Combustion and Emission Characteristics of Coconut-Based Biodiesel in a Liquid Fuel Burner
Abstract
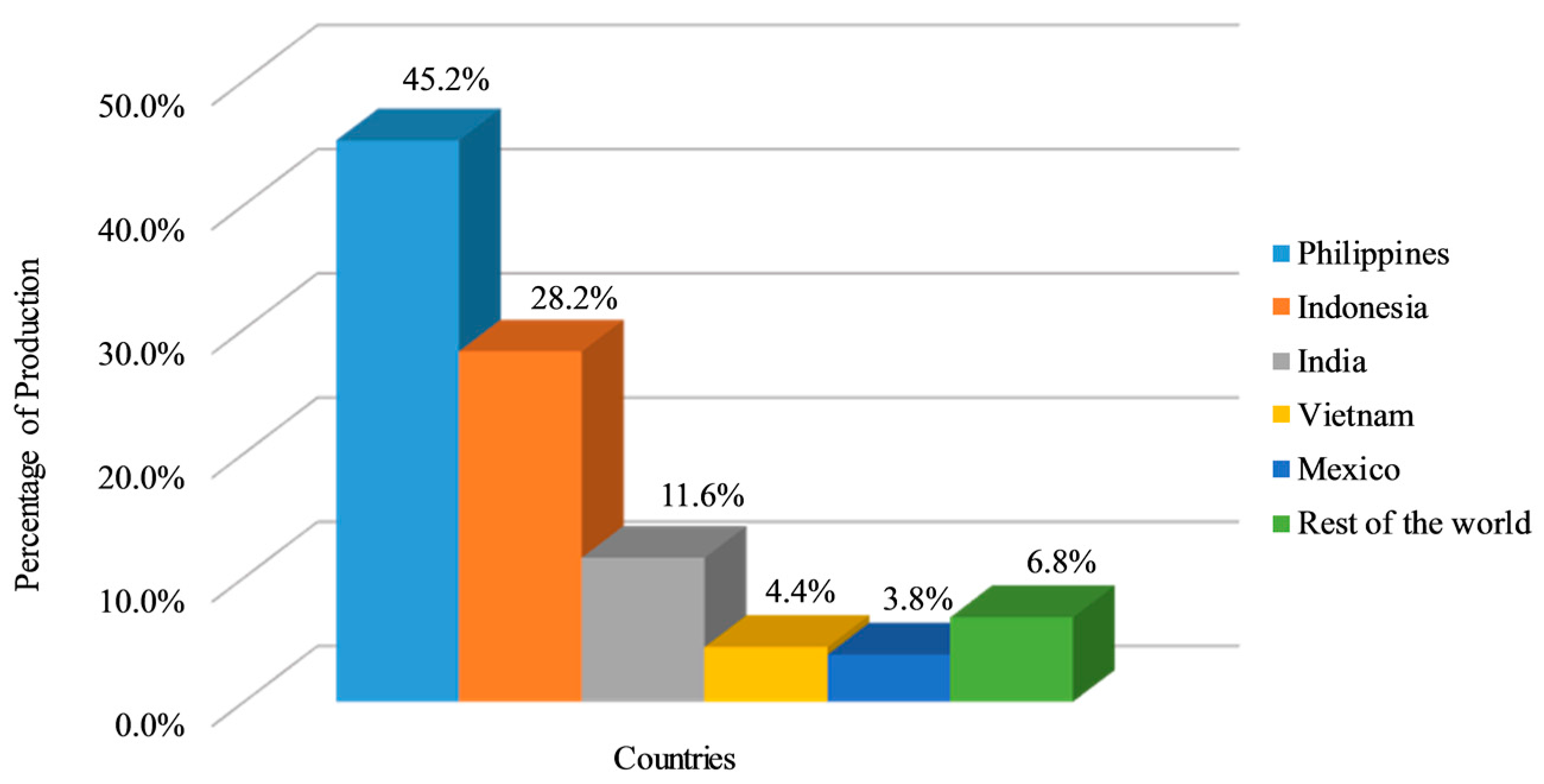
:1. Introduction
2. Methodology
2.1. Fuel Blends and Physical Properties
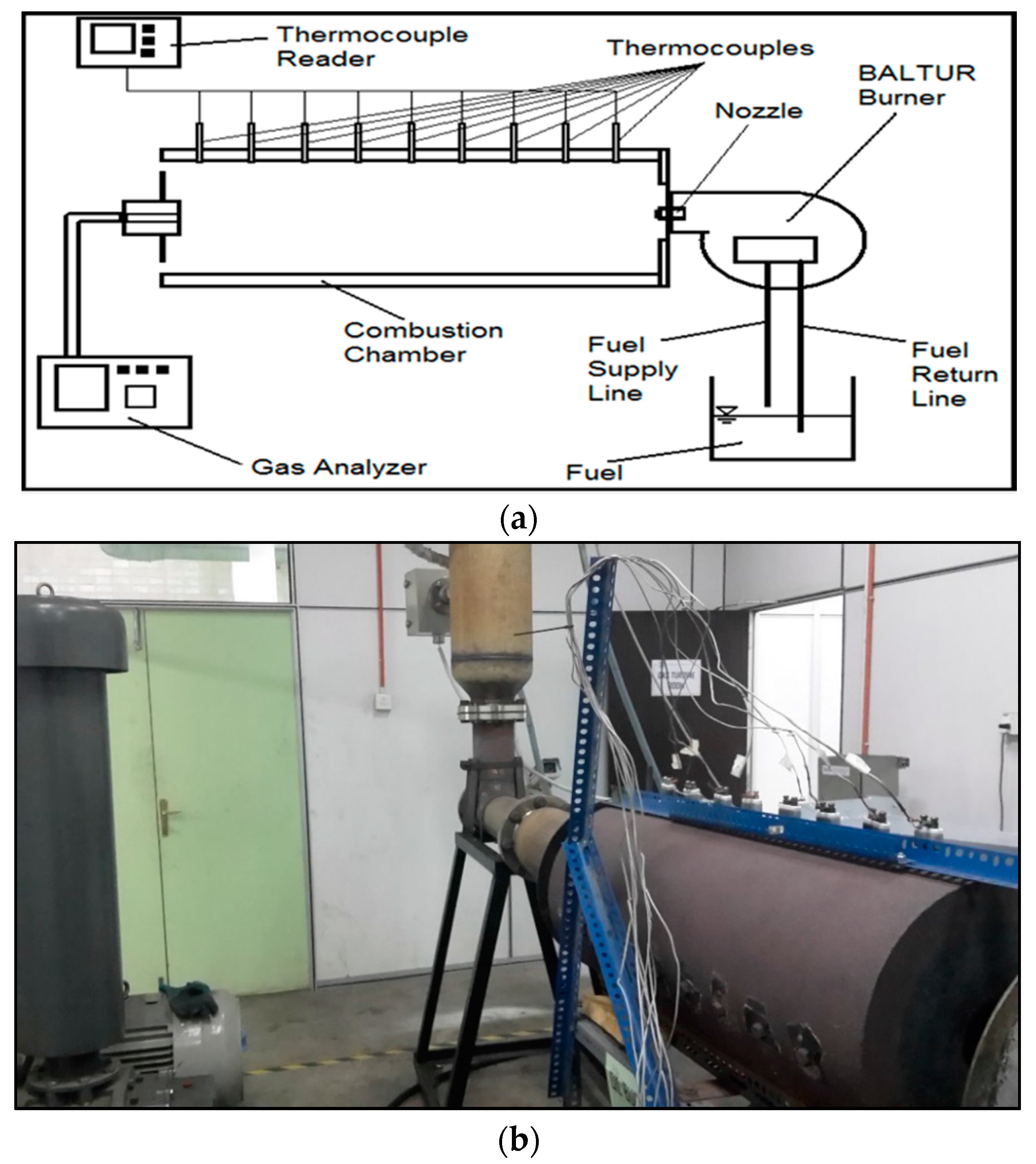
2.2. Experimental Set-Up
3. Results and Discussion
3.1. Fuel Properties
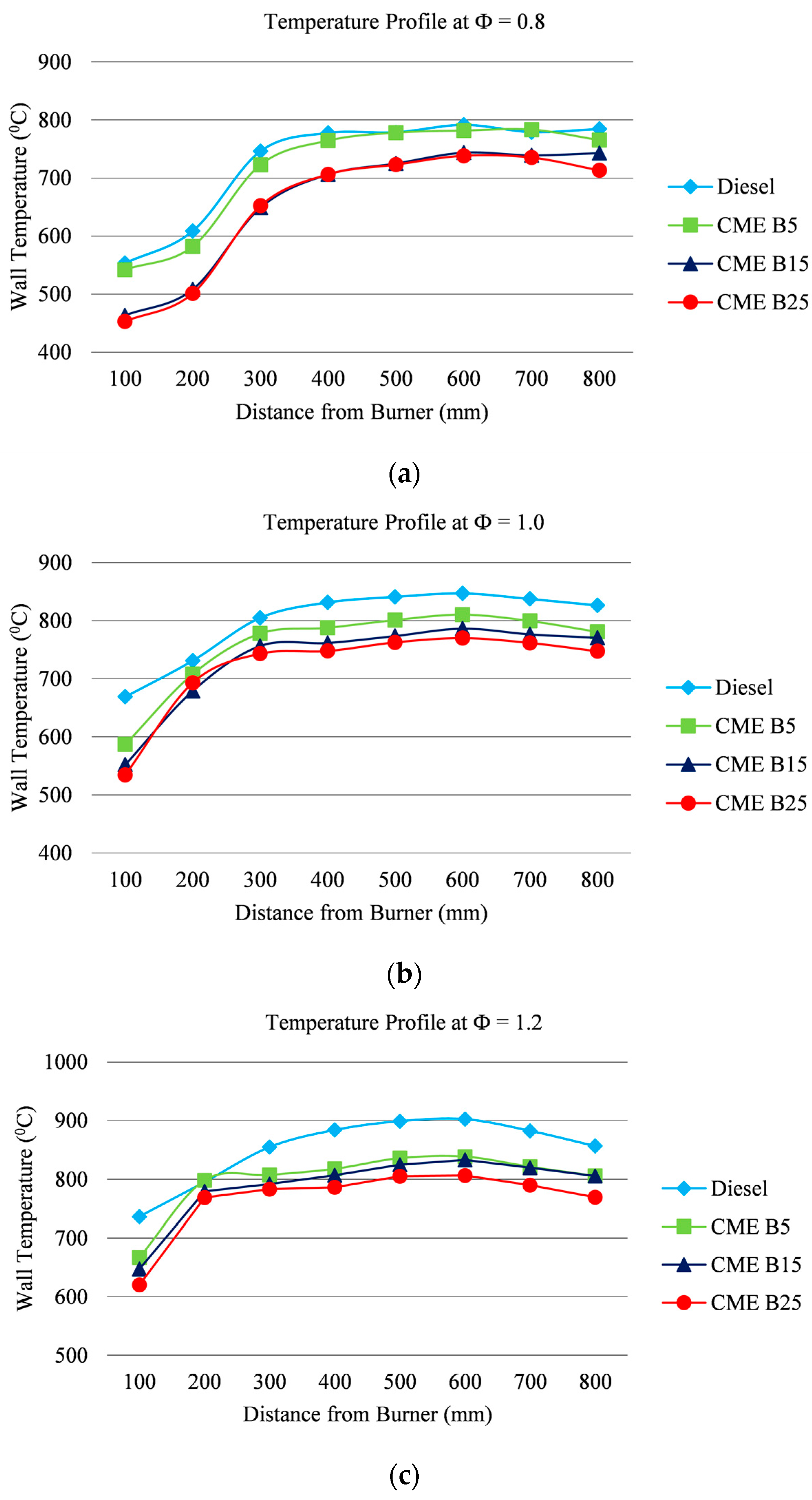
3.2. Wall Temperature Profile
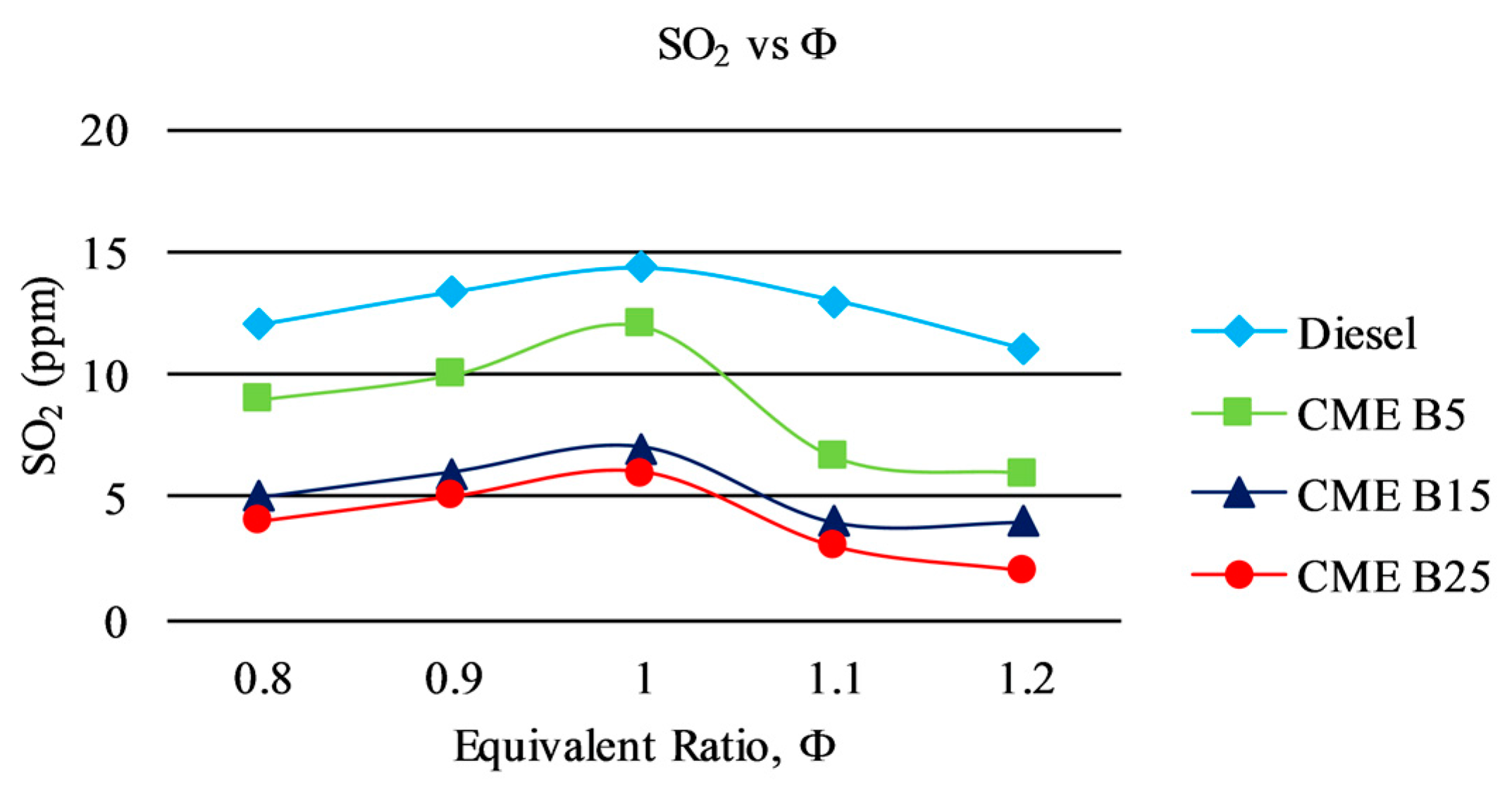
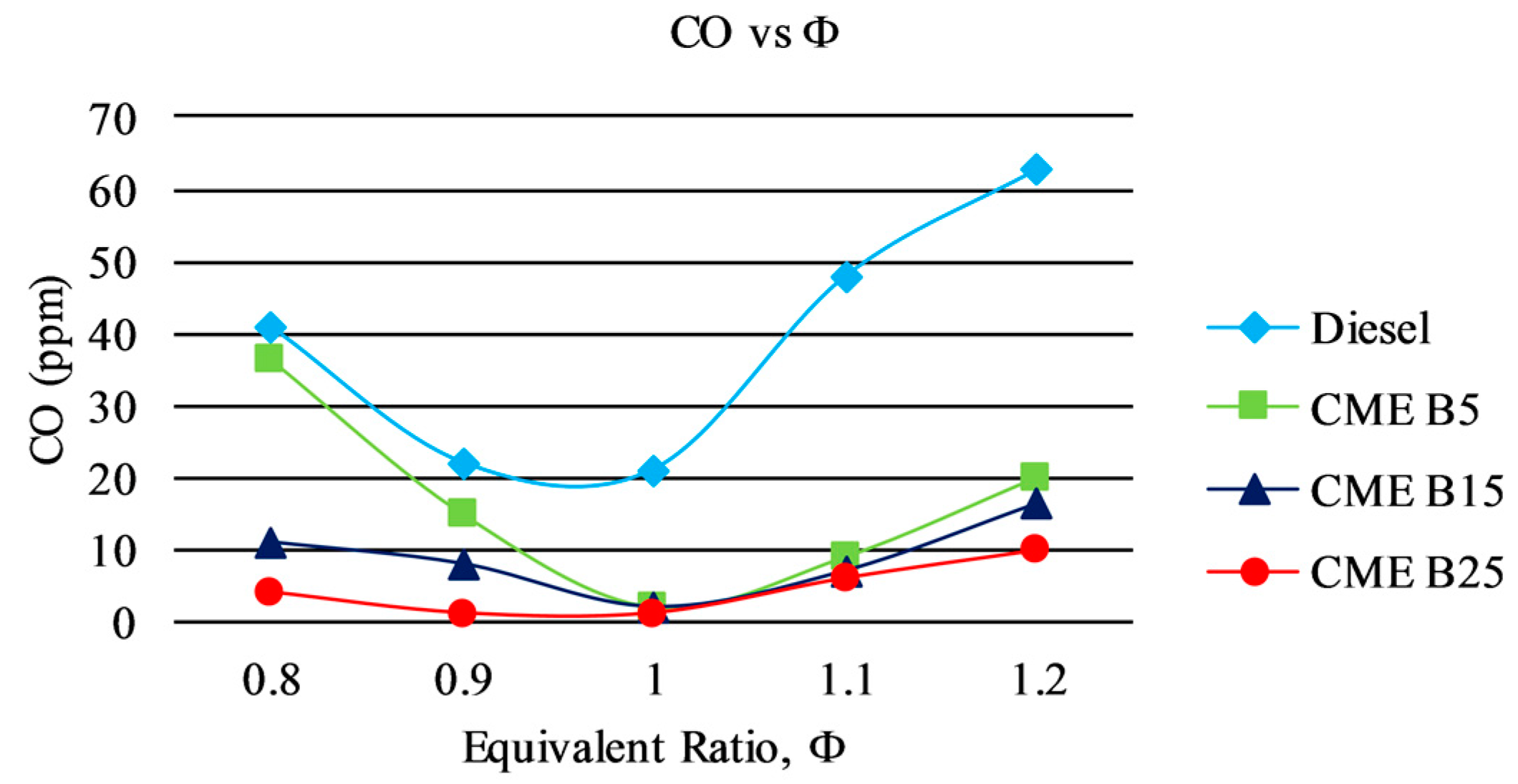
3.3. Gaseous Emission
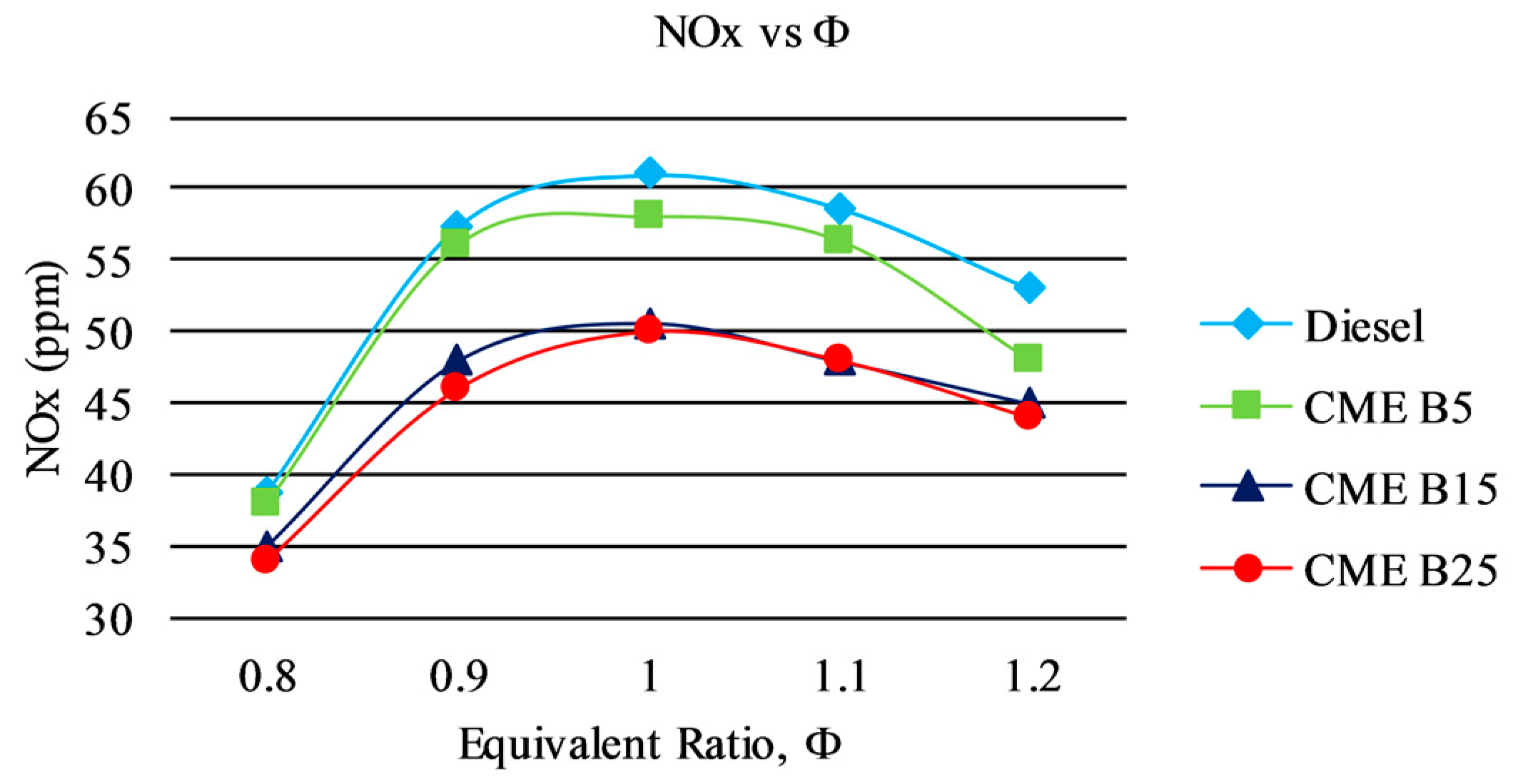
3.3.1. NOx Emission
3.3.2. SO2 Emission
3.3.3. CO Emission
4. Conclusions
- For all equivalence ratios, the CME biodiesel blends combust at lower temperatures than CDF.
- An increase in CME content reduces the combustion temperature.
- An increase in the fuel consumption results in a decrease in the air volume, which generates more heat energy during the combustion.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Puigjaner, L.; Pérez-Fortes, M.; Laínez-Aguirre, J. Towards a Carbon-Neutral Energy Sector: Opportunities and Challenges of Coordinated Bioenergy Supply Chains-A PSE Approach. Energies 2015, 8, 5613–5660. [Google Scholar] [CrossRef]
