Efficient Low Temperature Hydrothermal Carbonization of Chinese Reed for Biochar with High Energy Density
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Performance of HTC for Biochar
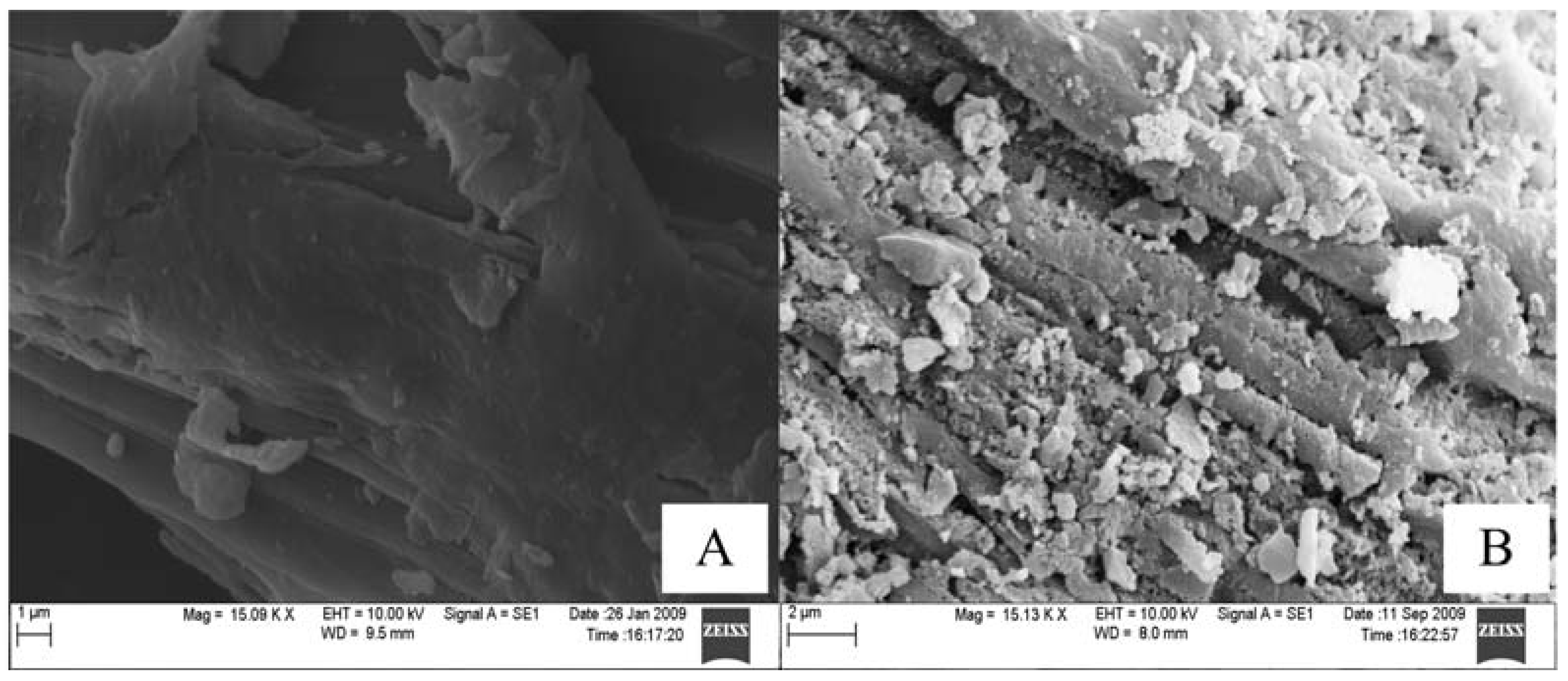
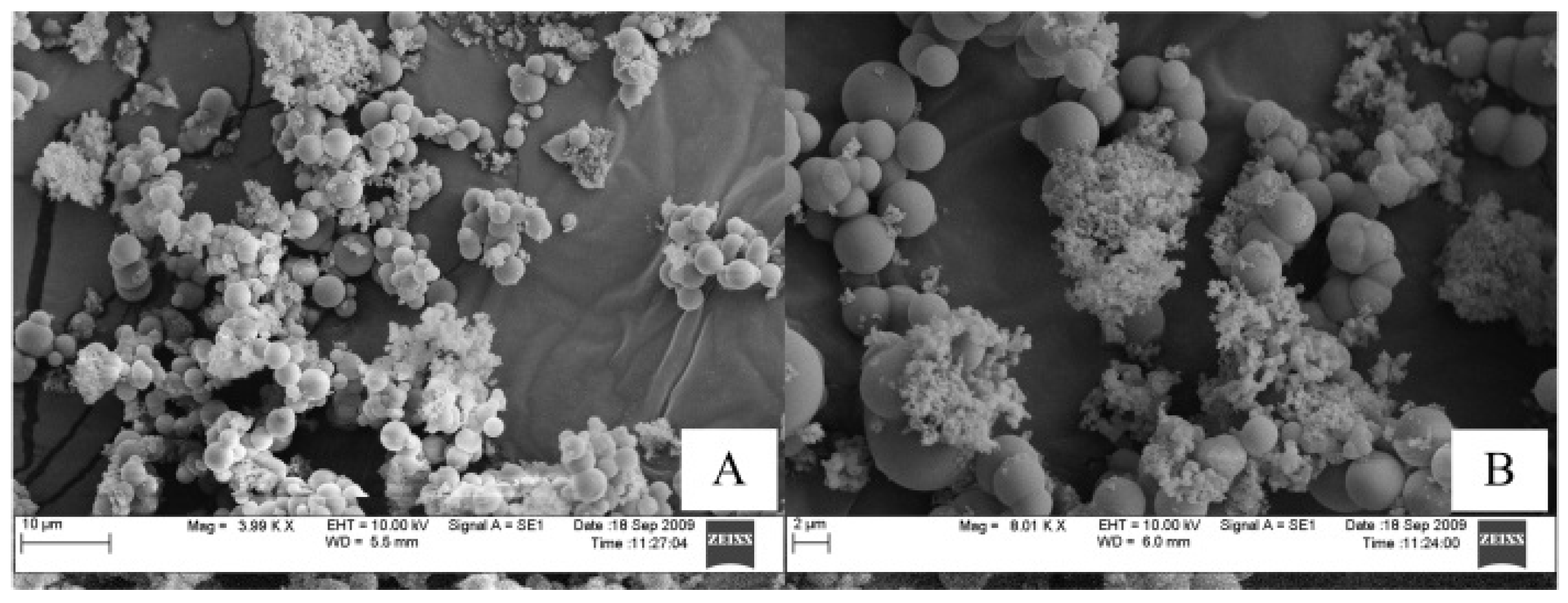
3.2. Characterization of Reed and Biochar
3.3. HTC of Biocrude for Biochar
4. Materials and Methods
4.1. Material
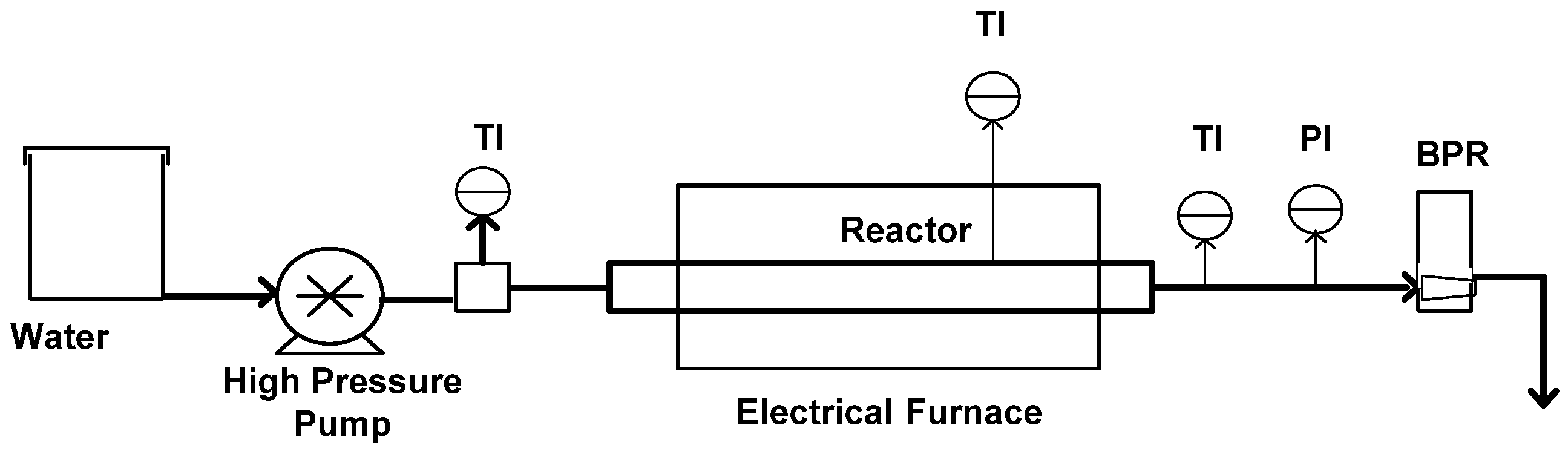
4.2. Apparatus and Procedure
4.3. Characterization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Matsumura, Y.; Sasaki, M.; Okuda, K. Supercritical water treatment of biomass for energy and material recovery. Combust. Sci. Technol. 2006, 178, 509–536. [Google Scholar] [CrossRef]
- Lee, I.; Kimm, M.; Ihm, S. Gasification of glucose in supercritical water. Ind. Eng. Chem. Res. 2002, 41, 1182–1188. [Google Scholar] [CrossRef]
- Motonobu, G.; Ryusaku, O.; Tsutomu, H. Hydrothermal conversion of municipal organic waste into resources. Bioresour. Technol. 2004, 93, 279–284. [Google Scholar]
- Kong, L.Z.; Li, G.M.; Wang, H. Hydrothermal catalytic conversion of biomass for lactic acid production. J. Chem. Technol. Biotechnol. 2008, 83, 383–388. [Google Scholar] [CrossRef]
- Selhan, K.; Thallada, B.; Akinori, M. Low-temperature catalytic hydrothermal treatment of wood biomass: Analysis of liquid products. Chem. Eng. J. 2005, 108, 127–133. [Google Scholar]
- Sevilla, M.; Macia-Agullo, J.A.; Fuertes, A.B. Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef]
- Liu, W.J.; Zeng, F.X.; Jiang, H. Preparation of high adsorption capacity bio-chars from waste biomass. Biomass Bioenergy 2011, 102, 8247–8252. [Google Scholar]
