1. Introduction
There are many variant forms of energy used for room heating, such as coal combusted in a boiler [
1], electric heating [
2], and even solar heating [
3,
4]. Due to the high cost of coal and electric heating, and the uncontrollability of solar heating, industrial waste heat has increasingly been found to be an alternative in room heating since it is a significant potential resource for nearby sinks. The waste heat resources are very abundant in industrial plants: industrial residual water (IRW), byproduct combustible gas, flue gas from furnaces, byproduct steam, and sensible heat carried on solid materials such as slag, scrap, and product. For example, Li et al. [
5] proposed a scheme to integrate industrial waste heat, including slag-flushing water, cooling water, and low-pressure steam, into a district heating network. A case study conducted by Togawa et al. [
6] indicates that waste heat provides more environmental benefits than the individual boiler system. By using waste heat from glass melting furnaces, gas turbine exhaust, steam boiler exhaust, etc., fuel oil consumption is reduced by 16.05 TJ/year, while total CO
2 emissions are reduced by 1204 ton/year, in the investigated waste heat system.
Due to the ease of access and the economic superiority of waste heat [
7], workshops in industrial plants are deservedly heated by waste heat, e.g., IRW, in most cases. Nevertheless, most of the potential heat is still emitted to the surroundings because industrial facilities are usually not optimally organized for IRW utilization. Most utilizations of IRW concentrate on the water with excess heat of high- and medium-temperature, such as flash evaporation power generation [
8,
9], or on stably supplied IRW with low-temperature, such as heating [
10] and driving a chemisorption chiller [
11]. Other forms of low-grade energy are also widely applied, mainly involving gaseous waste heat and geothermal water [
12,
13,
14]. However, few reports on the utilization of unstable low-temperature IRW are found. Actually, thermal energy storage (TES) is effective to solve the intermittent and unstable problems [
15] and is widely used in low-grade waste heat applications. It can store and transport the waste heat from technic process to other sections for heating and drying. Cheng et al. [
16] studied a kind of heat conduction-enhanced shape-stabilized phase change material (PCM) used in the under-floor heating system for house heating in winter. The TES with PCM has a comfortable temperature environment and the best economic benefits among the investigated three different heating types: phase change TES, non-phase change TES, and conventional air conditioning heating system. In addition, Xia and Zhang [
17] developed a new double-layer radiant floor system which can work in both summer and winter, storing thermal or cold energy in off-peak periods and using it in peak periods. The results of [
18] also indicate the advantages of using PCM.
Four main types of TES systems are: sensible heat storage, latent heat storage, quasi-latent heat storage, and adsorption storage of heat. Thermal energy that is involved in changing the temperature of materials is called sensible heat, and its amount is simply the product of the specific heat and the temperature change. Thus, this is a simple and widely-used TES method. Commonly used sensible heat storage materials include water [
19], rock [
20], soil [
21], and other high-temperature sintering mixtures such as Li
2O, Al
2O
3, and TiO
2. This type of TES has been used to control the temperature in living or working spaces. In some cases, the amount of storage material can be quite large, so that there is the obvious concern about its huge equipment limiting its further development. A different mechanism for the storage of thermal energy involves phase transitions with no change in chemical composition. In this case, latent heat is absorbed or supplied at a constant temperature, which is ease of control, rather than over a range of temperature, as it is with sensible heat. The PCMs can be divided into two types: high-temperature PCMs and low-temperature PCMs, according to the phase change temperature. High-temperature PCMs, including high-temperature molten salts [
22], salt-mixtures [
23], metals and alloys [
24], are mainly used for aerospace application; while low-temperature PCMs, such as calcium chloride hexahydrate [
25], sodium acetate trihydrate [
26], organic alcohol [
27], and paraffin [
28], are mainly used for waste heat recovery, solar energy utilization, domestic heating, and air conditioning system. Additionally, chemical reactions generally result in the generation or absorption of heat, similar to the thermal effects related to phase transitions in materials in which there are no changes in chemical composition. The thermal effects related to chemical reactions are often described in terms of quasi-latent heat [
29]. Though quasi-latent heat storage has the characteristics of large storage density and pollution free, it is not easy to commercially promote due to its high cost. Besides, there is another new type, adsorption heat storage, which stores and converts heat through the thermal effect of an adsorption process [
30,
31]. It has a large storage density of appropriate 800~1000 kJ/kg, loses less heat, is non-toxic and does not pollute. However, there are few applications because of its dissatisfied heat transfer characteristic and complex storage process.
Paraffin, as a most useful [
32] and frequently used [
33,
34] PCM for TES at low-temperature applications, is a byproduct of refining petroleum product, and is extracted from the wax distillate of crude oil. It is mixed by straight-chain alkanes and can be expressed by C
nH
2n+2. It has many advantages such as wide melting/solidification temperature ranges [
35], relatively large latent heat [
35], small volume change during the phase change process [
35,
36], chemically stable property [
35,
36], non-toxic [
35], and moderate price [
35,
37]. Compared with inorganic PCMs, paraffin has no problems of phase separation on melting and has good self-nucleating properties on freezing, so that nucleating agents are not required [
36,
38]. It also has no subcooling effects [
35] and corrosion problems of the conventional materials of construction [
35,
38]. Depended on the phase change temperature ranges, paraffin is widely used in industrial waste heat recovery, phase change building wall, heat storage floor, solar energy storage, etc., undergoing a phase change from solid to liquid, or vice versa [
36,
39].
Although each study in literature seems to address a specific aim with a corresponding architecture of the heating system, there is still the need for a workshop heating system that is specific enough to be used in a pharmaceutical plant, as well as a simulation model that is broad enough to analyze the thermal performance of a TES unit. In this study, a workshop heating system is designed to extract the waste heat from IRW, which is implemented through a TES unit with paraffin wax as a PCM. Then the thermal behaviors of the TES unit are modeled and discussed. The technical contribution of this work is identified within the designated system architecture, which solves the utilization of unstable low-temperature IRW that is rarely found in literature. The scientific contribution of this work is identified in the development of the thermal analysis model, consisting of the recurrence formulae of fluid temperature and the melting/solidification radius of paraffin wax during the bidirectional phase change process.
2. Problem Description and System Design
In a pharmaceutical plant, IRW is being used in the workshop heating system. Given the energy loss and pipe requirement, the temperature of IRW supplied to the workshop heating unit should be within 50 to 60 °C. Thus, the IRW, from a medicine production unit, with the original temperature of 70 °C passes through a temperature regulating valve before enters the workshop heating unit. If the production unit works at its rated load, only 20.4 h per day are needed to produce the medicine, according to the market orders. During the remaining 3.6 h, the medicine production unit stops work; thus, there is no IRW generated. In practice, to meet the requirement of continuous workshop heating, the medicine production unit operates at a relatively low load, approximately 85%, which consumes some extra energy consumption and cost.