- Golledge, N.R.; Kowalewski, D.E.; Naish, T.R.; Levy, R.H.; Fogwill, C.J.; Gasson, E.G.W. The Multi-Millennial Antarctic Commitment to Future Sea-Level Rise. Nature 2015, 526, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Argawal, A.; Gupta, A.K.; Pandey, A. Novel Combustion Concepts for Sustainable Energy Development; Springer: New Delhi, India, 2014. [Google Scholar]
- Chen, J.; Wang, W.H.; Liu, T.W.; Wu, F.H.; Zheng, H.L. Photosynthetic and Antioxidant Responses of Liquidambar Formosana and Schimasuperba Seedlings to Sulfuric-Rich and Nitric-Rich Simulated Acid Rain. Plant Physiol. Biochem. 2013, 64, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Uzkeser, M.; Seven, B.; Gundogdu, F.; Acemoglu, H.; Aksakal, E.; Varoglu, E. The Evaluation of Myocardial Damage in 83 Young Adults with Carbon Monoxide Poisoning in the East Anatolia Region in Turkey. Hum. Exp. Toxicol. 2006, 25, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G.; Dunn, R.O.; Bagby, M.O. Biodiesel: The Use of Vegetable Oils and Their Derivatives as Alternative Diesel Fuels. In Fuels and Chemicals from Biomass; Saha, B.C., Woodward, J., Eds.; American Chemical Society (ACS): Washington, DC, USA, 1997; pp. 172–208. [Google Scholar]
