energy Fuels 2007, 22, 207–217. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Reyes-Labarta, J.A.; Valdes, F.J. New Heterogeneous Catalytic Transesterification of Vegetable and Used Frying Oil. Ind. Eng. Chem. Res. 2010, 49, 9068–9076. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T. A review on supercritical fluids (SCF) technology in sustainable biodiesel production: Potential and challenges. Renew. Sustain. Energy Rev. 2011, 15, 2452–2456. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Huaping, Z.; Zongbin, W.; Yuanxiong, C.; Zhang, P.; Shijie, D.; Xiaohua, L.; Zongqiang, M. Preparation of biodiesel catalyzed by solid super base of calcium oxide and its refining process. Chin. J. Catal. 2006, 27, 391–396. [Google Scholar]
- Marrone, P.A.; Hodes, M.; Smith, K.A.; Tester, J.W. Salt precipitation and scale control in supercritical water oxidation—part B: Commercial/full-scale applications. J. Supercrit. Fluids 2004, 29, 289–312. [Google Scholar] [CrossRef]
- Sirisomboonchai, S.; Abuduwayiti, M.; Guan, G.; Samart, C.; Abliz, S.; Hao, X.; Kusakabe, K.; Abudula, A. Biodiesel production from waste cooking oil using calcined scallop shell as catalyst. Energy Convers. Manag. 2015, 95, 242–247. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J. Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Mahesh, S.E.; Ramanathan, A.; Begum, K.M.S.; Narayanan, A. Biodiesel production from waste cooking oil using KBr impregnated CaO as catalyst. Energy Convers. Manag. 2015, 91, 442–450. [Google Scholar] [CrossRef]