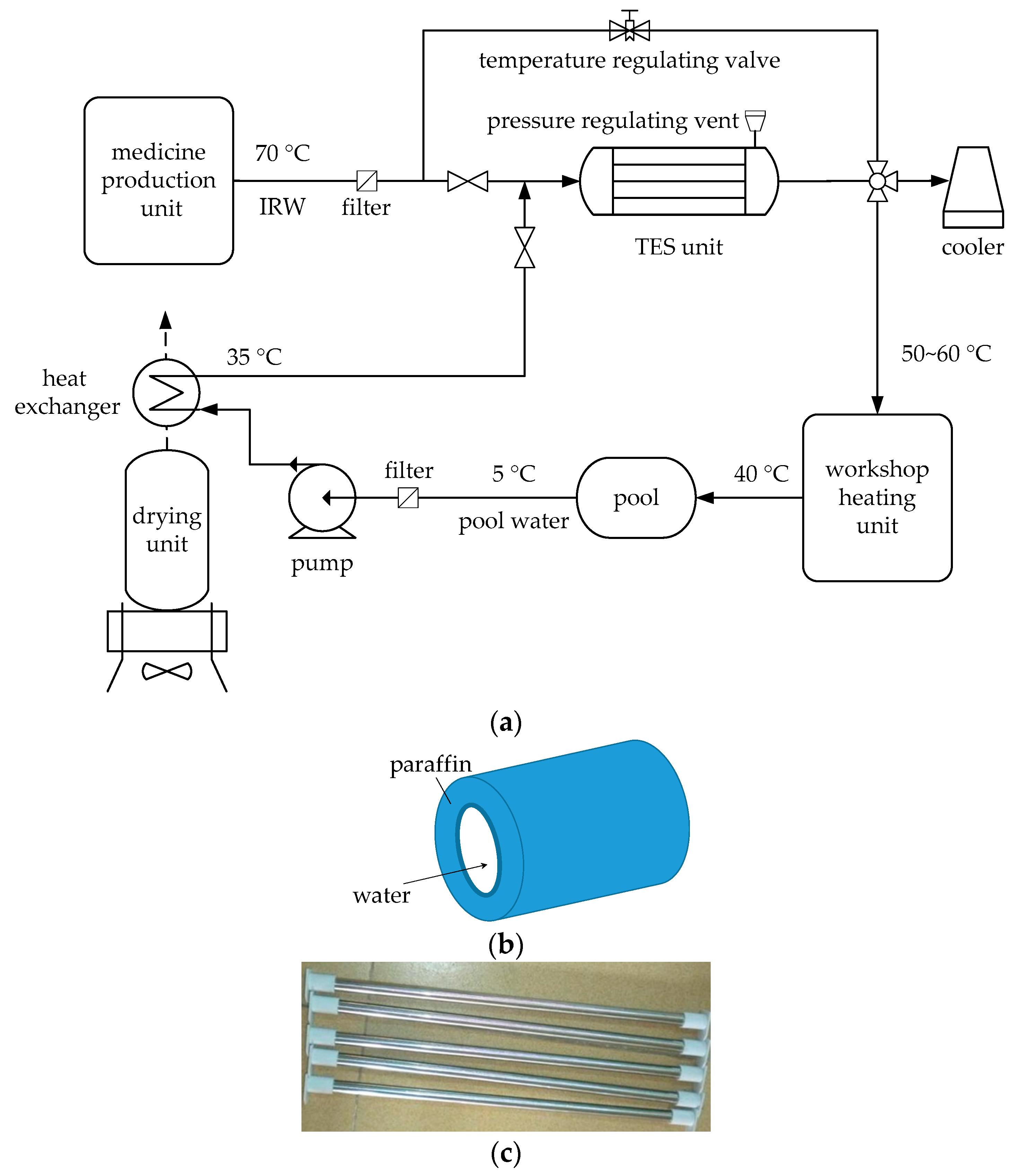
To solve this problem, a novel workshop heating system using IRW through a TES unit is proposed, as shown in
Figure 1. In the proposed system, the medicine production unit works at its rated load, in which point the energy efficiency of the production unit performs the best. Thus, the continuous low-load-operating of the medicine production unit is replaced by a batch-type rated-load-operating, leading to a corresponding intermittent of IRW generation. However, the heat requirement of the workshop heating unit is continuous. Thus, the TES unit, with paraffin wax as a PCM, plays a crucial role in the system. When the medicine production unit runs, the workshop heating unit receives filtered IRW passing through the temperature regulating valve, after which the temperature of IRW is regulated to 50 to 60 °C; meanwhile part of the IRW flows through the tube of the TES unit (see
Figure 1b), and the heat of this part of the IRW with the temperature of 70 °C is stored in the paraffin wax filled in the shell of the TES unit, leading to a phase change of the paraffin wax from solid to liquid. When the medicine production unit stops, there is no IRW produced from production unit, and the TES unit is in an exothermic state. The filtered water from a pool, with the temperature of 5 °C, is pumped into a heat exchanger, in which the pool water is preheated to 35 °C by the hot wind from a drying unit next to the medicine production unit. The preheated pool water enters the tube of the TES unit to extract the heat stored in the liquid paraffin wax in the previous cycle. After exchanging heat with paraffin wax, the pool water is finally heated to 50 to 60 °C and sent to the workshop heating unit; whereas the paraffin wax changes from liquid state to the solid. The TES unit is a set of shell-and-tube pipe columns (see
Figure 1c). Fluid, either IRW or pool water, flows in the tube after filtered; and paraffin wax is stored in the shell. The shell is made of carbon steel, while the tube is pure copper covered by thermal insulators. In addition, a pressure regulating vent [
37] is installed necessarily to protect the structural integrity of the TES unit from excessive pressure caused by volumetric expansion of the paraffin wax.