- Xie, H.; Song, L.; Xie, Y.; Pi, D.; Shao, C.; Lin, Q. An Experimental Study on the Macroscopic Spray Characteristics of Biodiesel and Diesel in a Constant Volume Chamber. Energies 2015, 8, 5952–5972. [Google Scholar] [CrossRef]
- Ejikeme, P.M.; Anyaogu, I.D.; Ejikeme, C.L.; Nwafor, N.P.; Egbuonu, C.A.C.; Ukogu, K.; Ibemesi, J.A. Catalysis in Biodiesel production by Transesterification Processes—An Insight. J. Chem. 2010, 7, 1120–1132. [Google Scholar] [CrossRef]
- Magin, L.; Rodríguez-Fernández, J.; Agudelo, J.R. Diesel Particulate Emissions from Used Cooking Oil Biodiesel. Bioresour. Technol. 2008, 99, 731–740. [Google Scholar]
- Mustafa, C.; Van Gerpen, J.H. Comparison of Engine Performance and Emissions for Petroleum Diesel Fuel, Yellow Grease Biodiesel, and Soybean Oil Biodiesel. Trans. ASAE 2003, 46, 937. [Google Scholar]
- Mohd Jaafar, M.N.; Eldrainy, Y.A.; Mat Ali, M.F.; Wan Omar, W.Z.; MohdHizam, M.F.A. Combustion Performance Evaluation of Air Staging of Palm Oil Blends. Environ. Sci. Technol. 2012, 46, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Ja’afar, M.N.M.; Omar, W.Z.W.; Rahim, M.R.; Azmi, I.; Abdullah, M.H. Study on Combustion Performance of Palm Oil Biodiesel Blend. J. Teknol. 2014, 69, 127–131. [Google Scholar]