- Leung, D.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from waste cooking oil via base-catalytic and supercritical methanol transesterification. Energy Convers. Manag. 2009, 50, 923–927. [Google Scholar] [CrossRef]
- Escobar, J.C.; Lora, E.S.; Venturini, O.J.; Yáñez, E.E.; Castillo, E.F.; Almazan, O. Biofuels: Environment, technology and food security. Renew. Sustain. Energy Rev. 2009, 13, 1275–1287. [Google Scholar] [CrossRef]
- Köckritz, A.; Martin, A. Oxidation of unsaturated fatty acid derivatives and vegetable oils. Eur. J. Lipid Sci. Technol. 2008, 110, 812–824. [Google Scholar] [CrossRef]
- Keith, F.W., Jr.; Blachly, F.E.; Sadler, F.S. Impurities in vegetable oil refining soapstock. J. Am. Oil Chem. Soc. 1954, 31, 298–302. [Google Scholar] [CrossRef]
- Ochoa, N.; Pagliero, C.; Marchese, J.; Mattea, M. Ultrafiltration of vegetable oils: Degumming by polymeric membranes. Sep. Purif. Technol. 2001, 22, 417–422. [Google Scholar] [CrossRef]
- Encinar, J.; Gonzalez, J.; Rodriguez, J.; Tejedor, A. Biodiesel fuels from vegetable oils: Transesterification of Cynara c ardunculus L. oils with ethanol. Energy Fuel. 2002, 16, 443–450. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Absalon, C.; Marias, F.; Aymonier, C.; Mench, M. Conversion of fern (Pteris vittata L.) biomass from a phytoremediation trial in sub-and supercritical water conditions. Biomass Bioenerg. 2011, 35, 872–883. [Google Scholar]
- He, H.; Wang, T.; Zhu, S. Continuous production of biodiesel fuel from vegetable oil using supercritical methanol process. Fuel 2007, 86, 442–447. [Google Scholar] [CrossRef]
