Experimental Study of Hydrogen Addition Effects on a Swirl-Stabilized Methane-Air Flame
Abstract
:1. Introduction
2. Experiments and Method
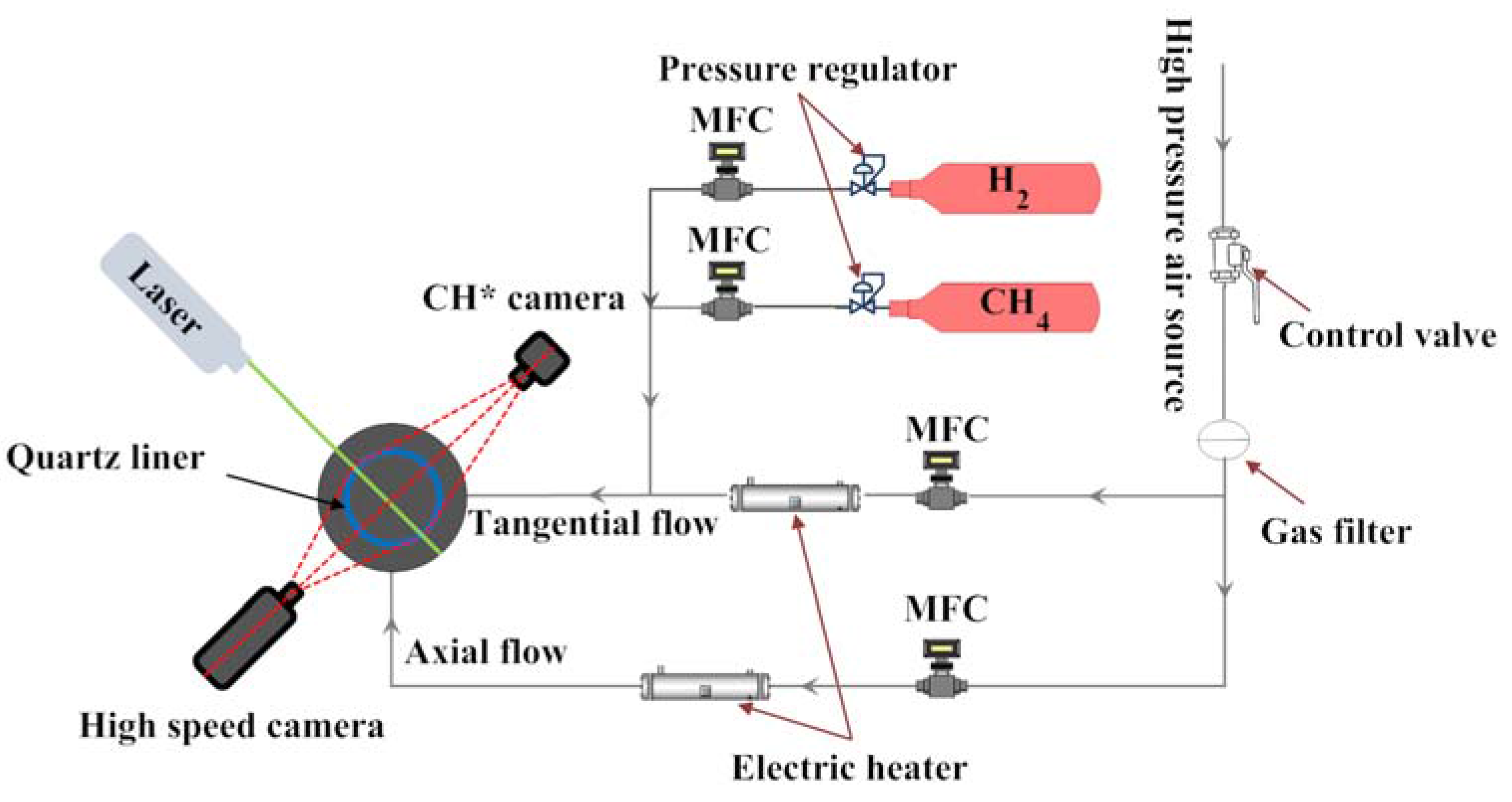
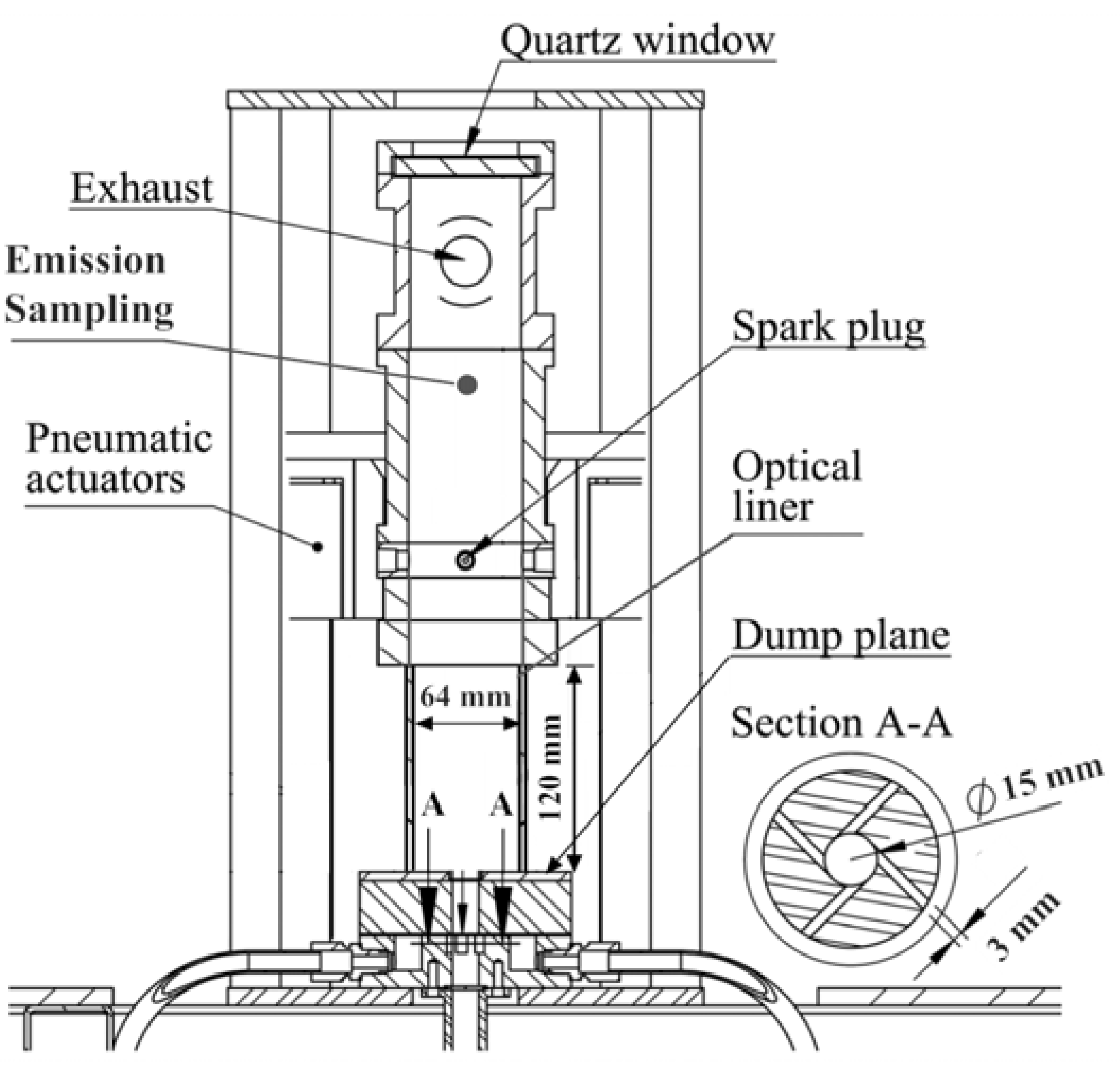
2.1. Flow Path and Equipment