- Ganjehkaviri, A.; Jaafar, M.N.M.; Hosseini, S.E.; Musthafa, A.B. Performance Evaluation of Palm Oil-Based Biodiesel Combustion in an Oil Burner. Energies 2016, 9, 97. [Google Scholar] [CrossRef]
- Raghavan, K. Biofuels from Coconuts; Fuels from Agriculture in Communal Technology (FACT) Foundation: Wageningen, The Netherlands, 2010. [Google Scholar]
- Price Policy for the 2016 Season Copra. Commission for Agricultural Costs and Prices; Ministry of Agriculture of India: New Delhi, India, 2015.
- Cloin, J. Coconut Oil as a Biofuel in Pacific Islands: Challenges and Opportunities; South Pacific Applied Geoscience Commission: Suva, Fiji, 2005. [Google Scholar]
- Saifuddin, N.; Fazlili, A.; Kumaran, P.; Pei-Jua, N.; Priathashini, P. The Production of Biodiesel and Bio-kerosene from Coconut Oil Using Microwave Assisted Reaction. Earth Environ. Sci. 2016, 32, 12039–12043. [Google Scholar] [CrossRef]
- Suryanto, A.; Suprapto, S.; Mahfud, M. The Production of Biofuels from Coconut Oil Using Microwave. Modern Appl. Sci. 2015, 9, 93. [Google Scholar] [CrossRef]