5. Results and Discussions
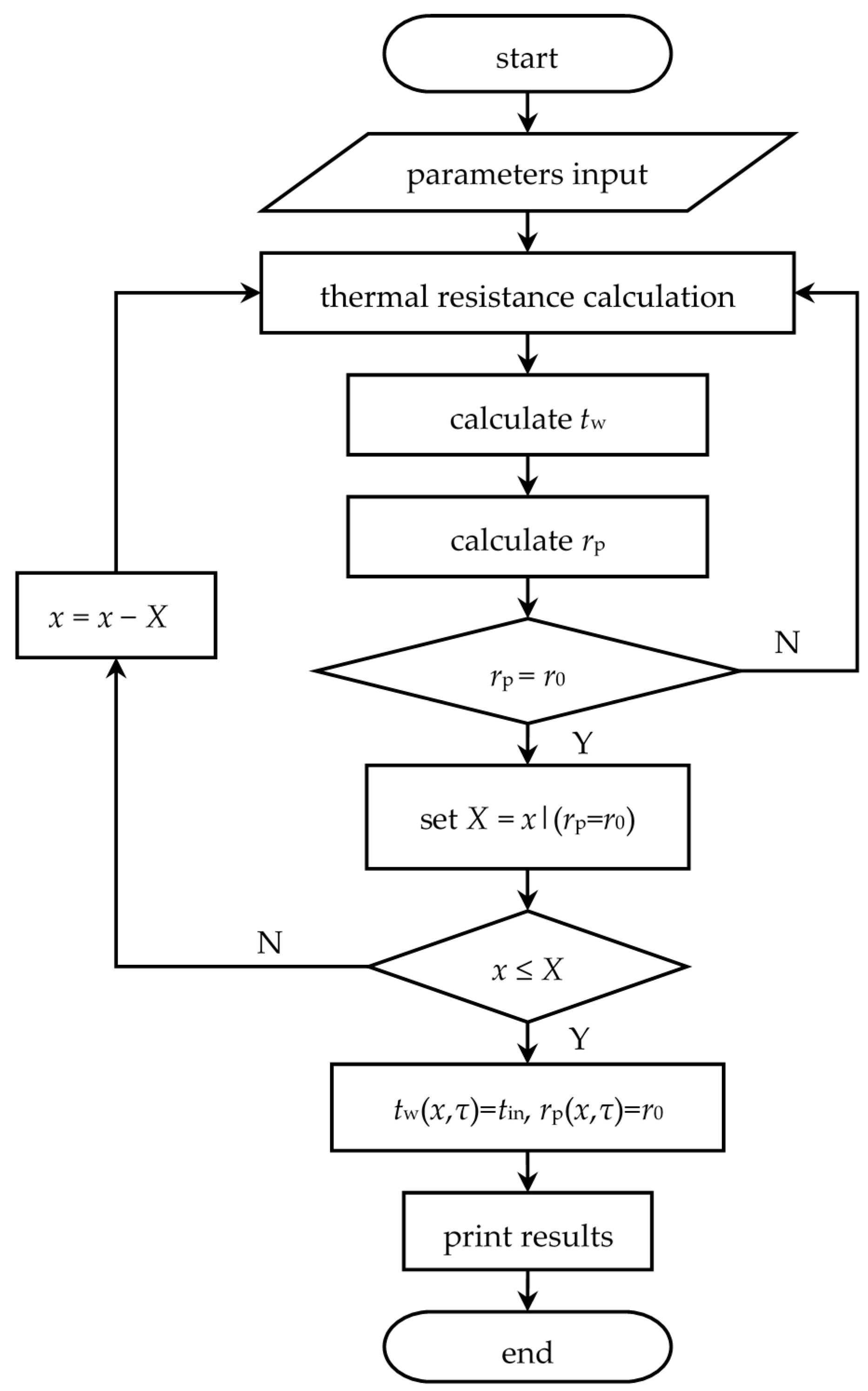
The real recurrence formulae of heat storage and release processes can be obtained by substituting parameters in
Table 1,
Table 2 and
Table 3 into Equations (11) to (14). The detailed solving method is shown in
Figure 7, with the axial step length
= 0.01 m and the time step length
= 1 s. A similar solving procedure for heat release process is not re-described here.
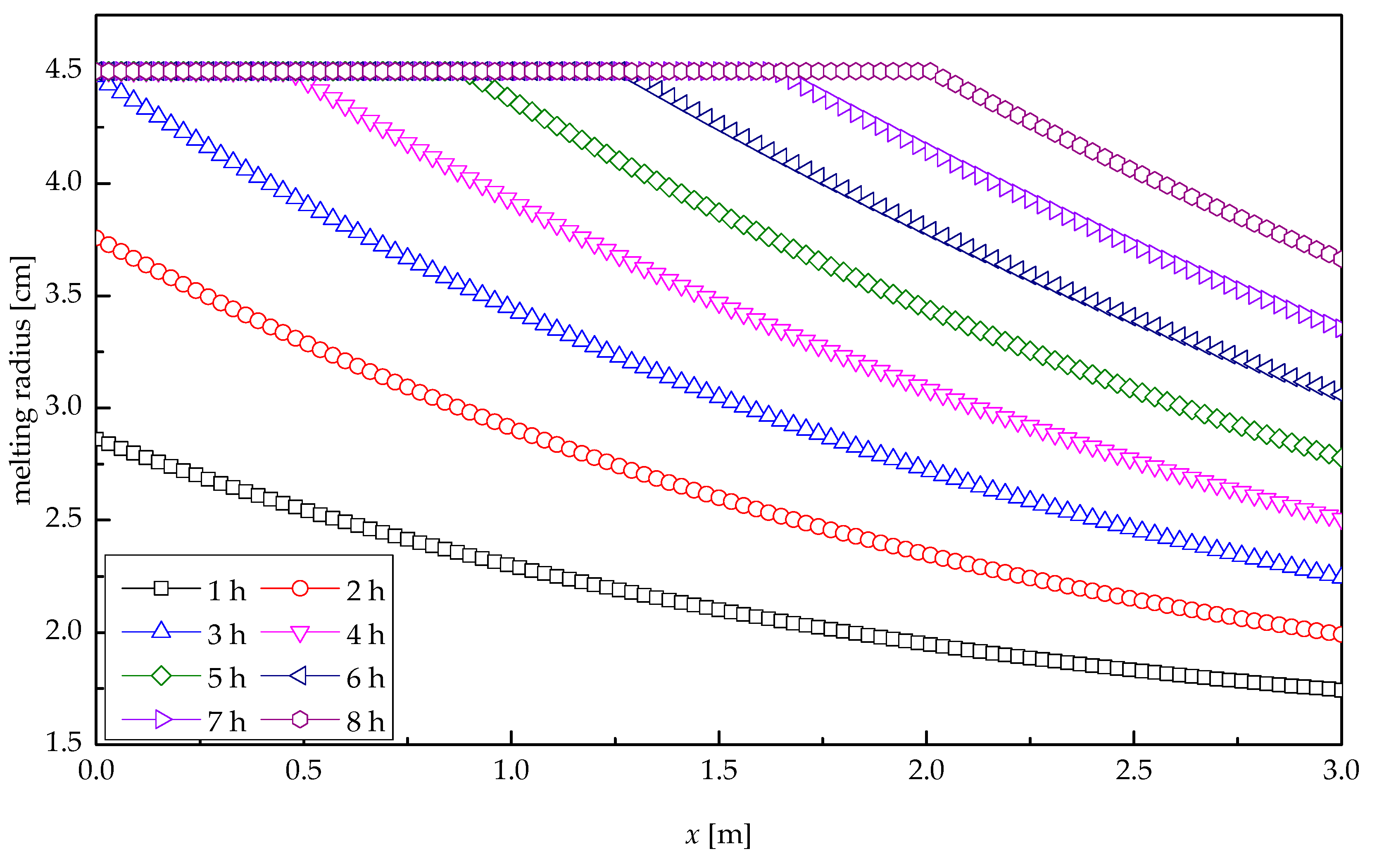
Figure 8 presents the profile of melting radius of paraffin wax along the tube length direction at various times for heat storage process. It was indicated that the paraffin wax at IRW entrance melted quickly, with the average melting velocity of 0.50 cm/h, because of the relatively great temperature difference between IRW and paraffin wax. The melting radius decreased with the increasing distance along the tube length. At the outlet of the tube, the melting velocity of paraffin wax was the lowest, with the average value of 0.08 cm/h, corresponding to the smallest melting radius. In the first 3.03 h, paraffin wax at the IRW entrance was not entirely melted, and the distance between two melting curves presented a shape of “from bigger to smaller”. At the moment of 3.03 h, the melting radius of paraffin wax at the inlet reached its maximum value, i.e., the paraffin wax at the IRW entrance melted completely. The heat storage process after 3.03 h could be regarded as a moving-back IRW entrance, and thus the melting curves also moved back. With the increasing time, the melting radius of paraffin wax at each location increased, resulting in a gradually reduced distance between two adjacent curves. After 8-h heat transfer, the melted paraffin wax accounted for 90.8%, and the melting radius at the outlet reached 3.66 cm.