- Silva, C.D.; Oliveira, J.V. Biodiesel production through non-catalytic supercritical transesterification: Current state and perspectives. Brazil. J. Chem. Eng. 2014, 31, 271–285. [Google Scholar] [CrossRef]
- Minami, E.; Saka, S. Kinetics of hydrolysis and methyl esterification for biodiesel production in two-step supercritical methanol process. Fuel 2006, 85, 2479–2483. [Google Scholar] [CrossRef]
- Demirbas, A. Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- Van Kasteren, J.; Nisworo, A. A process model to estimate the cost of industrial scale biodiesel production from waste cooking oil by supercritical transesterification. Resour. Conserv. Recycl. 2007, 50, 442–458. [Google Scholar] [CrossRef]



| Components | Reaction Method | |
|---|---|---|
| Catalyzed Method | Supercritical Fluid Method | |
| Molar Ratio (Oil: Alcohol) | 1:6 | 1:6 |
| Reaction temperature | 65 °C | 260 °C |
| Reaction pressure | 1 bar | 65 bar for methanol |
| 75 bar for ethanol | ||
| Reaction holding time | 60 min | 0 min |
| Catalyst | KOH | Not used |
| Instrument | Condition |
|---|---|
| GC | 6890 GC, Agilent |
| MSD | 5975, Agilent |
| Methods | |
| Column | DB-WAX (30 mm × 250 um, 0.25 um thickness) |
| Oven temperature | 250 °C at 5 °C/min |
| Carrier gas | He, 1 mL/min |
| Injection volume | 1 μL |
| Detector temperature | 250 °C |
| Mass scan range | 29–800 amu |
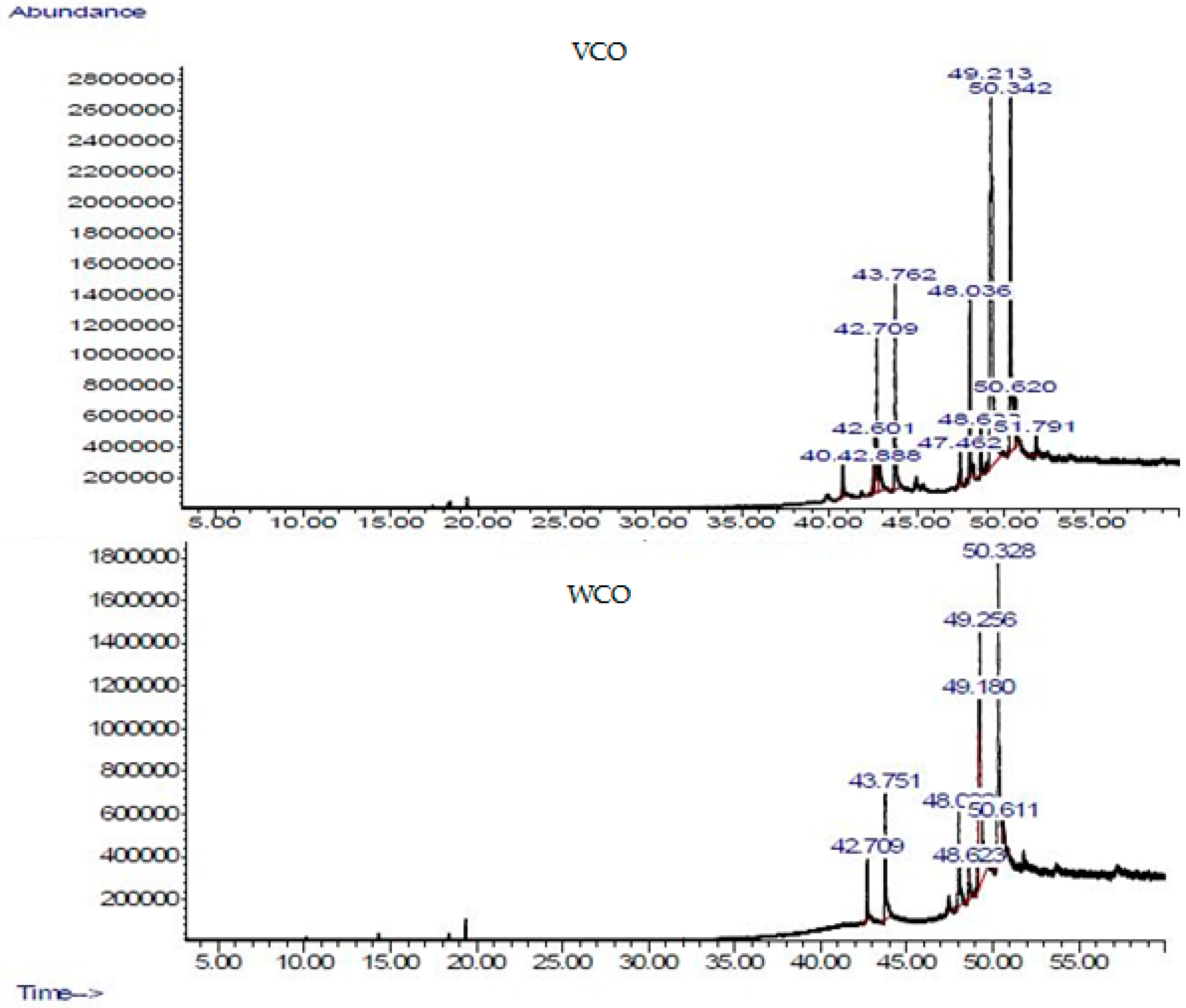
| Sample | Fatty Acid (FA) (wt %) | Free Fatty Acid (FFA) (wt %) | |||||
|---|---|---|---|---|---|---|---|
| 13:2 | 16:1 | 18:1 | 18:2 | 18:3 | Others | ||
| VCO | 6.49 | 9.86 | 2.41 | 13.58 | 58.48 | 9.19 | 0.28 |
| WCO | 3.38 | 9.89 | 2.41 | 36.70 | 43.21 | 4.42 | 3.15 |