- Llamas, A.; García-Martínez, M.J.; Al-Lal, A.M.; Canoira, L.; Lapuerta, M. Biokerosene from Coconut and Palm Kernel Oils: Production and Properties of Their Blends with Fossil Kerosene. Fuel 2012, 102, 483–490. [Google Scholar] [CrossRef]
- Giles, V. Pacific Regional Workshop on Biofuels: Opportunities and Implications for Sustainable Livelihoods in Pacific Island Countries; Pacific Regional Workshop: Nadi, Fiji, 2008. [Google Scholar]
- Liaquat, A.M.; Masjuki, H.H.; Kalam, M.A.; Fattah, I.R.M.; Hazrat, M.A.; Varman, M.; Mofijur, M.; Shahabuddin, M. Effect of Coconut Biodiesel Blended Fuels on Engine Performance and Emission Characteristics. Procedia Eng. 2013, 56, 583–590. [Google Scholar] [CrossRef]
- Woo, C.; Kook, S.; Hawkers, E.; Rogers, P.L.; Marquis, C. Engine Combustion and Emission of Coconut Oil Based Biodiesel and Diesel Blends. In Proceedings of the 19th Australasian Fluid Mechanics Conference, Melbourne, Australia, 8–11 September 2014. [Google Scholar]
- Salleh, M.M.N. Study on the Performance of Envo Diesel in a Combustion System. Master’s Thesis, Universiti Teknologi Malaysia, Skudai, Malaysia, 2015. [Google Scholar]