Figure 9 describes the profile of IRW temperature along the tube length direction at several times for heat storage process. At the inlet of the tube, the IRW revealed a substantial drop of temperature as a result of the marked heat exchange, for the big temperature difference between the IRW and paraffin wax. As the distance along the tube increased, the temperature difference between IRW and paraffin wax decreased. In the first 3.03 h, the paraffin wax at IRW entrance was melting, and the temperature change of IRW there varied from that at the outlet, because of the different thermal resistances corresponding to their individual melting radius. After 3.03 h, the paraffin wax at the entrance melted completely, and the melting cross-section presented a backward shift, which resulted in an expanding temperature difference and a rapid melting velocity; and thus the temperature of IRW at outlet of the tube varied appreciably with time. After 8-h heat transfer, the melting cross-section had moved to the location
x = 2 m, and the IRW temperature at the outlet had reached 64 °C, when the heat storage effect appeared rather unnoticeable.
Figure 10 shows the profile of solidification radius of paraffin wax along the tube length direction at various times for heat release process. It was indicated that the paraffin wax at pool water entrance solidified quickly, with the average solidification velocity of 0.60 cm/h. The solidification radius decreased with the increasing distance along the tube length. At the outlet of the tube, the solidification velocity of paraffin wax was the lowest, with the average value of 0.12 cm/h, corresponding to the smallest solidification radius. In the first 2.48 h, paraffin wax at the pool water entrance was not entirely solidified. At the moment of 2.48 h, the solidification radius of paraffin wax at the inlet reached its maximum value, i.e., the paraffin wax at the pool water entrance solidified completely, after which the solidification cross-section showed a moving-back shift. After 8-h heat transfer, the solidified paraffin wax accounted for 98.1%, and the solidification radius at the outlet reached 3.94 cm.
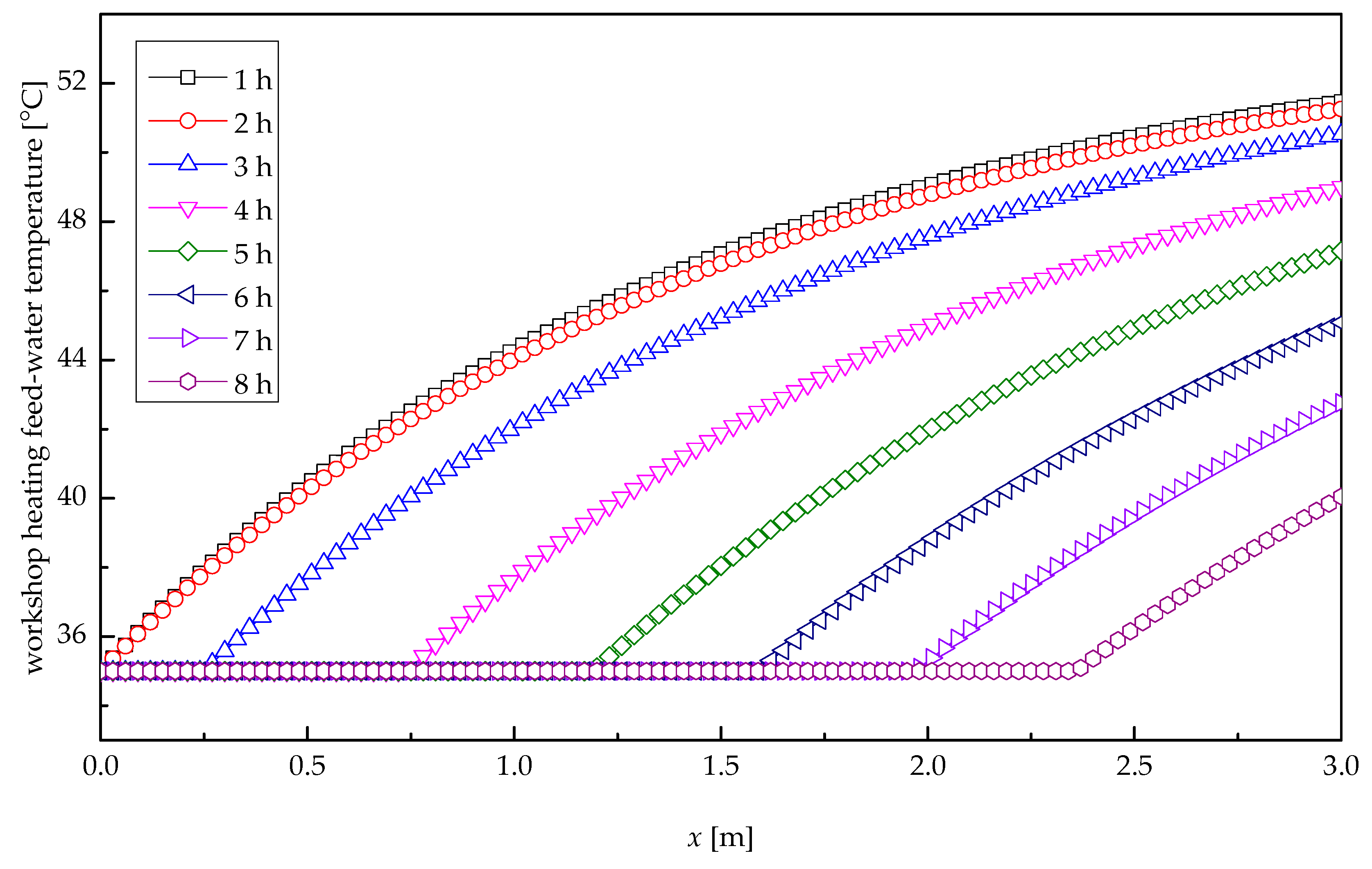