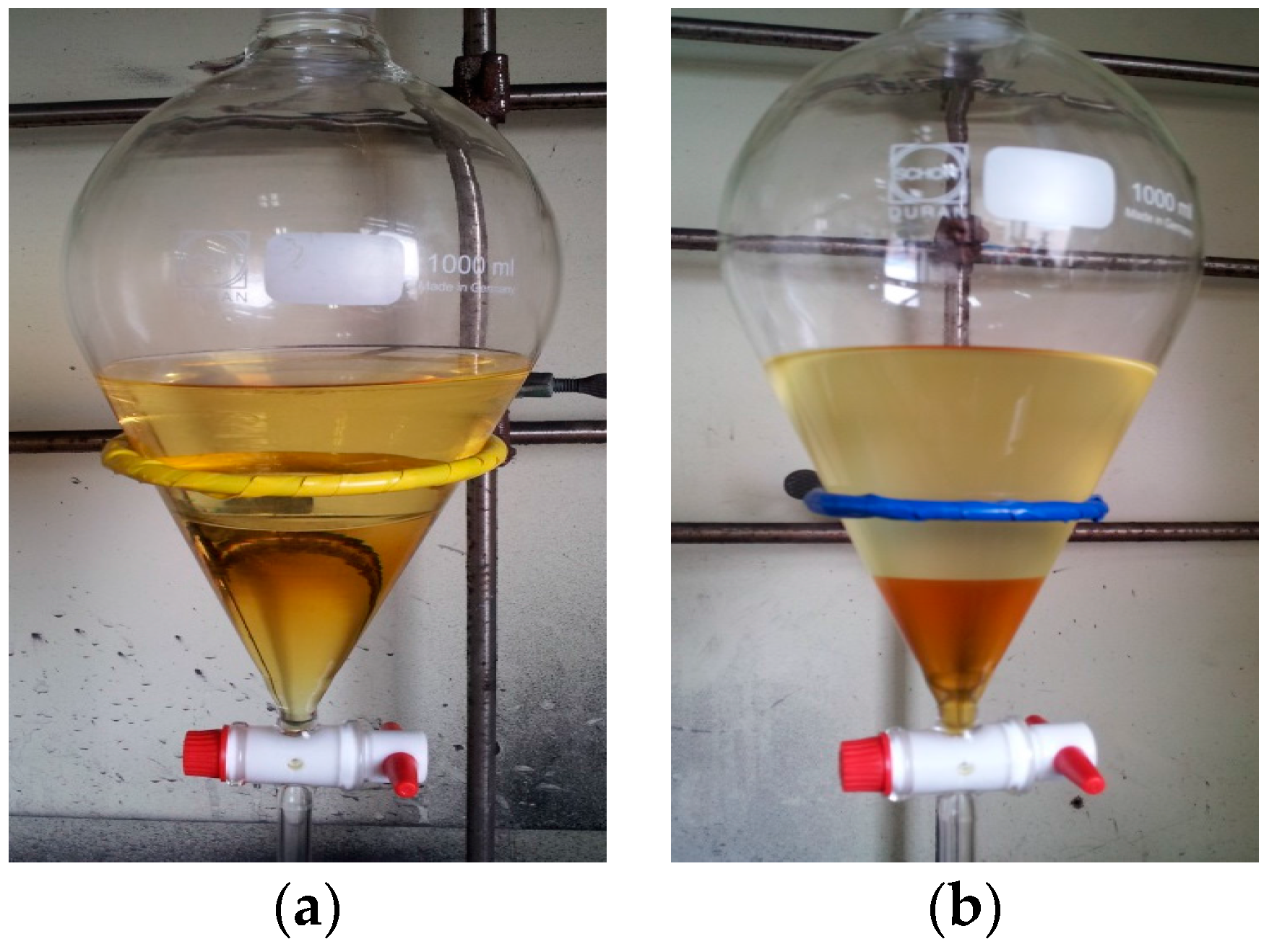
| Raw Material | Method | Product | Composition (wt %) | ||||
|---|---|---|---|---|---|---|---|
| FAMEs | FAEEs | FA | Glycerol | Others | |||
| VCO | Catalyst | Upper layer | 100 | 0 | 0 | 0 | 0 |
| Lower layer | 44.04 | 0 | 21.99 | 28.90 | 8.67 | ||
| NCM | Upper layer | 70.50 | 0 | 17.29 | 12.02 | 0.18 | |
| Lower layer | 96.23 | 0 | 2.35 | 0.16 | 1.26 | ||
| SCE | Upper layer | 0 | 89.40 | 4.10 | 2.11 | 4.39 | |
| Lower layer | 0 | 96.19 | 2.13 | 0 | 1.68 | ||
| WCO | Catalyst | Upper layer | 100 | 0 | 0 | 0 | 0 |
| Lower layer | 55.03 | 0 | 24.98 | 14.57 | 5.43 | ||
| NCM | Upper layer | 75.91 | 0 | 14.32 | 2.08 | 7.70 | |
| Lower layer | 93.67 | 0 | 4.54 | 0.28 | 1.51 | ||
| SCE | Upper layer | 0 | 90.27 | 5.95 | 0.38 | 3.40 | |
| Lower layer | 0 | 94.93 | 4.59 | 0 | 0.49 | ||
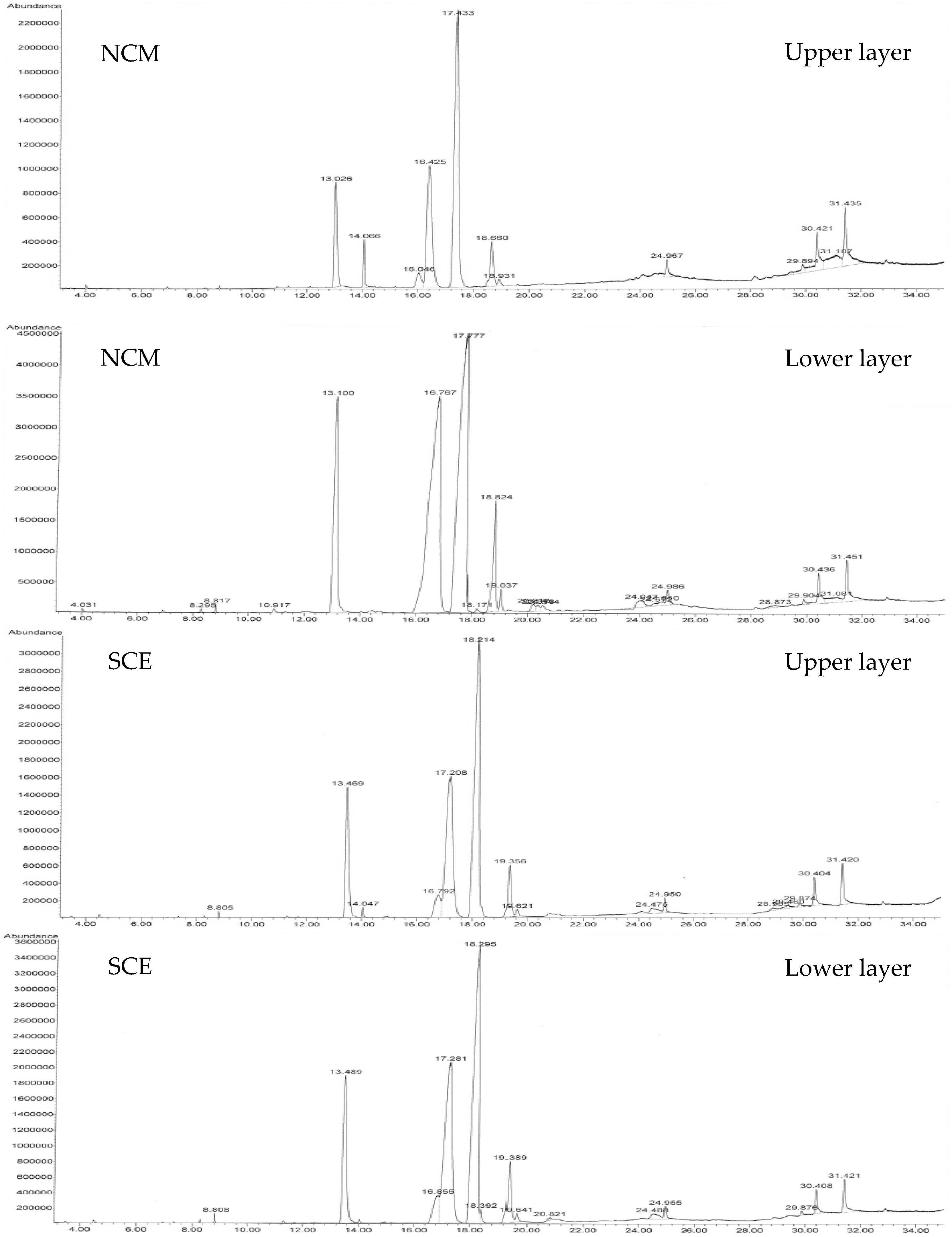
| Compound Name | Total Amount (area %) | |||||
|---|---|---|---|---|---|---|
| Upper Layer | Lower Layer | |||||
| Catalyzed | NCM | SCE | Catalyzed | NCM | SCE | |
| Octanoic Acid, Methyl/Ethyl Ester | - | 0.107 | 0.102 | - | 0.248 | - |
| 4-Decenoic Acid, Methyl/Ethyl Ester | - | 0.061 | - | - | 0.182 | 0.209 |
| 3-(Allyloxy)-2-cyclohexen-1-one | - | - | 0.093 | - | - | - |
| 2,4-Decadienal, [(E,E)/-] | - | 0.117 | 0.241 | - | 0.182 | 0.209 |
| Tetradecanoic Acid, Methyl/Ethyl Ester | - | 0.114 | - | - | - | - |
| Hexadecanoic Acid, Methyl/Ethyl Ester | 11.952 | 13.183 | 13.665 | 3.734 | 8.416 | 12.079 |
| Glycerin | - | 0.164 | - | 28.896 | 12.024 | 2.112 |
| Heptadecanoic Acid, Methyl/Ethyl Ester | - | 0.076 | 0.422 | - | - | - |