- Libra, J.A.; Ro, K.S.; Kammann, C. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 89–124. [Google Scholar] [CrossRef]
- Gupta, R.B.; Kumar, S.; Kong, L.Z. Biomass to Biochar Conversion in Subcritical Water. U.S. Patent 8,637,718 B2, 8 January 2014. [Google Scholar]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem. A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Back in the black: Hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007, 31, 787–788. [Google Scholar] [CrossRef]
- Phillip, E. Organic chemical reactions in supercritical water. Chem. Rev. 1999, 99, 603–621. [Google Scholar]
- Hu, B.; Yu, S.H.; Wang, K.; Liu, L.; Xu, X.W. Functional carbonaceous materials from hydrothermal carbonization of biomass: An effective chemical process. Dalton Trans. 2008, 6, 5414–5423. [Google Scholar] [CrossRef] [PubMed]
- Laginhas, C.; Valente Nabais, J.M.; Titirici, M.M. Activated carbons with high nitrogen content by a combination of hydrothermal carbonization with activation. Microporous Mesoporous Mater. 2016, 226, 125–132. [Google Scholar] [CrossRef]
- Kumar, S.; Loganathan, V.A.; Gupta, R.B.; Barnett, M.O. An Assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J. Environ. Manag. 2011, 92, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhou, Y.Y.; Meng, F.; Zhang, Y.H.; Liu, Z.H.; Zhang, W.Q.; Xue, G. Preparation and characterization of hydrochar from waste eucalyptus bark by hydrothermal carbonization. Energy 2016, 97, 238–245. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Mubarak, N.M.; Jayakumar, N.S.; Sahu, J.N.; Ganesan, P. Chemical, dielectric and structural characterization of optimized hydrochar produced from hydrothermal carbonization of palm shell. Fuel 2016, 163, 88–97. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zhang, F.S. Removal of copper (II) and phenol from aqueous solution using porous carbons derived from hydrothermal chars. Desalination 2011, 267, 101–106. [Google Scholar] [CrossRef]
- Watanabe, M.; Sato, T.; Inomata, H.; Smith, R.L., Jr.; Arai, K., Jr.; Kruse, A.; Dinjus, E. Chemical reactions of C1 compounds in near-critical and supercritical water. Chem. Rev. 2004, 104, 5803–5821. [Google Scholar]
- Savova, D.; Apak, E.; Ekinci, E.; Yardim, F.; Petrov, N.; Budinova, T.; Razvigorova, M.; Minkova, V. Biomass conversion to carbon adsorbents and gas. Biomass Bioenergy 2011, 21, 133–142. [Google Scholar] [CrossRef]
- Kong, L.Z.; Miao, P.J.; Qin, J.G. Characteristics and pyrolysis dynamic behaviors of hydrothermally treated micro crystalline cellulose. J. Anal. Appl. Pyrolysis 2013, 100, 67–74. [Google Scholar] [CrossRef]
- Sakanishi, K.; Ikeyama, N.; Sakaki, T. Comparison of the hydrothermal decompositionreactivities of chitin and cellulose. Ind. Eng. Chem. Res. 1999, 38, 2177–2181. [Google Scholar] [CrossRef]
- Kong, L.Z.; Li, G.M.; He, W.Z. Reutilization disposal of sawdust and maize straw by hydrothermal reaction. Energy Sources Part A 2009, 31, 876–887. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Devolatilization of biomass fuels and biomass components studied by TG/FTIR technique. Ind. Eng. Chem. Res. 2006, 45, 4486–4493. [Google Scholar] [CrossRef]