Figure 11 depicts the profile of the temperature of workshop heating feed-water along the tube length direction at several times for heat release process. At the inlet of the tube, the pool water presented a substantial rise of temperature as a result of the marked heat exchange, due to the big temperature difference between pool water and paraffin wax. With the increasing distance along the tube length, the temperature difference between pool water and paraffin wax decreased, resulting in a slow increase in the temperature of workshop heating feed-water. In the first 2.48 h, the outlet temperatures of pool water remained stable, and the temperature change of pool water there differed from that at the outlet, because their thermal resistances related to their solidification radii were greatly different. After 2.48 h, the paraffin wax at the entrance solidified completely, and the solidification cross-section presented a backward shift, which resulted in an expanding temperature difference and a rapid solidification velocity; thus, the temperature of pool water at the outlet of the tube varied appreciably with time. After 8-h heat transfer, the pool water temperature at the outlet was only 40 °C. It is after about 3.32-h heat exchange that the temperature of workshop heating feed-water reduced to 50 °C, i.e., the minimum required temperature of the heating system. Therefore, the meaningful heat release time was 3.32 h.
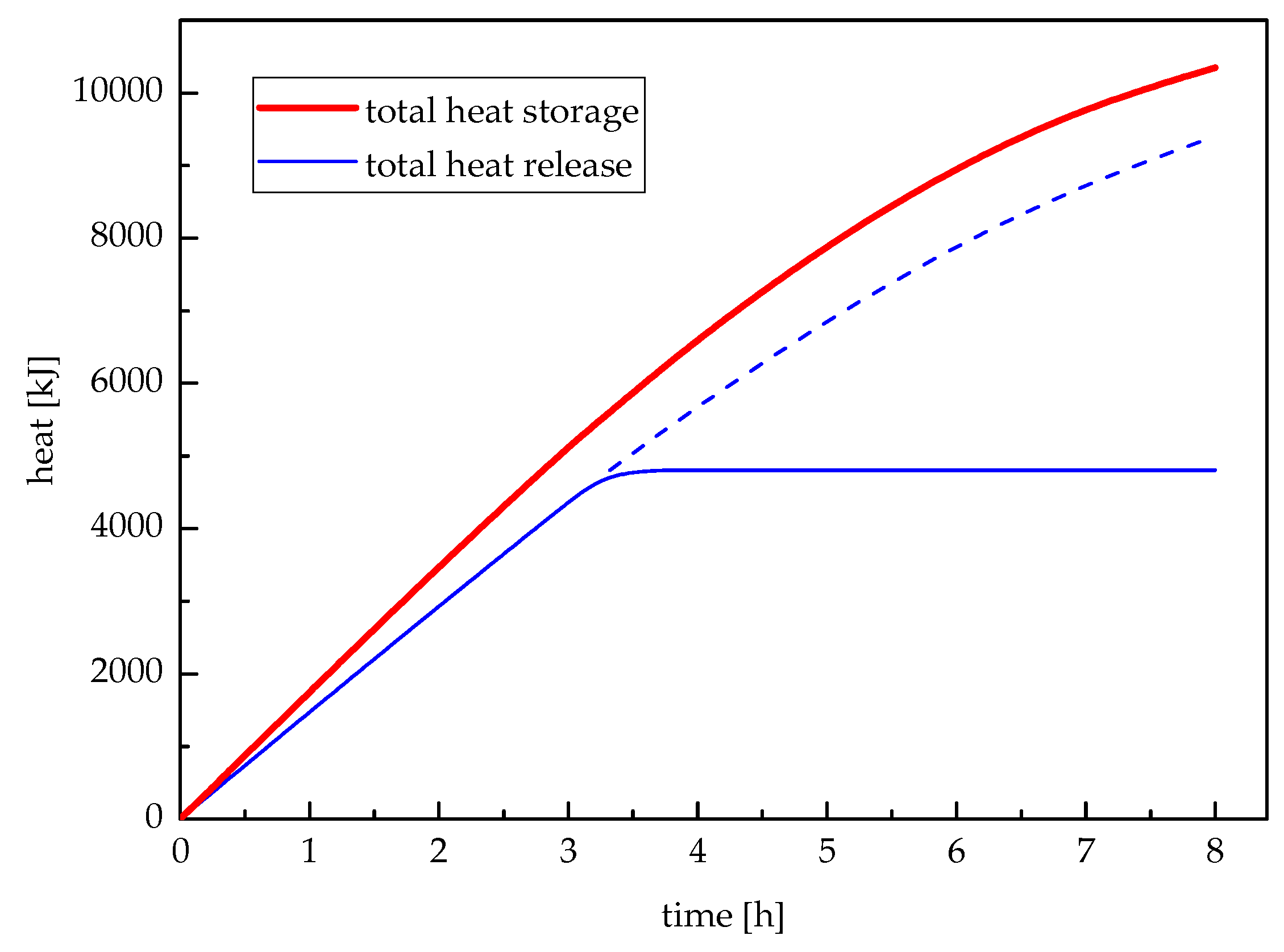
Figure 12 illustrates the total heat storage and total heat release varying with time. The total heat storage would continue increasing until the paraffin wax melted entirely; while the total heat release would continue decreasing until the last paraffin wax solidified. However, the change rates of heat storage and release were gradually becoming slow. During the meaningful heat release period, the total heat release was 4805 kJ. After the first 3.32 h, the temperature of workshop heating feed-water was less than 50 °C which did not satisfy the requirement of workshop heating unit.
