Sludge Acts as a Catalyst for Coal during the Co-Combustion Process Investigated by Thermogravimetric Analysis
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. TG Analysis
2.3. Data Analysis Methods
3. Results and Discussion
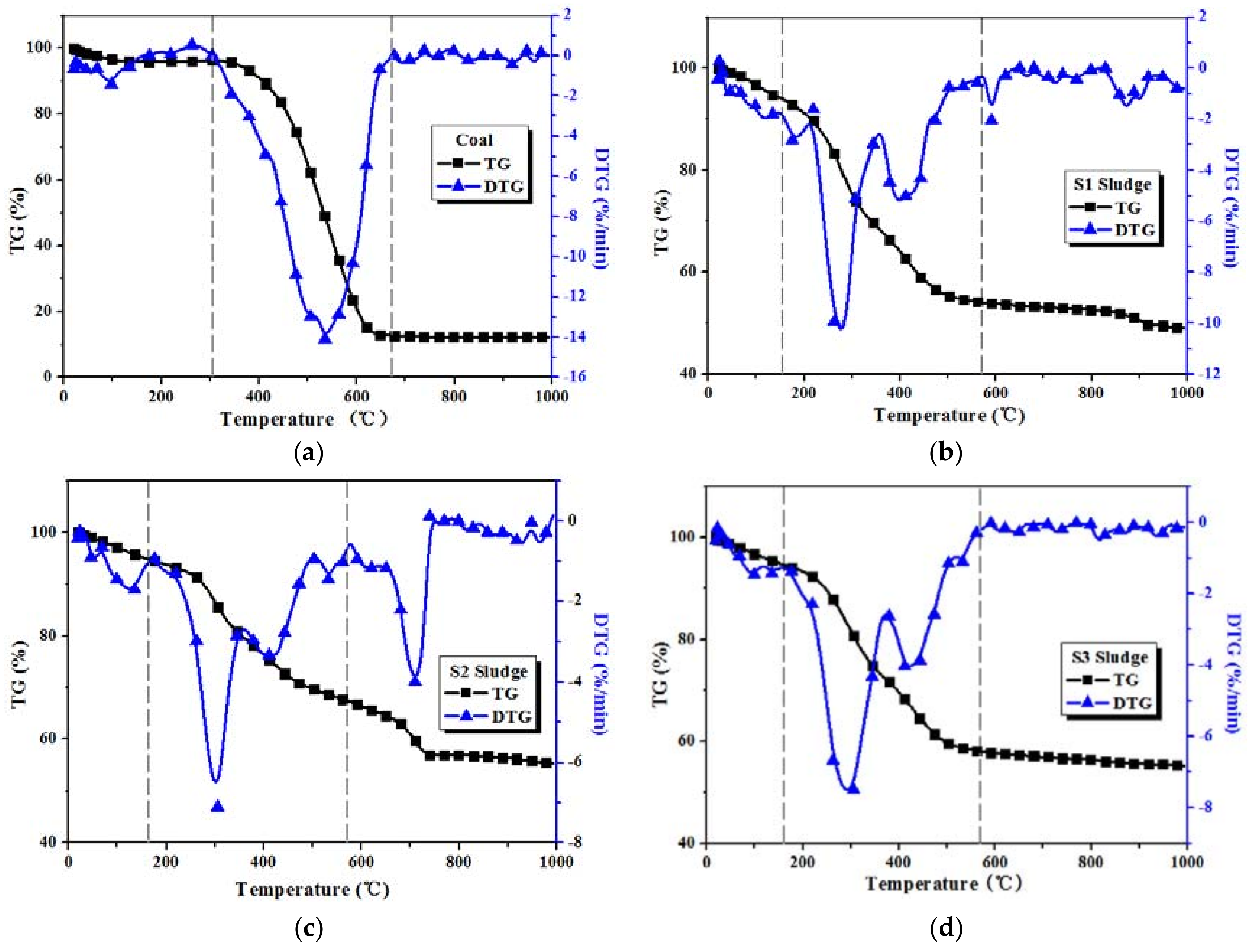
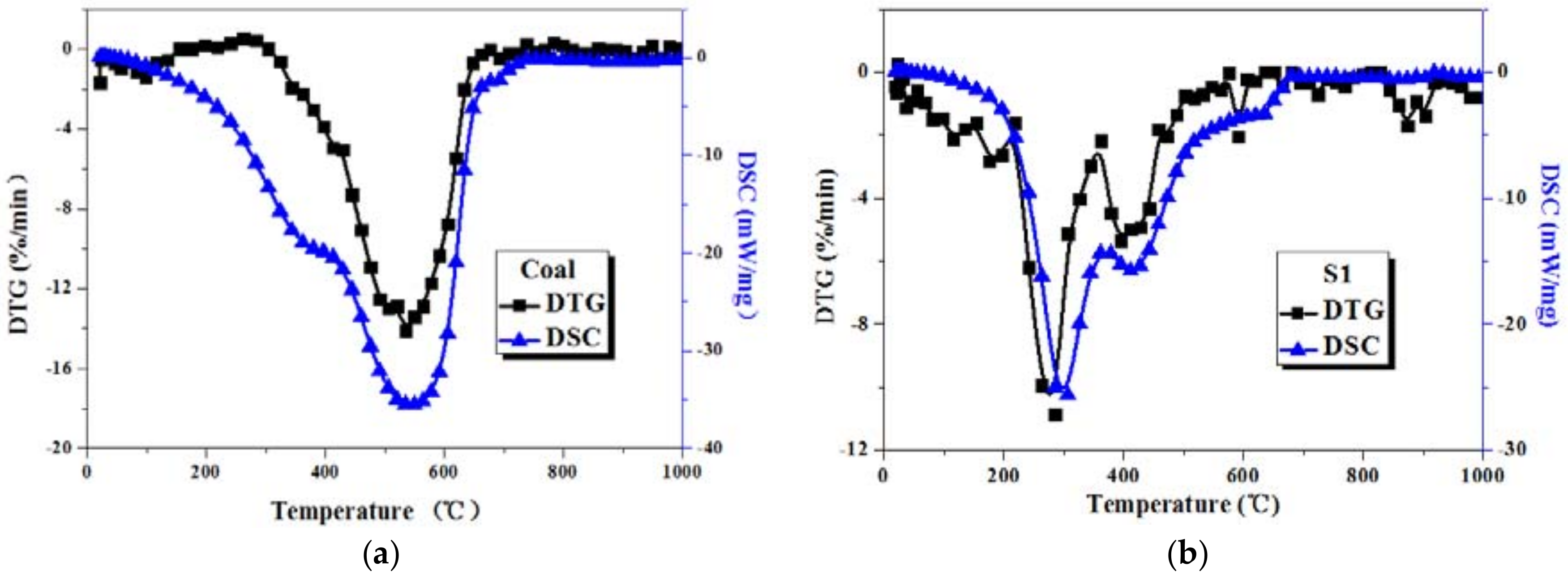
3.1. Combustion Characteristics of Coal and Sludge Samples
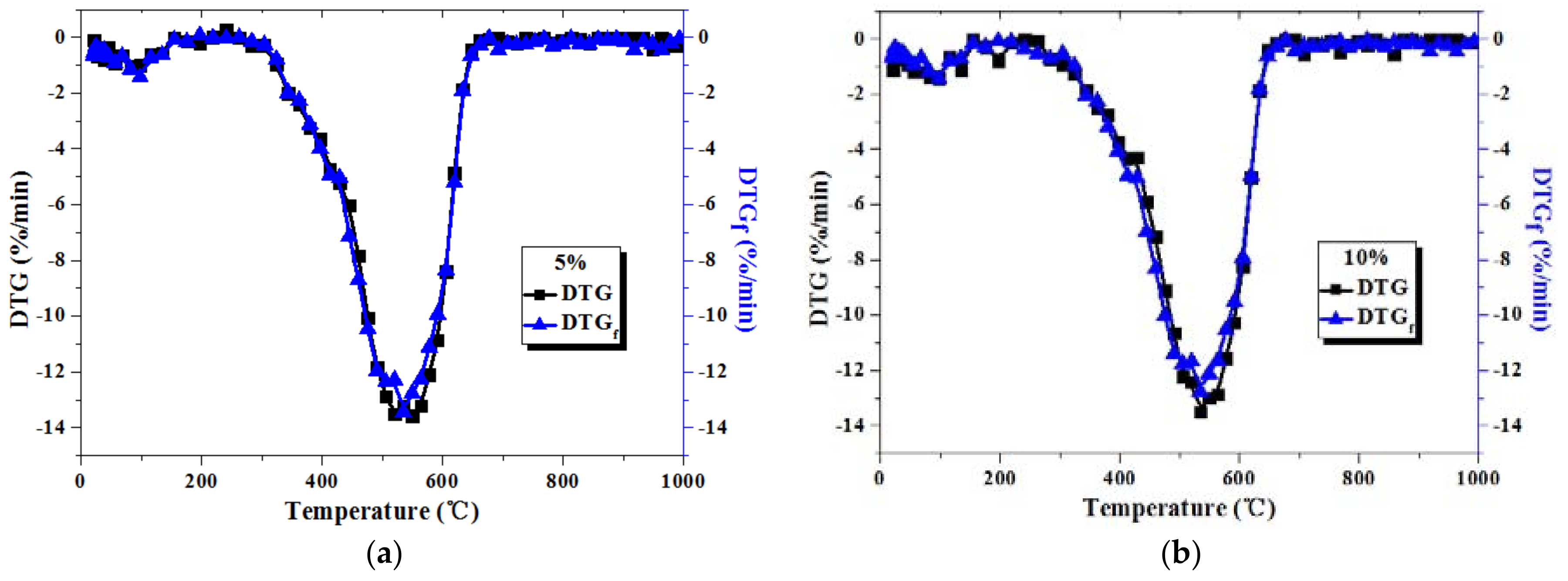
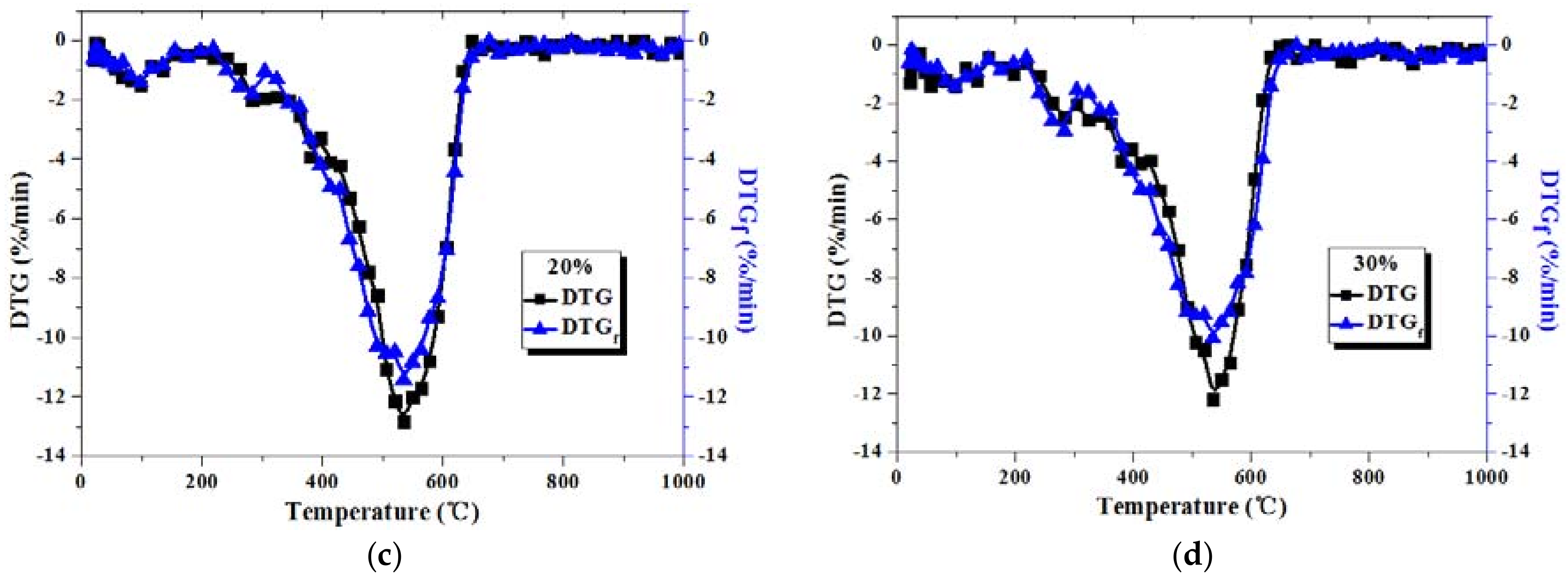
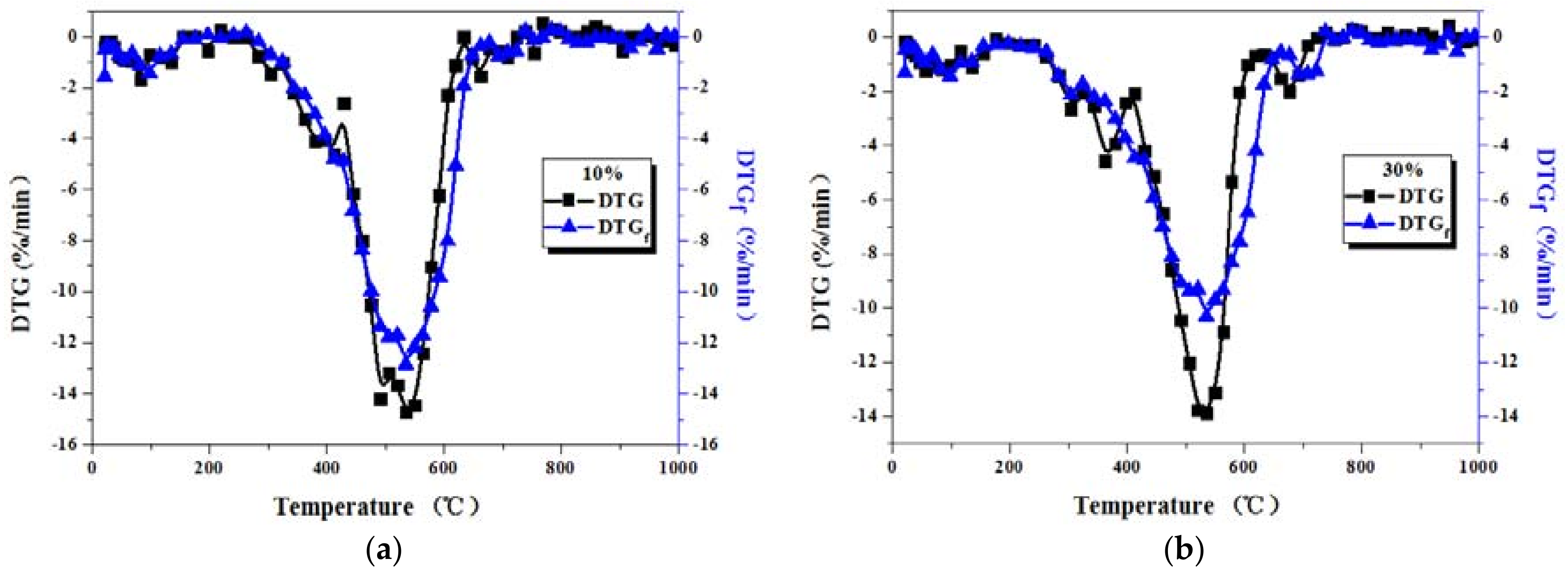
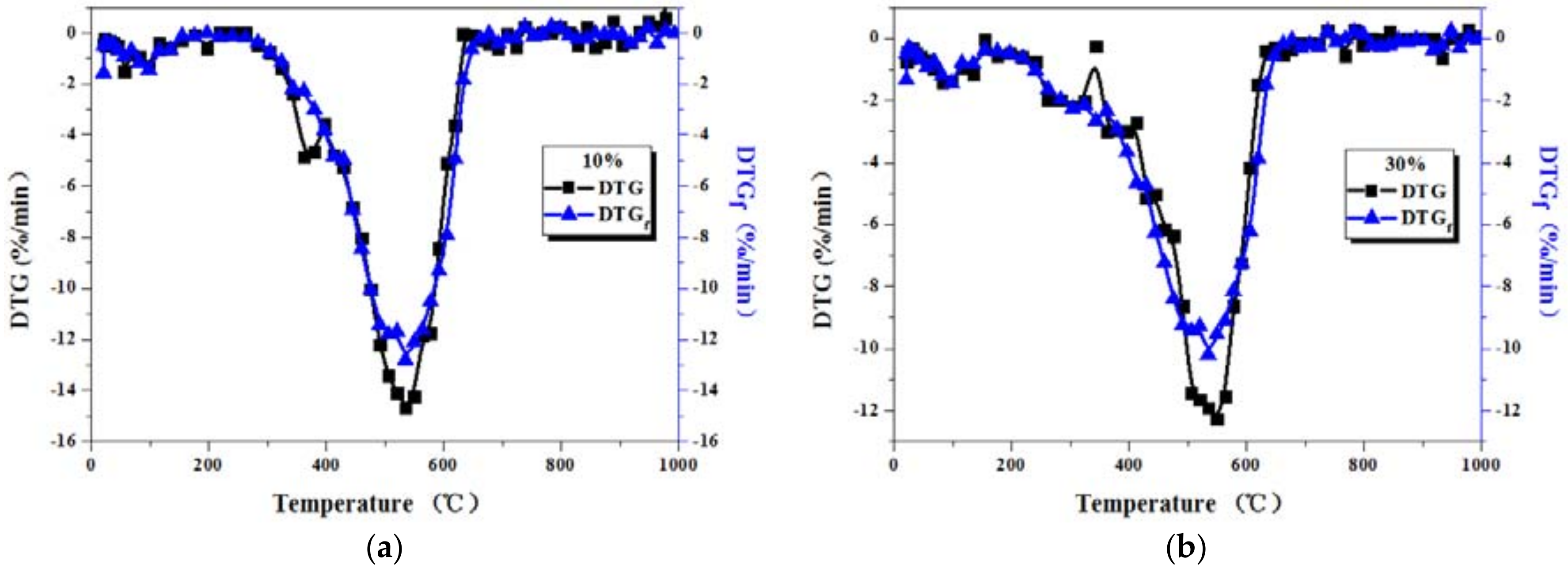
3.2. Comparison between Actual and Fitted DTG Curves for Coal-Sludge Blends
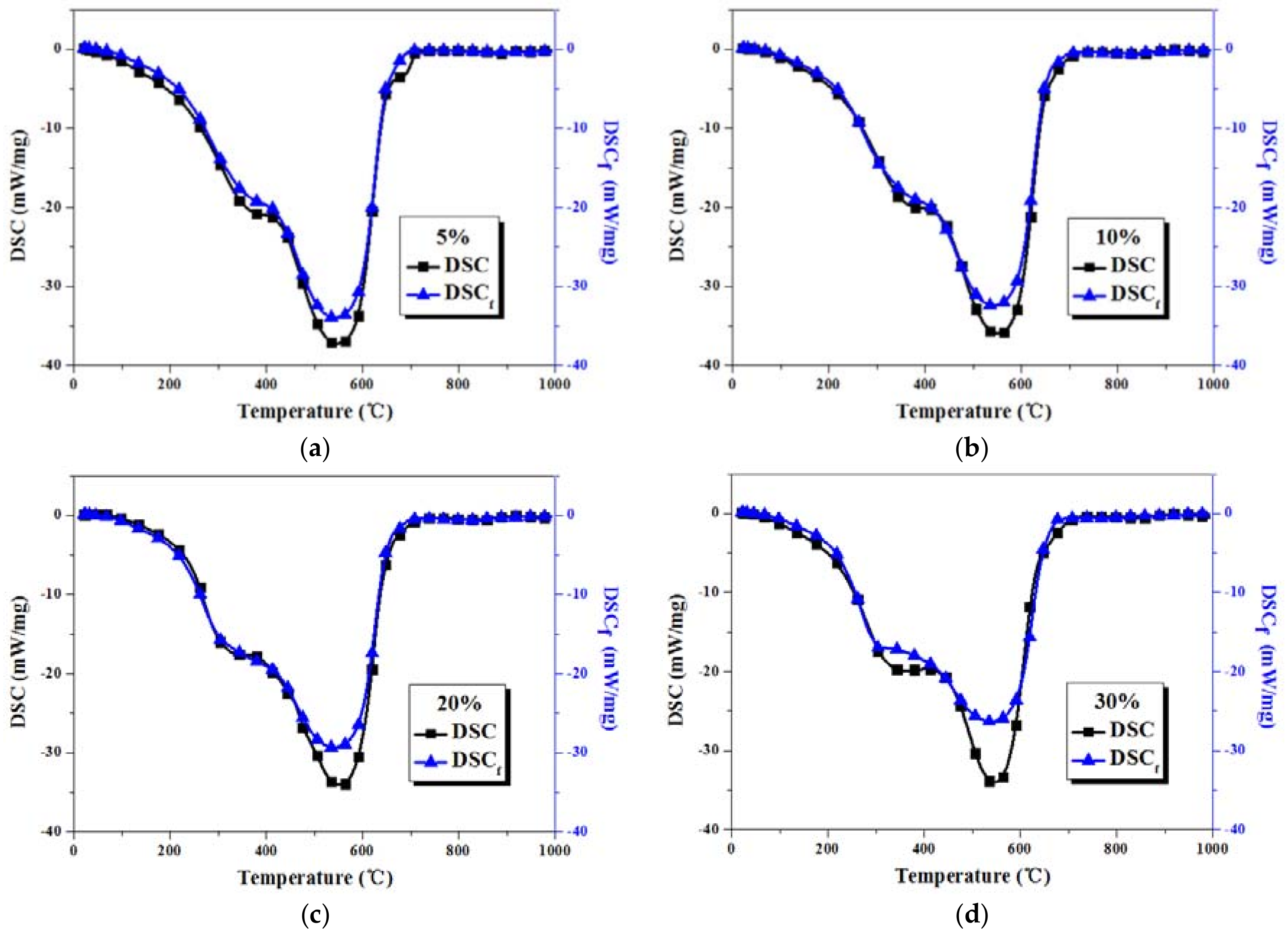
3.3. Comparison between Actual and Fitted DSC Curves of Coal-Sludge Blends
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J. On Development & Study of Urban Sludge Disposal New Technology. China Munic. Eng. 2013, 4, 41–44. [Google Scholar]
- Werther, J.; Ogada, T. Sewage sludge combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Xia, J.; Chen, G. A study on the feasibility of co-combustion of sewage sludge and coal in the coal-fired power plant. In Proceedings of the International Conference on Power Engineering, Tokyo, Japan, 16–20 November 2009; pp. 311–316. [Google Scholar]