- Kobayashi, N.; Okada, N.; Hirakawa, A.; Sato, T.; Kobayashi, J.; Hatano, S.; Itay, Y.; Mori, S. Characteristics of solid residues obtained from hot-compressed-water treatment of woody biomass. Ind. Eng. Chem. Res. 2009, 48, 373–379. [Google Scholar] [CrossRef]
- Zhu, N.Y.; Yan, T.M.; Qiao, J. Adsorption of arsenic, phosphorus and chromium by bismuth impregnated biochar: Adsorption mechanism and depleted adsorbent utilization. Chemosphere 2016, 164, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kothari, U.; Lee, Y.Y.; Gupta, R.B. Hydrothermal pretreatment of switchgrass and corn stover for production of ethanol and carbon microspheres. Biomass Bioenergy 2011, 35, 956–968. [Google Scholar] [CrossRef]


| Properties | Mass |
|---|---|
| Moisture (wt.%) | 2.6 |
| Lignin (wt.%) | 23.5 |
| Cellulose (wt.%) | 39.5 |
| Hemicellulose (wt.%) | 26.7 |
| Carbon (wt.%) | 44.6 |
| HHV (kJ/g) | 17.1 |
| Run | Temp. (°C) | Time (h) | P (MPa) | Yield (wt.%) | HHV (kJ/g) | Energy Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 200 | 2.0 | 17.2 | 59.8 | 21.5 | 75.2 |
| 2 | 200 | 2.0 | 27.6 | 66.7 | 20.9 | 81.5 |
| 3 | 200 | 4.0 | 17.2 | 63.2 | 21.5 | 79.5 |
| 4 | 200 | 4.0 | 27.6 | 60.6 | 21.8 | 77.2 |
| 5 | 230 | 2.0 | 17.2 | 48.9 | 26.9 | 76.9 |
| 6 | 230 | 4.0 | 27.6 | 45.6 | 24.3 | 64.8 |
| 7 | 230 | 2.0 | 17.2 | 52.8 | 25.7 | 79.4 |
| 8 | 230 | 4.0 | 27.6 | 49.1 | 25.4 | 72.9 |
| 9 | 200 | 4.0 | 2.4 | 66.4 | 20.3 | 78.9 |
| 10 | 230 | 4.0 | 4.1 | 37.8 | 23.5 | 51.9 |
| 11 | 260 | 4.0 | 6.5 | 28.4 | 25.9 | 43.0 |