2.2. Operating Condition, LBO, and CO Emission Measurements
2.3. Investigation of the Flame and Flow Field
2.4. Mathematical Description for the Proper Orthogonal Decomposition (POD)
2.5. Computational Implementation: Method of Snapshots
3. Results and Discussion
3.1. LBO Limits and The Averaged Features of Flames and Flow Field
3.2. Instantaneous Velocity Fields
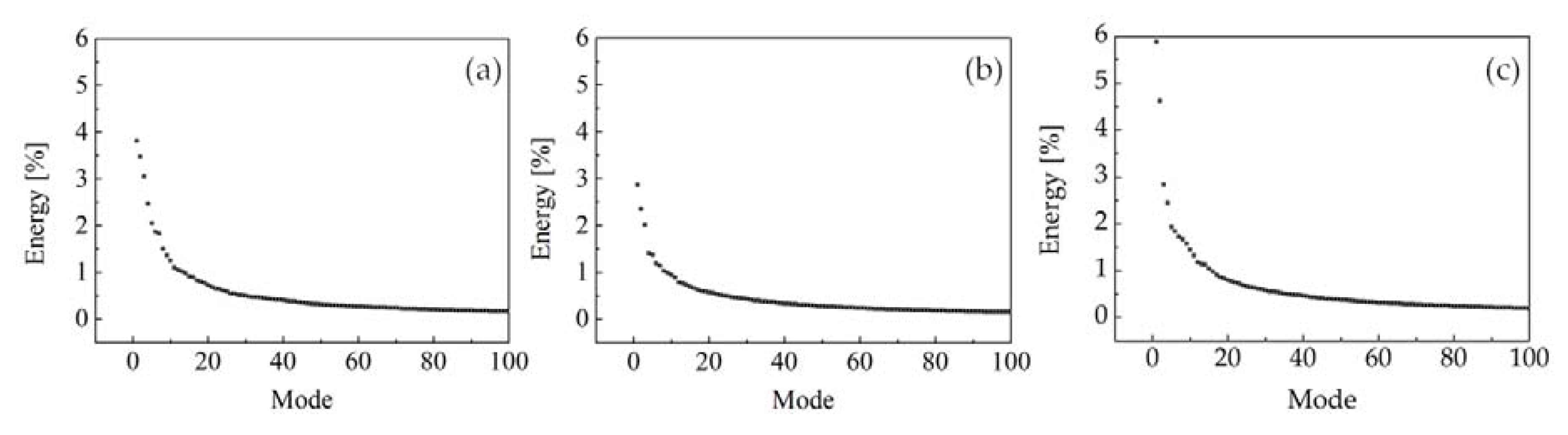
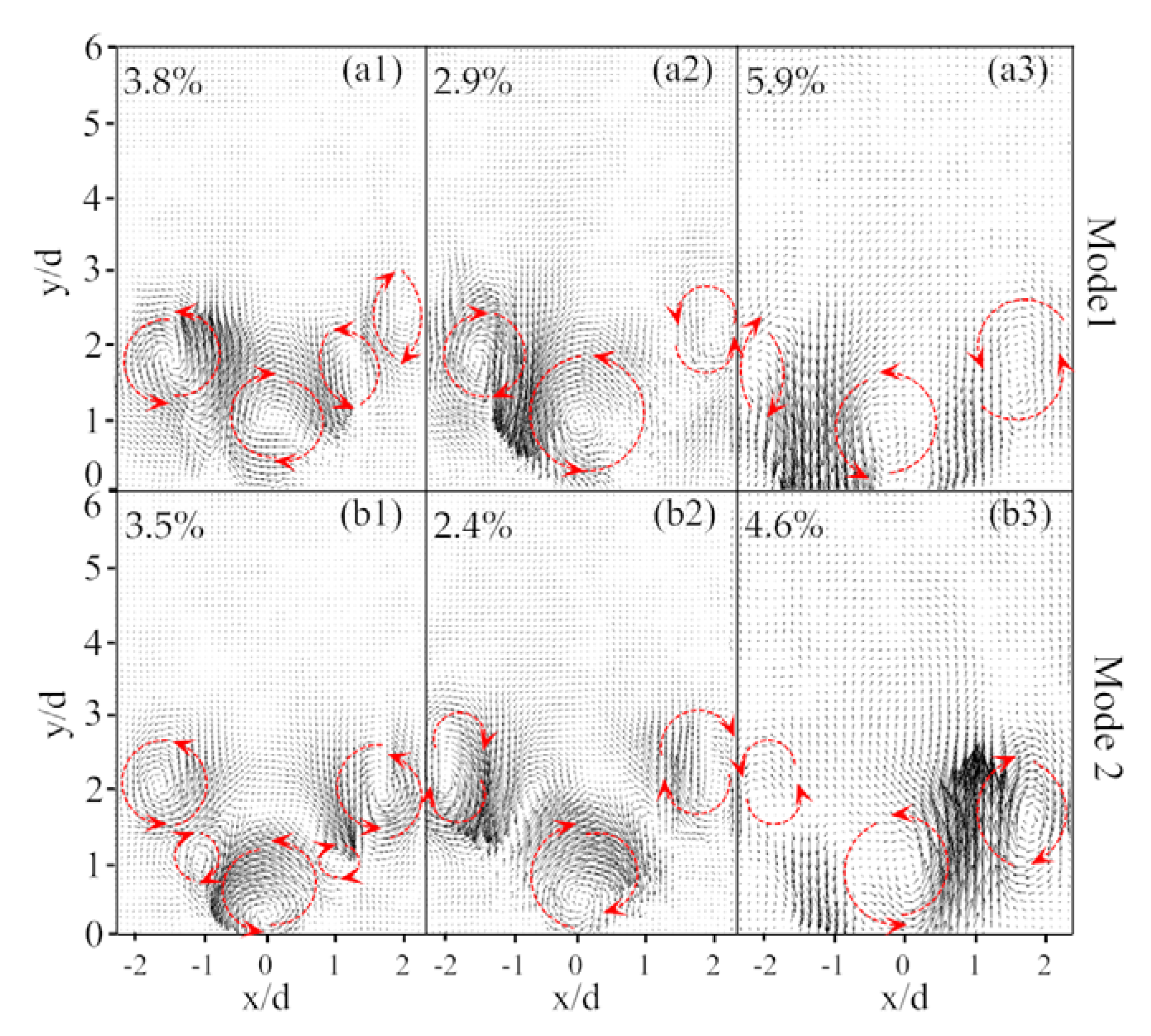
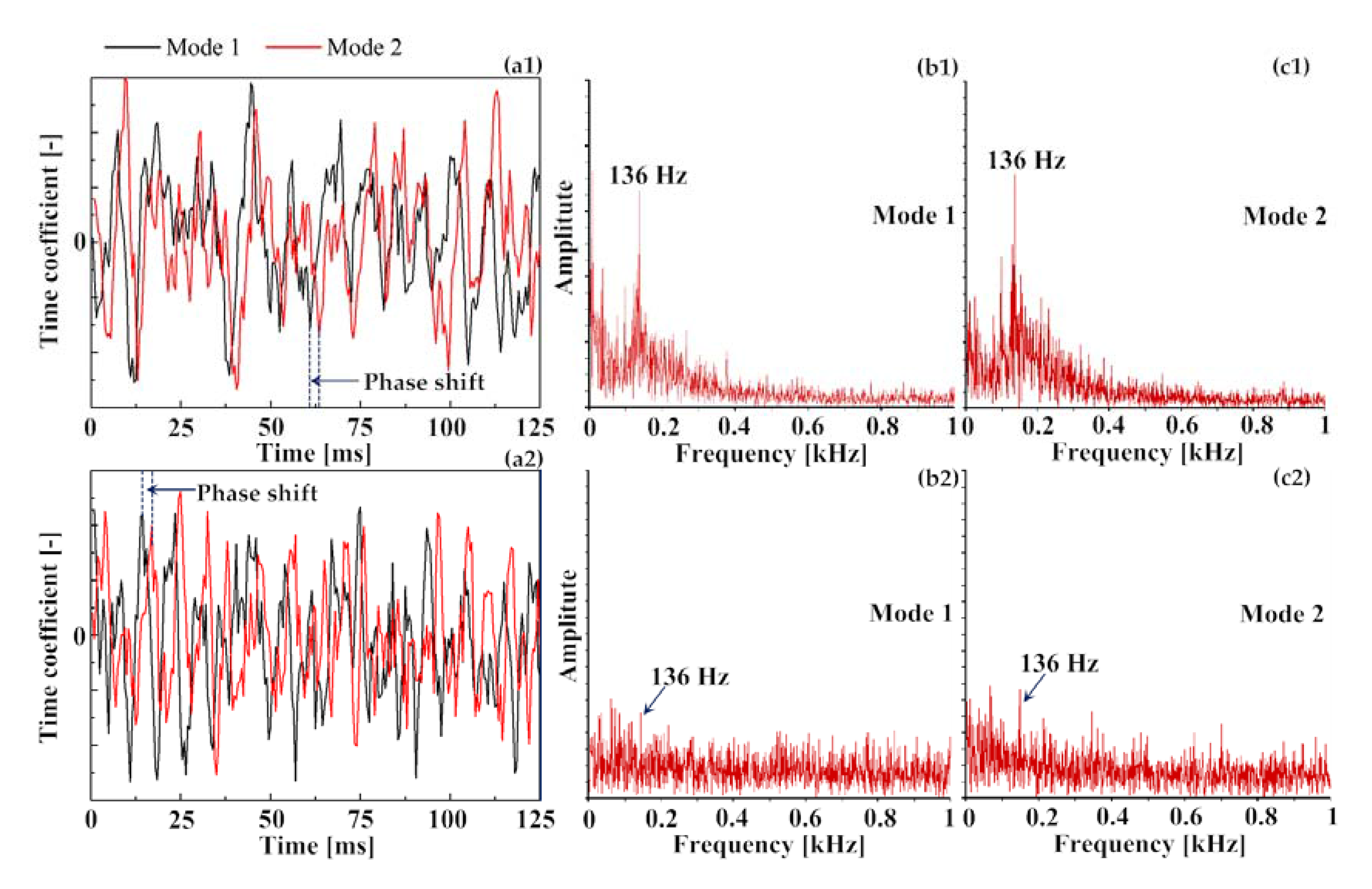
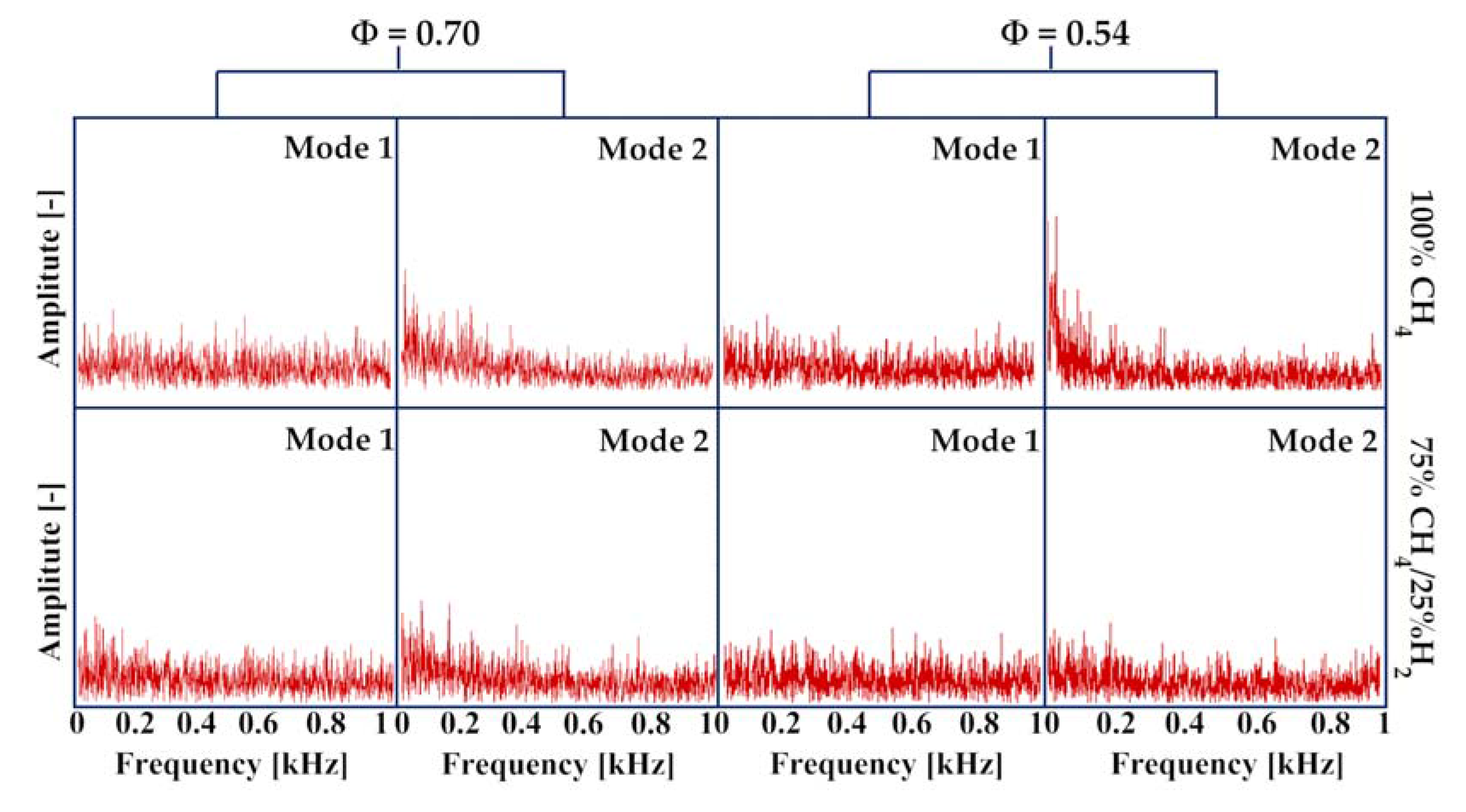