- Hu, Z.; Ma, X.; Chen, Y.; Liao, Y.; Wu, J.; Yu, Z.; Li, S.; Yin, L.; Xu, Q. Co-combustion of coal with printing and dyeing sludge: Numerical simulation of the process and related NOX emissions. Fuel 2015, 139, 606–613. [Google Scholar] [CrossRef]
- Lopes, M.H.; Abelha, P.; Oliveira, J.F.S.; Cabrita, I.; Gulyurtlu, I. Heavy metals behavior during monocombustion and co-combustion of sewage sludge. Environ. Eng. Sci. 2005, 22, 205–220. [Google Scholar] [CrossRef]
- Karlsson, S.; Aring, L.E.; Liske, J. Reducing high-temperature corrosion on high-alloyed stainless steel superheaters by co-combustion of municipal sewage sludge in a fluidised bed boiler. Fuel 2015, 139, 482–493. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Zhang, X.; Xing, H.; Hu, H.; Yao, H. Novel utilization of conditioner CaO for gas pollutants control during co-combustion of sludge and coal. Fuel 2017, 206, 541–545. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, C.; Li, Y.; Lu, D.; Chen, X.; Duan, Y. Pollutants emission during co-combustion of petrochemical sludge with coal in circulating fluidized bed incinerator. In Proceedings of the 6th International Symposium on Coal Combustion, Wuhan, China, 1–4 December 2007; pp. 465–470. [Google Scholar]