- Steinen Nozzle Catalog. Industrial Mining Oil Burner; Steinen Mfg. Co.: Parsippany, NJ, USA, 2016. [Google Scholar]
- Myers, R.L. The Basics of Chemistry, 1st ed.; Greenwood Pub Group: Santa Barbara, CA, USA, 2003. [Google Scholar]
- Ramalho, E.F.S.M.; Filho, J.R.C.; Albuquerque, A.R.; de Oliveira, S.F.; Cavalcanti, E.H.S.; Stragevitch, L.; Santos, I.M.G.; Souza, A.G. Low Temperature Behavior of Poultry Fat Biodiesel: Diesel Blends. Fuel 2012, 93, 601–605. [Google Scholar] [CrossRef]
- Refaat, A.A. Correlation between the Chemical Structure of Biodiesel and Its Physical Properties. Int. J. Environ. Sci. Technol. 2009, 6, 677–694. [Google Scholar] [CrossRef]
- Puhan, S.; Saravanan, N.; Nagarajan, G.; Vedaraman, N. Effect of Biodiesel Unsaturated Fatty Acid on Combustion Characteristics of a DI Compression Ignition Engine. Biomass Bioenergy 2016, 34, 1079–1088. [Google Scholar] [CrossRef]
- Sivaramakrishnan, K. Determination of Higher Heating Value of Biodiesels. Int. J. Eng. Sci. Technol. 2011, 3, 7981–7987. [Google Scholar]
- Musthafa, A.B. Study on the Performance of Palm Methyl Ester in a Combustion System. Master’s Thesis, Universiti Teknologi Malaysia, Skudai, Malaysia, 2015. [Google Scholar]