3.3. Identification of Precessing Vortex Core by POD Analysis
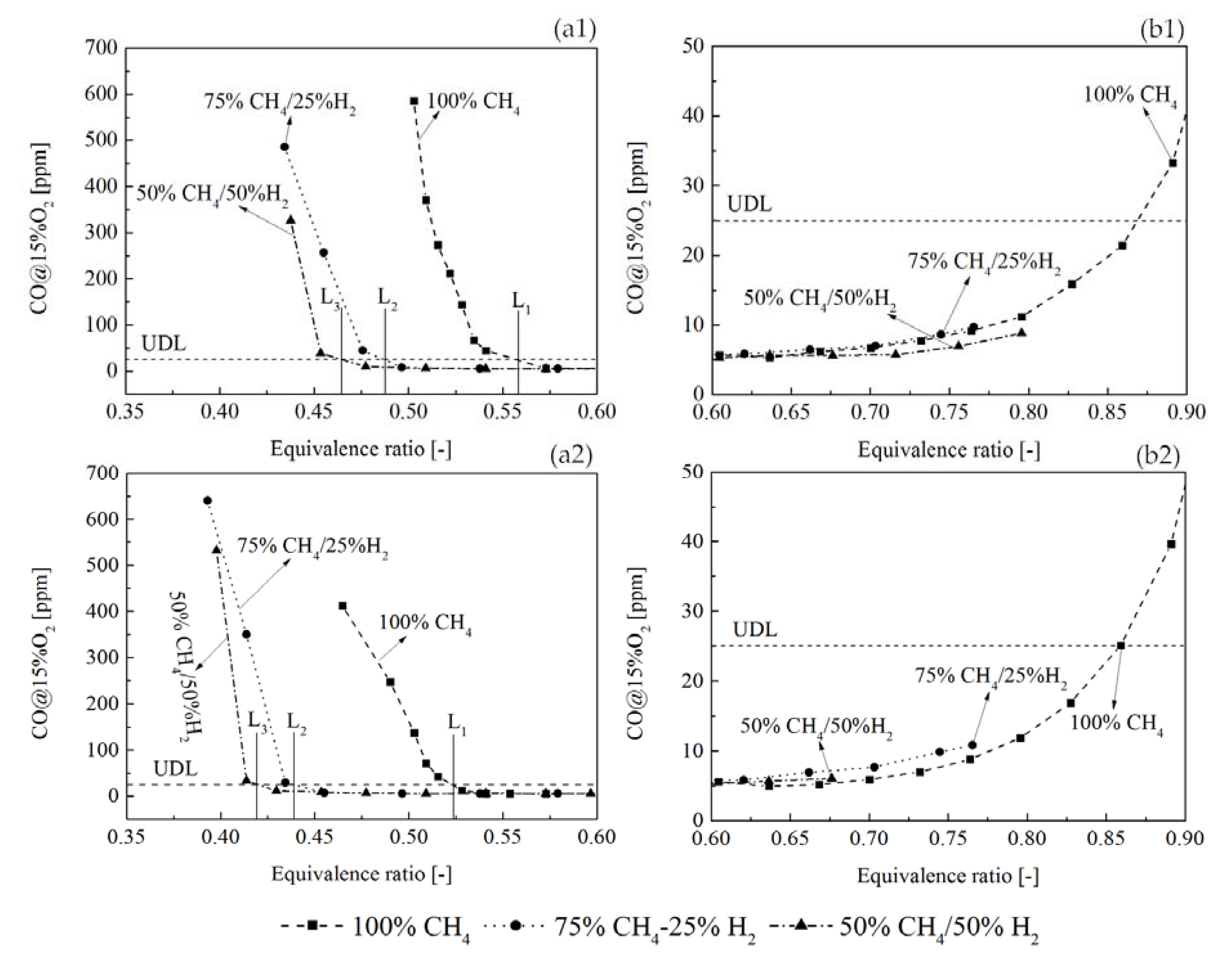
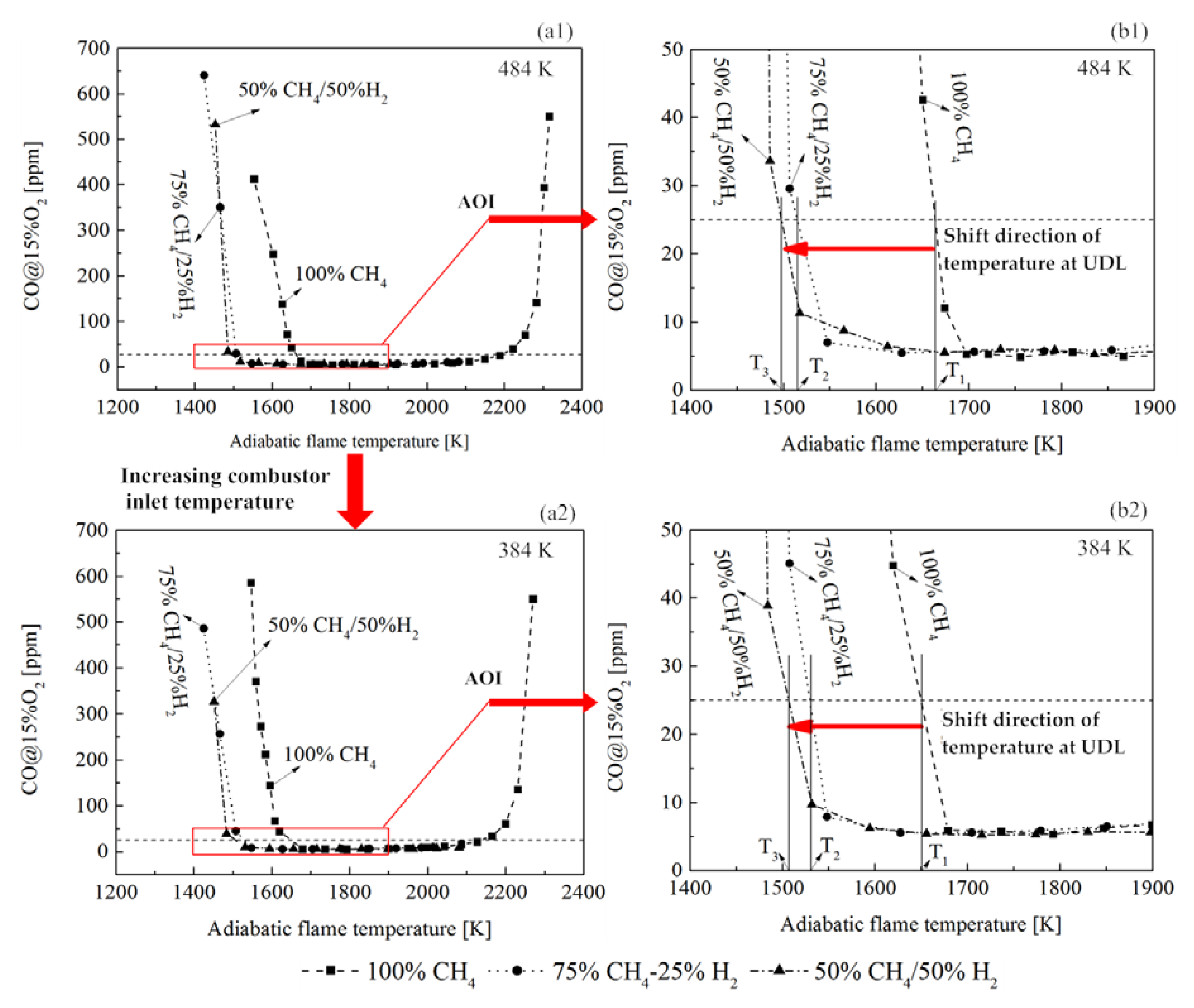
3.4. CO Emissions
4. Conclusions
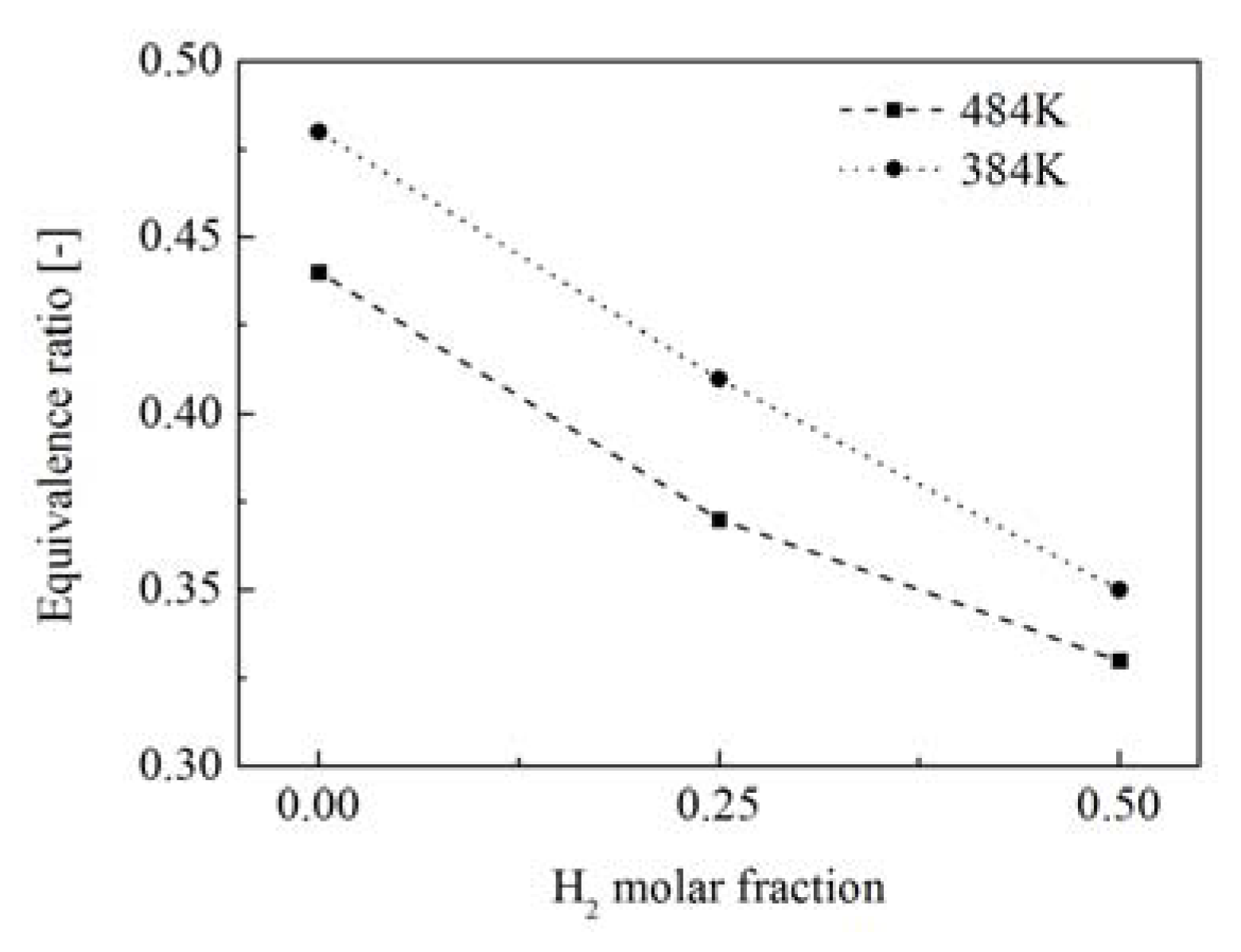