- Tan, P.; Ma, L.; Xia, J.; Fang, C.; Zhang, C.; Chen, G. Co-firing sludge in a pulverized coal-fired utility boiler: Combustion characteristics and economic impacts. Energy 2017, 119, 392–399. [Google Scholar] [CrossRef]
- Chen, W.S.; Han, J.; Qin, L.; Furuuchi, M.; Mitsuhiko, H. The Emission Characteristics of PAHs during Coal and Sewage Sludge Co-Combustion in a Drop Tube Furnace. Aerosol Air Qual. Res. 2014, 14, 1160–1167. [Google Scholar] [CrossRef]
- Rong, H.; Teng, W.; Min, Z.; Hao, W.; Haobo, H. Combustion Characteristics and Slagging during Co-Combustion of Rice Husk and Sewage Sludge Blends. Energies 2017, 10, 438. [Google Scholar] [CrossRef]
- Liu, J.Y.; Huang, L.; Buyukad, M.; Evrendilek, F. Response surface optimization, modeling and uncertainty analysis of mass loss response of co-combustion of sewage sludge and water hyacinth. Appl. Therm. Eng. 2017, 125, 328–335. [Google Scholar] [CrossRef]
- Lin, Y.; Liao, Y.; Yu, Z.S.; Fang, S.; Ma, X.Q. The investigation of co-combustion of sewage sludge and oil shale using thermogravimetric analysis. Thermochim. Acta 2017, 653, 71–78. [Google Scholar] [CrossRef]