- Croiset, E.; Thambimuthu, K.V. NO and SO Emissions from O/CO Recycle Coal Combustion. Fuel 2016, 80, 2117–2121. [Google Scholar] [CrossRef]
- Hasenberg, L.; Bender, R. Corrosion Handbook: Corrosive Agents and Their Interaction with Materials: V. 10: Sulfur Dioxide, Sodium Sulfate; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008. [Google Scholar]
- Gumus, M.; Kasifoglu, S. Performance and Emission Evaluation of a Compression Ignition Engine Using a Biodiesel (Apricot Seed Kernel Oil Methyl Ester) and Its Blends with Diesel Fuel. Biomass Bioenergy 2010, 34, 134–139. [Google Scholar] [CrossRef]
- Habibullah, M.; Rizwanul Fattah, I.M.; Masjuki, H.H.; Kalam, M.A. Effects of Palm–Coconut Biodiesel Blends on the Performance and Emission of a Single-Cylinder Diesel Engine. Energy Fuels 2015, 29, 734–743. [Google Scholar] [CrossRef]


| Properties | Standard | Unit | Fuels | ||||
|---|---|---|---|---|---|---|---|
| CDF | B5 | B15 | B25 | B100 | |||
| CME Volume | L | 0 | 0.5 | 1.5 | 2.5 | - | |
| Diesel Volume | L | 10 | 9.5 | 8.5 | 7.5 | - | |