| Octadecanoic Acid, Methyl/Ethyl Ester | 5.412 | - | - | 1.01 | 2.115 | 3.44 |
| 7-Octadecenoic Acid, Methyl/Ethyl Ester | 28.314 | - | - | - | - | - |
| CIS-Linoleic Acid Methyl/Ethyl Ester | 45.507 | - | - | - | - | - |
| 8-Octadecenoic Acid (Z)-, Methyl/Ethyl Ester | - | - | - | - | 16.53 | - |
| 9-Octadecenoic Acid (Z)-, Methyl/Ethyl Ester | - | 35.381 | - | 7.858 | - | - |
| Methyl/Ethyl Oleate (Oleic Acid Methyl/Ethyl Ester) | - | - | 33.986 | - | - | 23.457 |
| Methyl/Ethyl Linoleate (Linoleic Acid Methyl/Ethyl Ester) | - | - | 41.162 | - | - | 43.885 |
| 9,12-Octadecadienoic Acid, Methyl/Ethyl Ester | - | - | - | - | 37.155 | - |
| 9,12-Octadecadienoic Acid (Z,Z)-, Methyl/Ethyl Ester | 0.348 | 36.908 | - | 18.489 | - | - |
| 11,14,17-Eicosatrienoic Acid, Methyl/Ethyl Ester | - | - | 0.246 | - | - | - |
| 9,12,15-Octadecatrienoic Acid, Methyl/Ethyl Ester | - | - | 0.68 | - | - | - |
| 9,12,15-Octadecatrienoic Acid, Methyl/Ethyl Ester, (Z,Z,Z) | - | 0.156 | 0.276 | 2.455 | 6.04 | 6.538 |
| 9,11-Octadecadienoic Acid, Methyl/Ethyl Ester, (E,E)- | - | - | 0.317 | |||
| Eicosanoic Acid, Methyl/Ethyl Ester | - | 0.177 | 0.104 | - | - | - |
| 11-Eicosenoic Acid, Methyl/Ethyl Ester | - | 0.64 | - | - | - | - |
| Docosanoic Acid, Methyl Ester | - | - | 0.768 | - | - | - |
| 1,2-Epoxy-1-vinylcylododecene | - | 2.191 | 1.081 | - | - | - |
| Methyl/Ethyl 2,3,4,6 -Tetra-O-Methyl/Ethyl-.Alpha.-D-Glucoside | - | - | 0.246 | - | - | - |
| Hexadecanoic Acid; Palmatic Acid | - | - | 0.68 | 3.087 | 1.957 | 0.517 |
| 15-Crown-5 | - | 0.156 | 0.276 | - | - | - |
| 1,4,7,10,13,16-Hexaoxacyclooctadecane | - | 0 | 0.317 | - | - | 0.398 |