- Xie, Z.Q.; Ma, X.Q. The thermal behaviour of the co-combustion between paper sludge and rice straw. Bioresour. Technol. 2013, 146, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Moradian, F.; Pettersso, A.; Svärd, S.H.; Richards, T. Co-Combustion of Animal Waste in a Commercial Waste-to-Energy BFB Boiler. Energies 2013, 6, 6170–6187. [Google Scholar] [CrossRef]
- Aziz, M.; Budianto, D.; Oda, T. Computational Fluid Dynamic Analysis of Co-Firing of Palm Kernel Shell and Coal. Energies 2016, 9, 137. [Google Scholar] [CrossRef]
- Somorin, T.O.; Kolios, A.J.; Parker, A.; McAdam, E.; Williams, L.; Tyrrel, S. Faecal-wood biomass co-combustion and ash composition analysis. Fuel 2017, 203, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.F.; Ma, X.Q. Thermogravimetric analysis of the co-combustion of coal and paper mill sludge. Appl. Energy 2010, 87, 3526–3532. [Google Scholar]
- Otero, M.; Calvoa, L.F.; Gila, M.V.; Garcíaa, A.I.; Morána, A. Co-combustion of different sewage sludge and coal: A non-isothermal thermogravimetric kinetic analysis. Bioresour. Technol. 2008, 99, 6311–6319. [Google Scholar] [CrossRef] [PubMed]
- Magdziarz, A.; Wilk, M. Thermogravimetric study of biomass, sewage sludge and coal combustion. Energy Convers. Manag. 2013, 75, 425–430. [Google Scholar] [CrossRef]