| Density at 15 °C | ASTM D941 | kg/m3 | 830.1 | 831.5 | 834.3 | 837.1 | 858.2 |
| Kinematic Viscosity at 40 °C | ASTM D445 | mm2/s | 3.5018 | 3.4678 | 3.3600 | 3.2594 | 2.8396 |
| Surface Tension at 15 °C | ASTM D971 | N/m | 0.0295 | 0.0296 | 0.0296 | 0.0297 | 0.0305 |
| Gross Calorific Value | ASTM D240 | kJ/kg | 45,290 | 44,734 | 43,891 | 43,136 | 37,654 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Malik, M.S.; Shaiful, A.I.M.; Mohd. Ismail, M.S.; Mohd Jaafar, M.N.; Mohamad Sahar, A. Combustion and Emission Characteristics of Coconut-Based Biodiesel in a Liquid Fuel Burner. Energies 2017, 10, 458. https://doi.org/10.3390/en10040458
Abdul Malik MS, Shaiful AIM, Mohd. Ismail MS, Mohd Jaafar MN, Mohamad Sahar A. Combustion and Emission Characteristics of Coconut-Based Biodiesel in a Liquid Fuel Burner. Energies. 2017; 10(4):458. https://doi.org/10.3390/en10040458
Chicago/Turabian StyleAbdul Malik, Muhammad Syahiran, Ashrul Ishak Mohamad Shaiful, Mohd Shuisma Mohd. Ismail, Mohammad Nazri Mohd Jaafar, and Amirah Mohamad Sahar. 2017. "Combustion and Emission Characteristics of Coconut-Based Biodiesel in a Liquid Fuel Burner" Energies 10, no. 4: 458. https://doi.org/10.3390/en10040458