| 6-Octadecenoic Acid, (Z)-; Petroselinic acid | - | 0.177 | 0.104 | - | - | - |
| 9-Octadecenoic Acid (Z)-; Oleic Acid | - | 0.64 | - | 7.8 | 3.401 | 1.505 |
| 9,12-Octadecadienoic Acid (Z,Z)-; Linoleic Acid | - | - | 0.768 | 11.107 | 11.932 | 2.08 |
| Tetradecanedioic Acid Dimethyl/Diethyl Ester | - | - | - | 1.885 | - | - |
| Methyl Pentadecyl Ether | - | - | - | 5.007 | - | - |
| Bicyclo[10.1.0]tridec-1-ene | - | - | - | 8.673 | - | - |
| 21-Krone-7 | - | - | - | - | 3.78 | |
| Compound Name | Total Amount (area %) | |||||
|---|---|---|---|---|---|---|
| Upper Layer | Lower Layer | |||||
| Catalyzed | NCM | SCE | Catalyzed | NCM | SCE | |
| 2,4-Decadienal, [(E,E)/-] | - | - | 0.152 | - | - | 0.152 |
| Hexadecanoic Acid, Methyl/Ethyl Ester | 6.301 | 9.315 | 12.846 | 6.301 | 9.315 | 12.846 |
| Glycerin | 13.942 | 2.075 | 0.382 | 13.942 | 2.075 | 0.382 |
| Octadecanoic Acid, Methyl/Ethyl Ester | 1.938 | 2.478 | 3.87 | 1.938 | 2.478 | 3.87 |
| 9-Octadecenoic Acid (Z), Methyl/Ethyl Ester | 14.311 | 21.051 | - | 14.311 | 21.051 | - |
| 10-Octadecenoic Acid, Methyl/Ethyl Ester | 0.786 | - | - | 0.786 | - | - |
| Methyl/Ethyl Oleate (Oleic Acid Methyl/Ethyl Ester) | - | - | 28.822 | - | - | 28.822 |
| Methyl/Ethyl Linoleate (Linoleic Acid Methyl/Ethyl Ester) | - | - | 39.298 | - | - | 39.298 |
| 9,12-Octadecadienoic Acid, Methyl/Ethyl Ester | 26.334 | - | - | 26.334 | - | - |
| 9,12-Octadecadienoic Acid (Z,Z)-, Methyl/Ethyl Ester | - | 37.439 | - | - | 37.439 | - |
| 9,12,15-Octadecatrienoic Acid, Methyl/Ethyl Ester | - | 4.982 | - | - | 4.982 | - |