- Otero, M.; Dı́ez, C.; Calvo, L.F.; Garcı́a, A.I.; Morán, A. Analysis of the co-combustion of sewage sludge and coal by TG-MS. Biomass Bioenergy 2002, 22, 319–329. [Google Scholar] [CrossRef]
- Otero, M.; Sánchez, M.E.; García, A.; Morán, A. EmSimultaneous thermogravimetric-mass spectrometric study on the co-combustion of coal and sewage sludges. J. Therm. Anal. Calorim. 2006, 86, 489–495. [Google Scholar] [CrossRef]
- Ninomiya, Y.; Zhang, L.; Sakano, T.; Kanaoka, C.; Masui, M. Transformation of mineral and emission of particulate matters during co-combustion of coal with sewage sludge. Fuel 2004, 83, 751–764. [Google Scholar] [CrossRef]
- Folgueras, A.B.; Dı́az, R.M.; Xiberta, J.; Prieto, I. Thermogravimetric analysis of the co-combustion of coal and sewage sludge. Fuel 2003, 82, 2051–2055. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Vamvuka, D.; Salpigidou, N.; Kastanaki, E.; Sfakiotakis, S. Possibility of using paper sludge in co-firing applications. Fuel 2009, 88, 637–643. [Google Scholar] [CrossRef]
- Yeboah, Y.D.; Xu, Y.; Sheth, A.; Godavarty, A.; Agrawal, P.K. Catalytic gasification of coal using eutectic salts: Identification of eutectics. Carbon 2003, 41, 203–214. [Google Scholar] [CrossRef]