Heat storage rate of this system is defined as the amount of heat stored in the paraffin wax per unit time, and heat release rate is the amount of heat released from the paraffin wax per unit time. The changes of heat storage rate and release rate with time are shown in
Figure 13. It can be seen that both heat storage rate and heat release rate decreased with the increasing time, which is because the temperature difference between paraffin wax and heat exchange fluid was bigger in the first period and smaller in the rest. It again confirmed the change of thermal resistance in paraffin wax caused by the changing melting radius and solidification radius of paraffin wax as shown in
Figure 9 and
Figure 11.
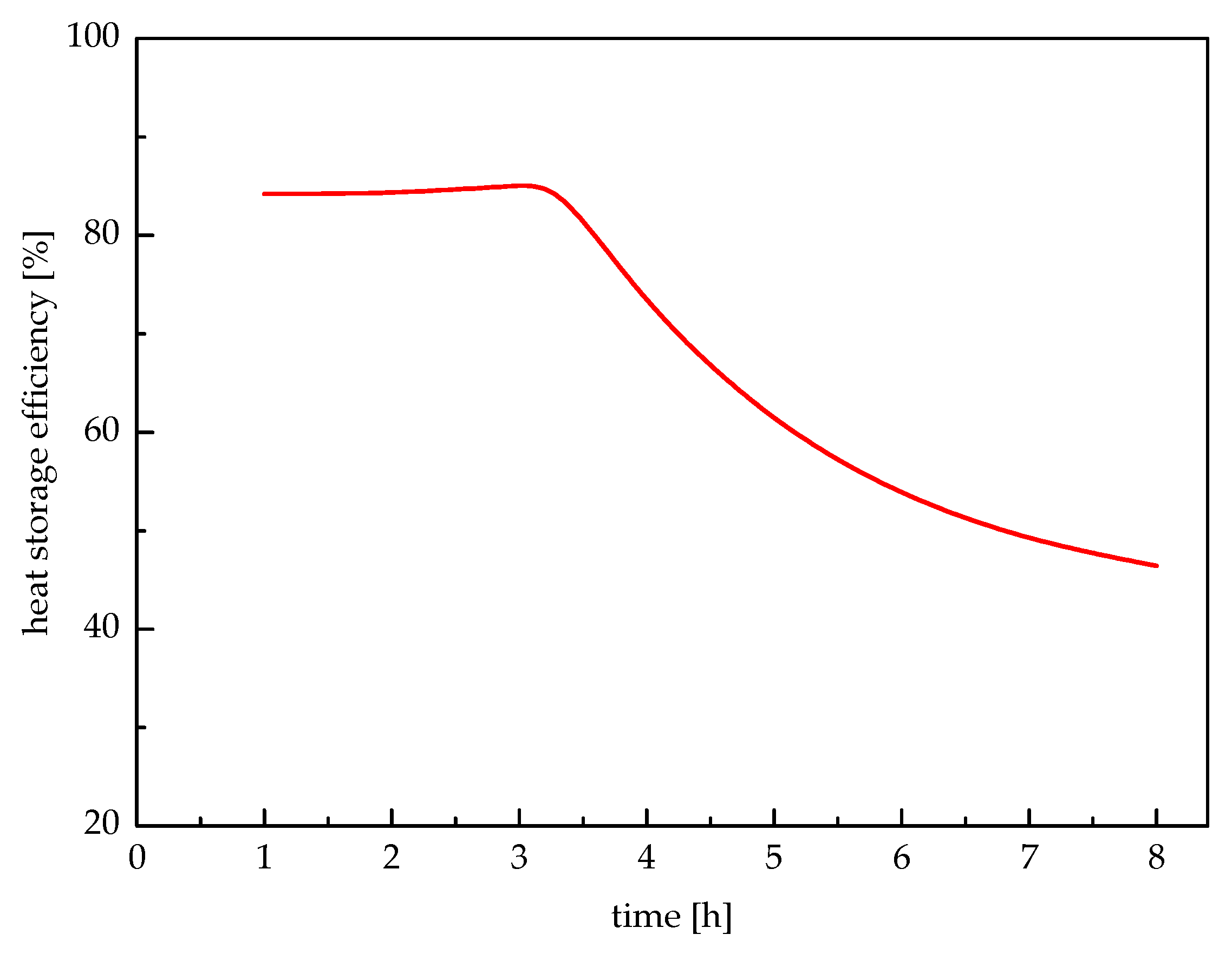
Storage efficiency of the system is defined as the ratio of the heat extracted to the heat stored in the paraffin wax. The storage efficiency of the system is illustrated in
Figure 14. It can be seen that in the first stage, the storage efficiency maintained at a higher level of appropriate 85% and had a very small increase with the increasing time. However, after the first 3.32 h, the storage efficiency decreased as the heat storage time increased. This is because that the meaningful heat extraction time was only 3.32 h. It is indicated that the storage efficiency of the proposed system was 43.78% for a storage cycle of 20.68-h storage and 3.32-h release.