- The flame LBO limits can be extended to lower equivalence ratios by adding H2 in the fuel flow and improving the combustor inlet temperature. Higher H2 molar fraction in the fuel mixture can benefit the combustor operation with an extended equivalence ratio range.
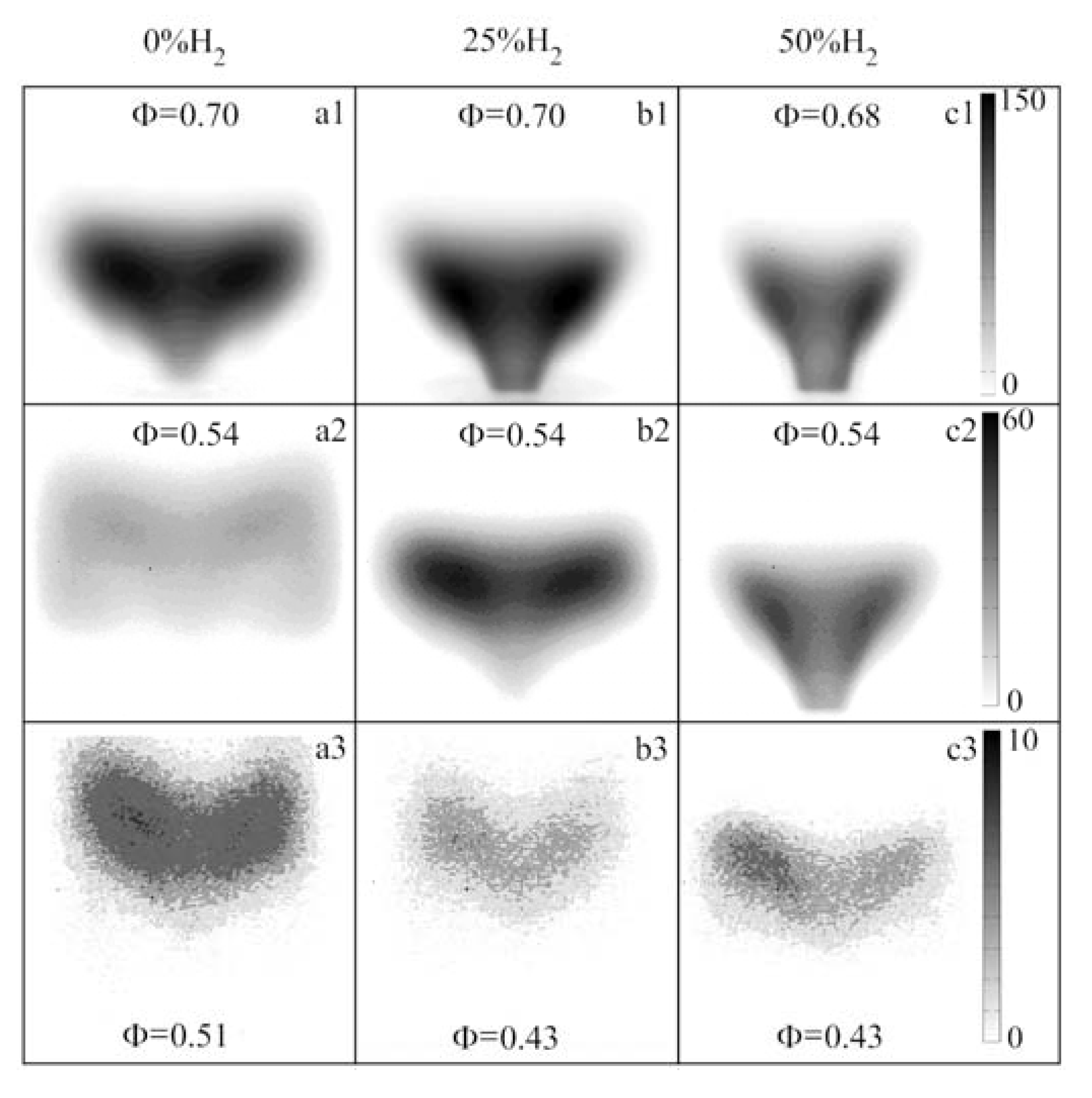
- Time-averaged CH chemiluminescence indicated that the flame reaction zone traveled and stabilized upstream with the increase of equivalence ratios and H2 molar fraction in the fuel mixture. The attached ‘V’ shape flame, ‘M’ shape flame, and a lifted ‘V’ shape flame was found along the decrease of the equivalence ratio without H2 addition. With 25% and 50% H2 addition, the flame shape changed from the attached ‘V’ shape to the lifted ‘V’ shape. The ‘M’ shape flame was not found.