- Sheth, A.; Yeboah, Y.D.; Godavarty, A.; Xu, Y.; Agrawal, P.K. Catalytic gasification of coal using eutectic salts: Reaction kinetics with binary and ternary eutectic catalysts. Fuel 2003, 82, 305–317. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Zhou, R.; Hu, J.; Wang, F.C.; Wang, J. Calcium-promoted catalytic activity of potassium carbonate for steam gasification of coal char: Influences of calcium species. Fuel 2012, 99, 64–71. [Google Scholar] [CrossRef]
- Domazetis, G.; Liesegang, J.; James, B.D. Studies of inorganics added to low-rank coals for catalytic gasification. Fuel Process. Technol. 2005, 86, 463–486. [Google Scholar] [CrossRef]
- Popa, T.; Fan, M.; Argyle, M.D.; Dyar, M.D.; Gao, Y.; Tang, J.; Speicher, E.A.; Kammene, D.M. H2 and COx generation from coal gasification catalyzed by a cost-effective iron catalyst. Appl. Catal. A Gen. 2013, 464, 207–217. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, D.; Wang, Y.; Argyle, M.D.; Fan, M. CO2 gasification of Powder River Basin coal catalyzed by a cost-effective and environmentally friendly iron catalyst. Appl. Energy 2015, 145, 295–305. [Google Scholar] [CrossRef]