| 9,12,15-Octadecatrienoic Acid, Methyl/Ethyl Ester, (Z,Z,Z)- | 2.996 | 0.643 | 5.437 | 2.996 | 0.643 | 5.437 |
| Hexadecanoic Acid; Palmatic Acid | 4.366 | 1.586 | 1.056 | 4.366 | 1.586 | 1.056 |
| 1,4,7,10,13,16-Hexaoxacyclooctadecane | 0.778 | 7.7 | 2.902 | 0.778 | 7.7 | 2.902 |
| 2-(bromomethyl) -2-octyl-15-crown-5 | - | - | 0.345 | - | - | 0.345 |
| Octadecanoic Acid | 1.473 | - | - | 1.473 | - | - |
| cis-7,cis-11-Hexadecadien-1-yl Acetate | 2.72 | - | - | 2.72 | - | - |
| 9-Octadecenoic Acid (E)-; trans-Oleic Acid | - | - | 2.001 | - | - | 2.001 |
| 9-Octadecenoic Acid (Z)-; Oleic Acid | 10.103 | 5.343 | - | 10.103 | 5.343 | - |
| Octaethylene Glycol Monododecyl Ether | 1.695 | - | - | 1.695 | - | - |
| 9,12-Octadecadienoic Acid (Z,Z)-; Linoleic Acid | 7.96 | 7.389 | 2.891 | 7.96 | 7.389 | 2.891 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, J.; Karki, S.; Sanjel, N.; Shah, M.; Oh, S.C. Comparison of Biodiesel Obtained from Virgin Cooking Oil and Waste Cooking Oil Using Supercritical and Catalytic Transesterification. Energies 2017, 10, 546. https://doi.org/10.3390/en10040546
Poudel J, Karki S, Sanjel N, Shah M, Oh SC. Comparison of Biodiesel Obtained from Virgin Cooking Oil and Waste Cooking Oil Using Supercritical and Catalytic Transesterification. Energies. 2017; 10(4):546. https://doi.org/10.3390/en10040546
Chicago/Turabian StylePoudel, Jeeban, Sujeeta Karki, Nawaraj Sanjel, Malesh Shah, and Sea Cheon Oh. 2017. "Comparison of Biodiesel Obtained from Virgin Cooking Oil and Waste Cooking Oil Using Supercritical and Catalytic Transesterification" Energies 10, no. 4: 546. https://doi.org/10.3390/en10040546