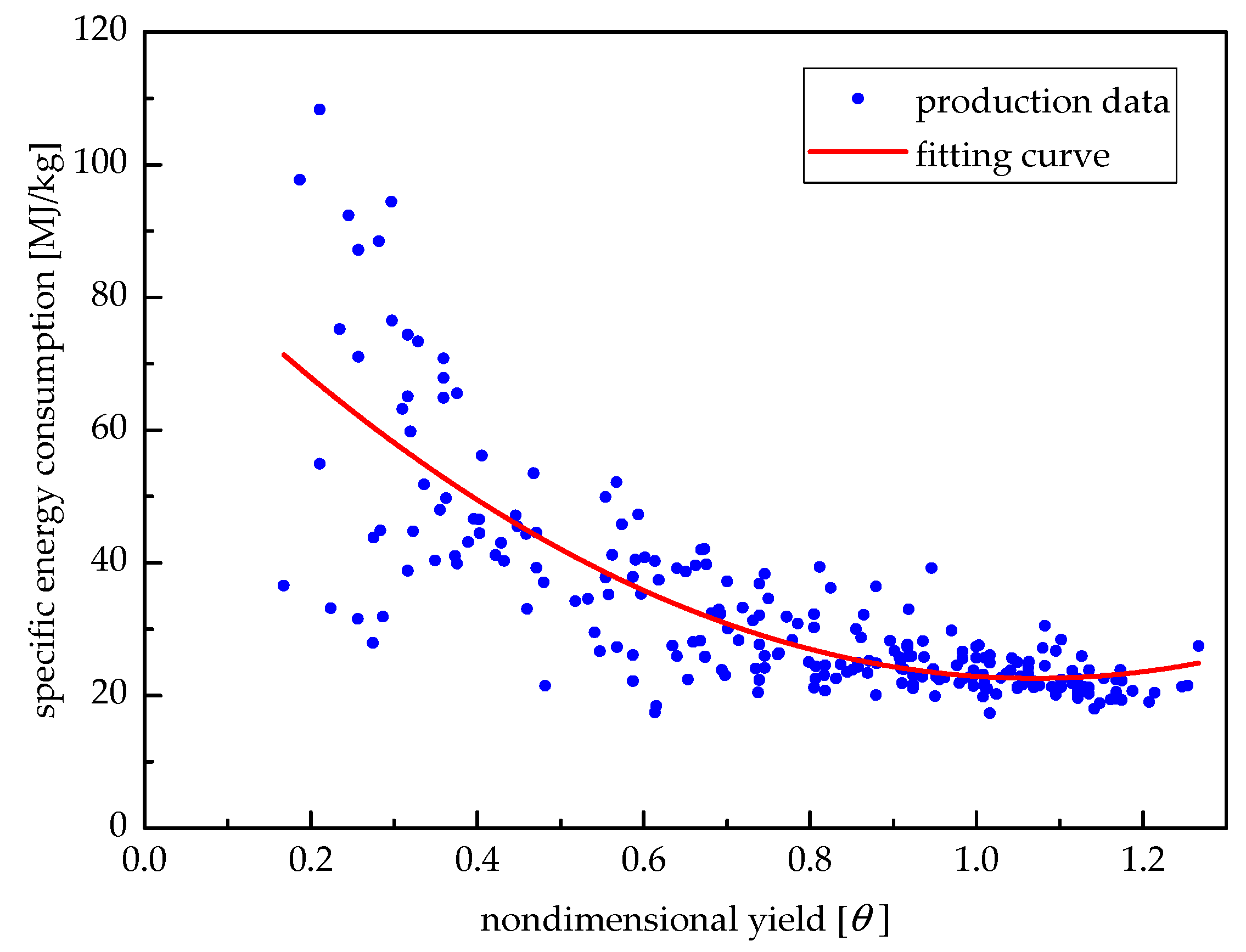
Figure 15 shows the relationship between the specific energy consumption of the medicine production unit and the corresponding yield. According to the production data from January to August of 2016, the fitting formula is given as:
where
b is the specific energy consumption, and
θ is the non-dimensional yield expressed as:
where
P and
Pr are the real yield and rated yield of the medicine production unit, respectively.
As reported in
Section 2, the low-load-operated continuous medicine production unit works under the load rate of appropriate 85%, while the proposed system is batch-type rated-load-operated. From Equation (16), the energy saving rate of the proposed system is
Though some of the results are qualitatively easy to understand, it is still scientifically significant to obtain the quantitative changing trends. The main contribution of this paper is to quantitatively determine the fluid temperature, the melting/solidification radius of paraffin wax along the tube, the total heat stored and released, the heat storage/release rate, the storage efficiency during the meaningful period, and the energy saving rate of the investigated application.
It should be noted that there are several types of heating system for industrial workshops. These heating systems in workshops can affect the thermal performance of TES unit. Thus, there are also limitations of this proposed workshop heating system for the design parameters. For instance, the pool water of 5 °C can be used to extract heat from the TES unit only if it can be preheated to 35 °C before entering the TES unit. However, in some medicine production processes, the heat used to preheat pool water is not available. Moreover, the flow rates of IRW and hot wind should match the heat requirements of the workshop heating unit and drying unit, respectively.