- Fan, S.; Yuan, X.Z.; Zhao, L.; Xu, L.H.; Kang, T.J.; Kim, H.T. Experimental and kinetic study of catalytic steam gasification of low rank coal with an environmentally friendly, inexpensive composite K2CO3-eggshell derived CaO catalyst. Fuel 2016, 165, 397–404. [Google Scholar] [CrossRef]
- Monterroso, R.; Fan, M.; Argyle, M.D.; Varga, K.; Dyar, D.; Tang, J.; Sun, Q.; Towler, B.; Elliot, K.W.; Kammen, D. Characterization of the mechanism of gasification of a powder river basin coal with a composite catalyst for producing desired syngases and liquids. Appl. Catal. A Gen. 2014, 475, 116–126. [Google Scholar] [CrossRef]

| Samples | Sludges | Coal | ||
|---|---|---|---|---|
| S1 | S2 | S3 | ||
| Proximate analysis (wt %) | ||||
| Moisture | 5.16 | 3.70 | 3.37 | 3.55 |
| Volatiles | 40.74 | 38.78 | 35.76 | 30.69 |
| Ashes | 52.76 | 56.93 | 56.87 | 13.20 |
| Fixed carbon | 1.34 | 0.59 | 4.00 | 52.56 |
| Ultimate analysis (wt %) | ||||
| C | 20.98 | 15.31 | 16.82 | 67.97 |
| H | 3.31 | 2.49 | 3.36 | 4.04 |
| N | 2.50 | 1.50 | 3.23 | 1.05 |
| Stotal | 4.32 | 0.81 | 1.03 | 0.48 |
| O | 10.97 | 19.26 | 15.32 | 9.71 |
| Higher heating values (MJ/kg) | ||||
| HHV | 9.251 | 5.934 | 8.446 | 21.935 |
| Samples | Dried Sludge Weight Percentages (%) | Heating Rate (°C/min) |
|---|---|---|
| S1 and C | 0, 10, 30, 100 | 20 |