| 12 | 280 | 4.0 | 8.3 | 19.0 | 28.1 | 31.2 |
| 13 | 280 | 0.5 | 7.2 | 20.7 | 24.7 | 29.9 |
| 14 | 280 | 1.0 | 7.2 | 19.2 | 28.3 | 31.8 |
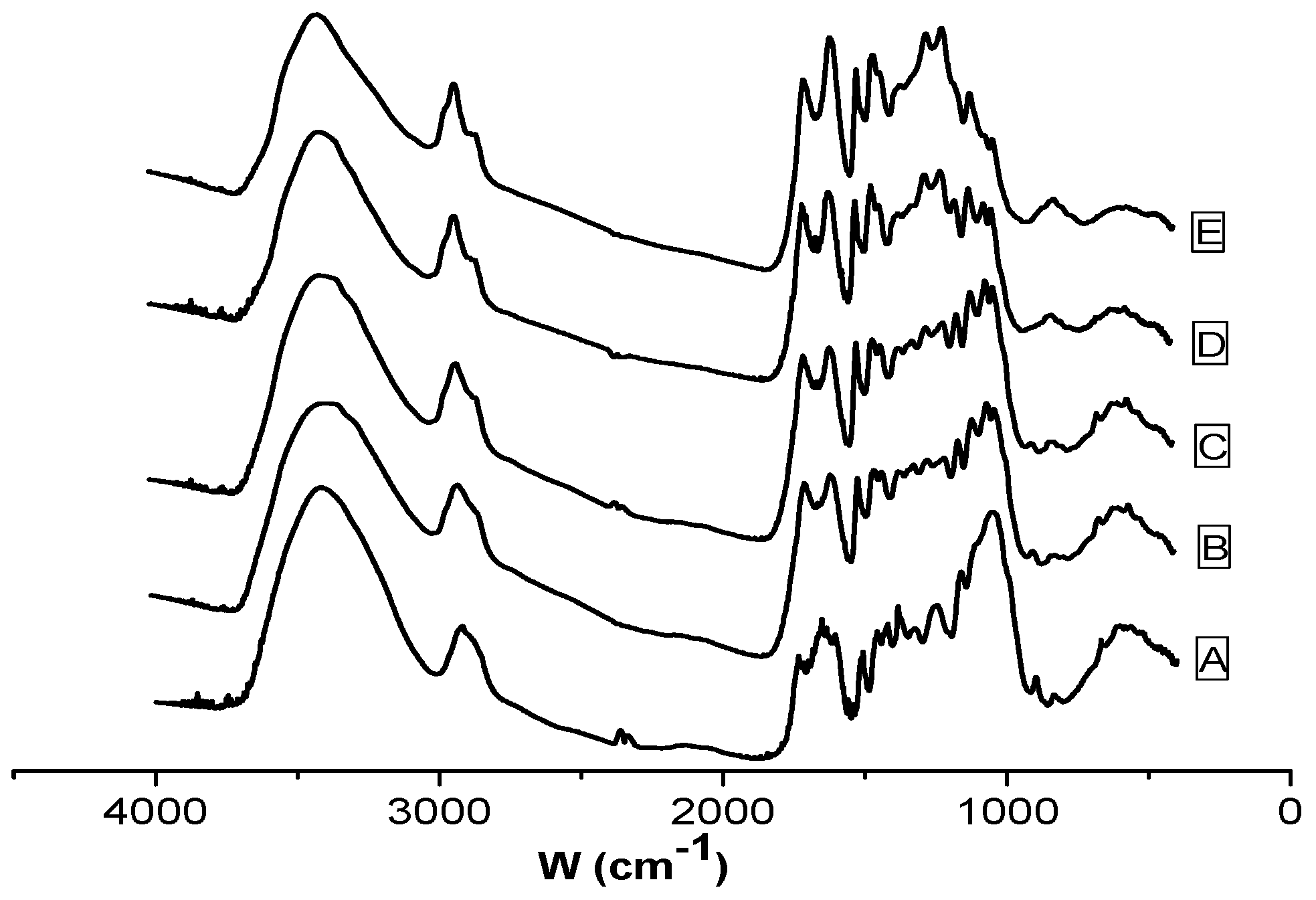
| Position of the Bands (cm−1) | Functional Group |
|---|---|
| 3401 | O–H stretching vibration |
| 2920 | CH, CH2 stretching vibration |
| 1700 | Carbonyl C=O stretching vibration |
| 1600 | C=C stretching vibration |
| 1513 | Benzene ring stretching vibration |
| 1424 | CH2 shearing, CH2 bending vibration |
| 1383 | CH bending vibration |
| 1267 | C–O–C stretching vibration in alkyl aromatic |
| 1160 | C–O–C asymmetry stretching vibration |
| 1060 | C–O stretching vibration |
| Composition | 200 °C | 230 °C | 260 °C | 280 °C |
|---|---|---|---|---|
| Glucose (g/L) | 2.7 | 1.2 | 0.4 | 0.3 |
| Lactic acid (g/L) | 3.2 | 3.5 | 3.6 | 2.7 |
| Acetic acid (g/L) | - | 0.6 | 1.1 | 0.9 |
| HMF (g/L) | - | - | - | 0.7 |
| Furan (g/L) | - | - | - | 1.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Huang, X.; Kong, L. Efficient Low Temperature Hydrothermal Carbonization of Chinese Reed for Biochar with High Energy Density. Energies 2017, 10, 2094. https://doi.org/10.3390/en10122094
Liu C, Huang X, Kong L. Efficient Low Temperature Hydrothermal Carbonization of Chinese Reed for Biochar with High Energy Density. Energies. 2017; 10(12):2094. https://doi.org/10.3390/en10122094
Chicago/Turabian StyleLiu, Chang, Xin Huang, and Lingzhao Kong. 2017. "Efficient Low Temperature Hydrothermal Carbonization of Chinese Reed for Biochar with High Energy Density" Energies 10, no. 12: 2094. https://doi.org/10.3390/en10122094