6. Conclusions
To use the IRW in a pharmaceutical plant, a workshop heating system using IRW through a TES unit with paraffin wax as a PCM is designed. In the proposed system, the medicine production unit works at its rated load, rather than the present low-load-operating of appropriate 85%. In the TES unit, paraffin wax is filled in the shell; IRW and pool water flows in the interior tube.
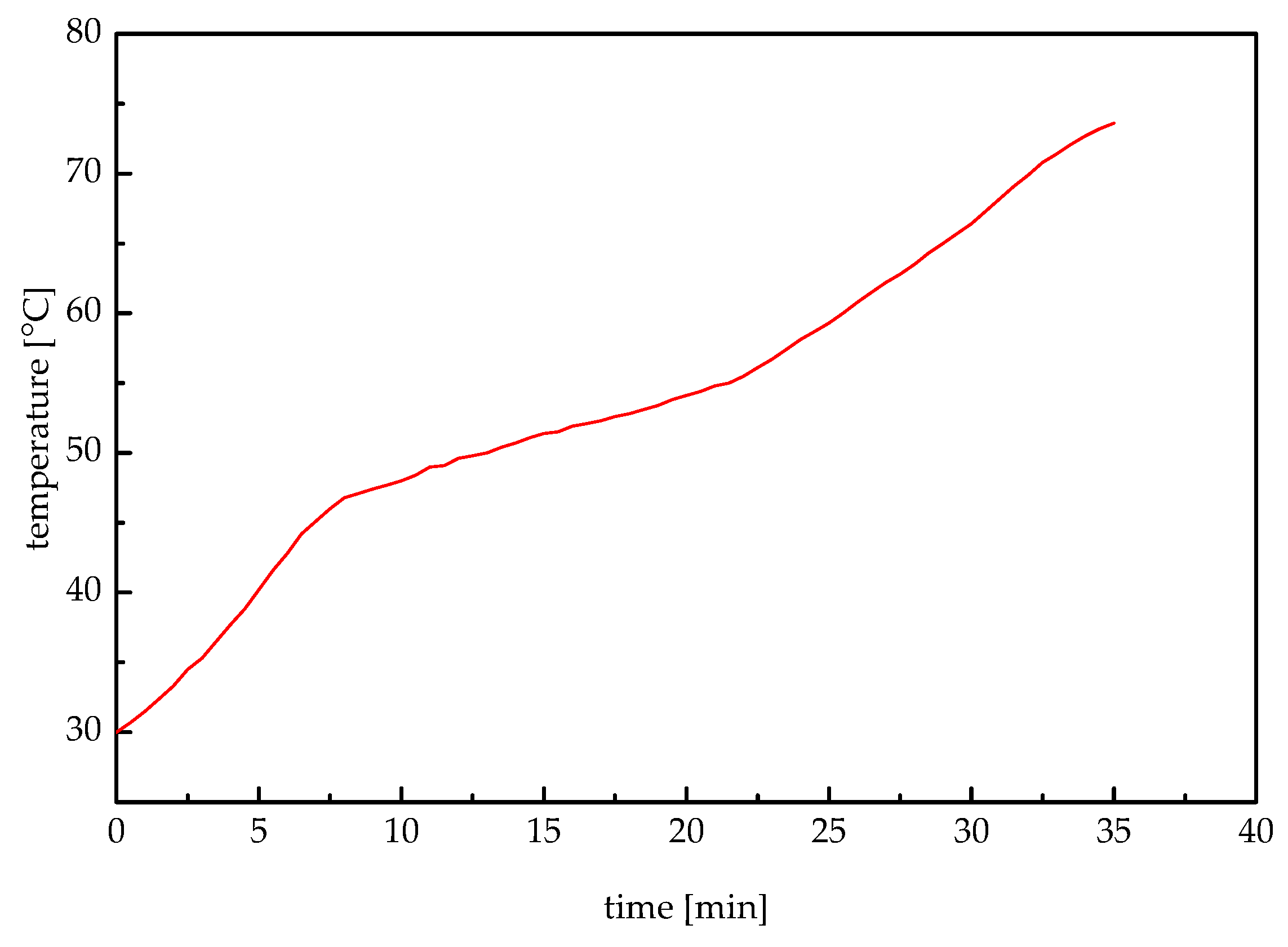
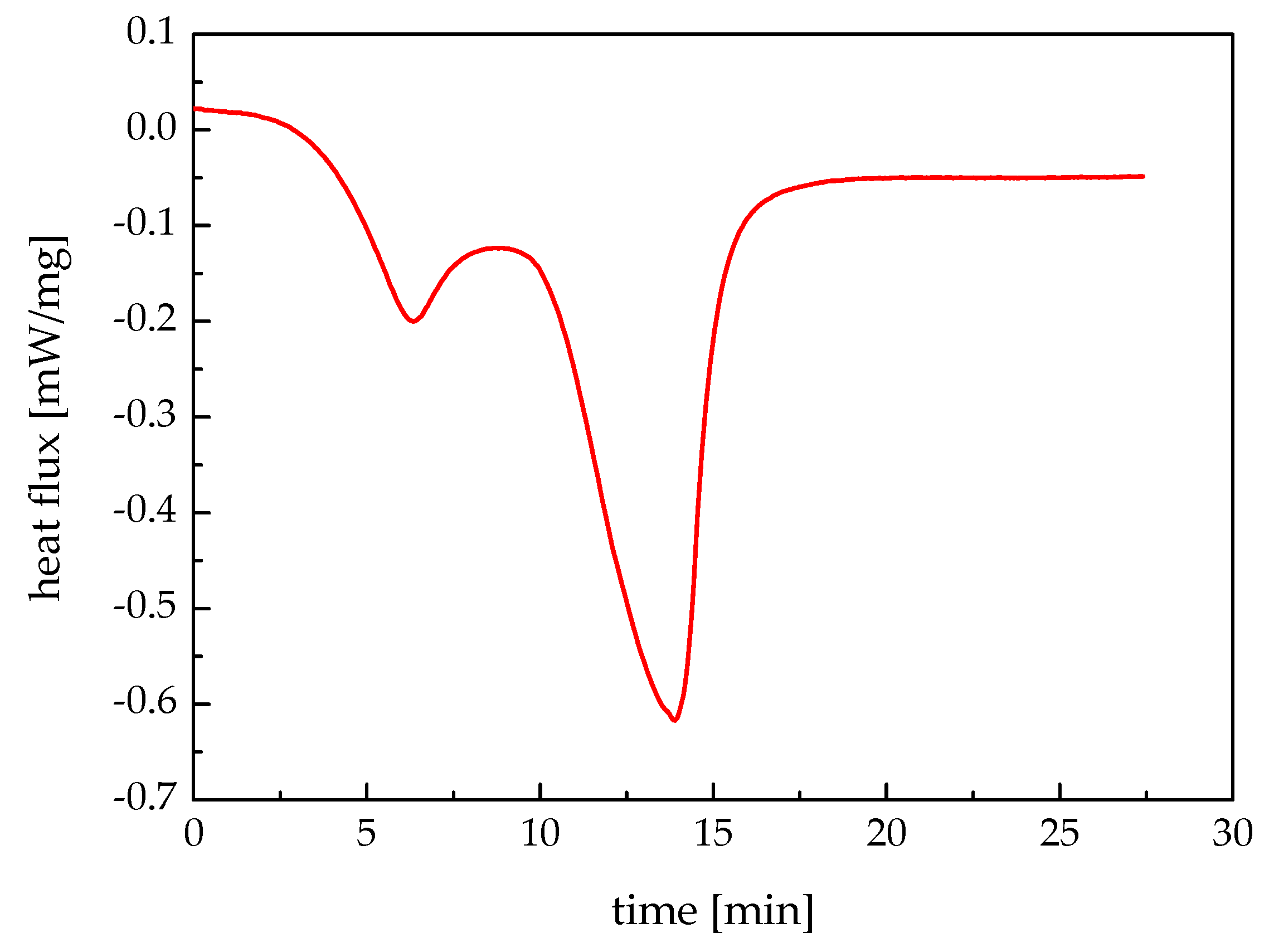
The mathematical models of heat storage and heat release processes are established, and the iterative formulae of the fluid temperature, the melting radius, and the solidification radius are obtained. In addition, the melting temperature range and the latent heat are measured by performing DSC. The melting temperature range of the investigated paraffin wax was 47 °C to 56 °C, and its latent heat was 171.4 kJ/kg.
The relationships between some quantities, such as melting radius, solidification radius, IRW temperature and workshop heating feed-water temperature, the pipe length, and time in the storage and release processes are simulated. It is concluded that the meaningful heat release time was 3.32 h in the investigated application. In addition, total heat storage and total heat release increased with the increasing time. During the meaningful heat release period, the total heat release was 4805 kJ. However, both heat storage rate and heat release rate decreased with the increasing time. The storage efficiency of this system maintained at a higher level of appropriate 85% at the first 3.32 h and decreased quickly as the heat storage time increased. For the system designed in this work, the entire storage efficiency was 43.78%. Additionally, the medicine production unit in this system had an energy saving rate of 10.25% compared with before.