| 0, 5, 10, 20, 30, 100 | 30 | |
| 0, 10, 30, 100 | 40 | |
| S2 and C | 0, 10, 30, 100 | 30 |
| S3 and C | 0, 10, 30, 100 | 30 |
| Dried Sludge Weight Percentage (%) | Heating Rate (°C/min) | Maximum Weight Loss Rate of Fitted Curves (%/min) | Maximum Weight Loss Rate of Actual Curves (%/min) | Change Rate (%) |
|---|---|---|---|---|
| 10 | 20 | 10.641 | 11.075 | 4.08 |
| 30 | 12.765 | 13.511 | 5.84 | |
| 40 | 14.635 | 15.777 | 7.80 | |
| 30 | 20 | 8.396 | 9.264 | 10.34 |
| 30 | 10.284 | 12.088 | 17.54 | |
| 40 | 11.471 | 13.915 | 21.31 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Wang, F.; Kanhar, A.H. Sludge Acts as a Catalyst for Coal during the Co-Combustion Process Investigated by Thermogravimetric Analysis. Energies 2017, 10, 1993. https://doi.org/10.3390/en10121993
Chen W, Wang F, Kanhar AH. Sludge Acts as a Catalyst for Coal during the Co-Combustion Process Investigated by Thermogravimetric Analysis. Energies. 2017; 10(12):1993. https://doi.org/10.3390/en10121993
Chicago/Turabian StyleChen, Wendi, Fei Wang, and Altaf Hussain Kanhar. 2017. "Sludge Acts as a Catalyst for Coal during the Co-Combustion Process Investigated by Thermogravimetric Analysis" Energies 10, no. 12: 1993. https://doi.org/10.3390/en10121993