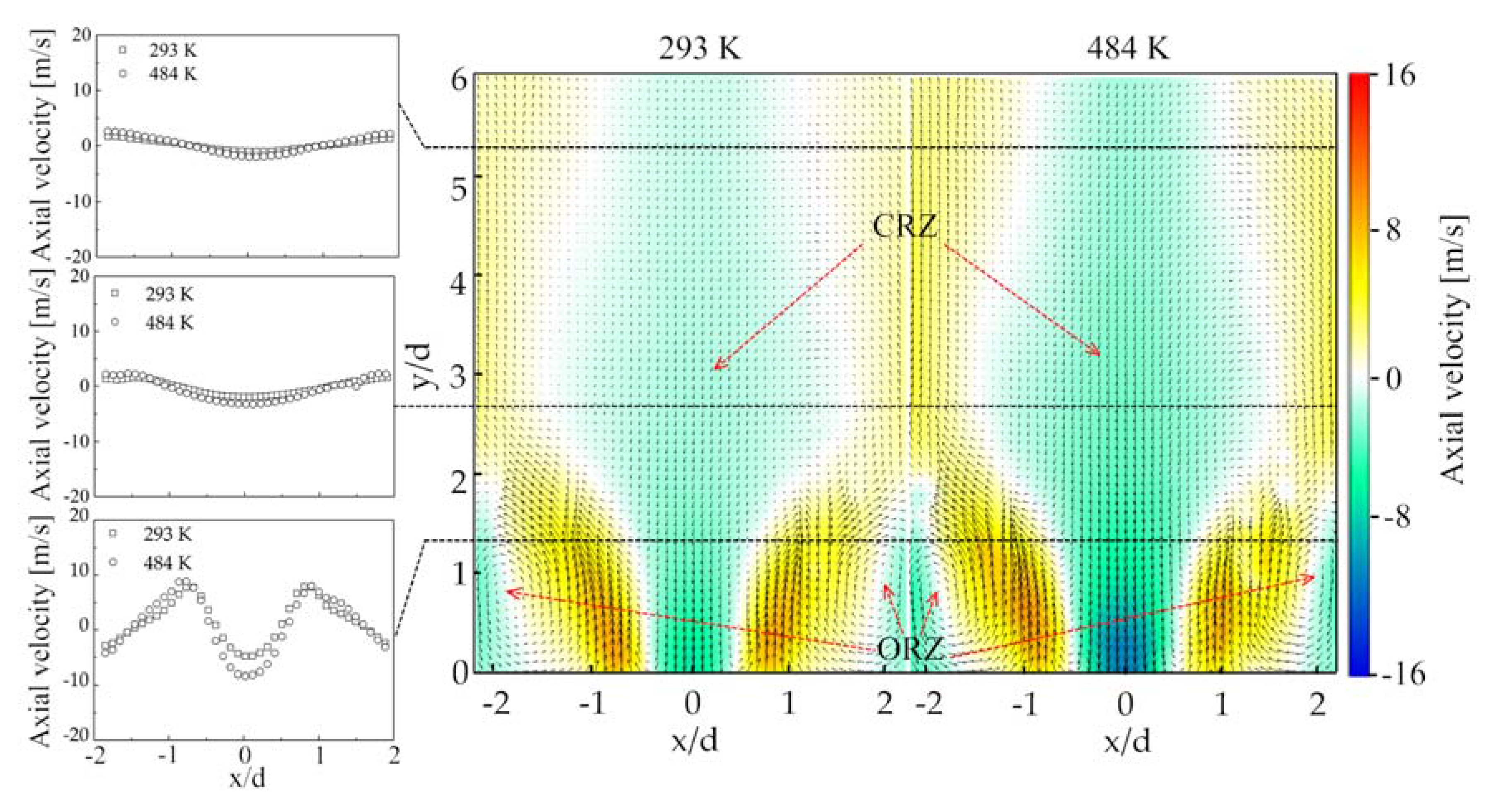
- The time-average velocity field of non-reacting flow revealed the presence of center recirculation zone (CRZ) and outer recirculation zone (ORZ) due to the vortex breakdown and the geometry of the dump combustor, respectively. An annular jet flow was found to be located between the CRZ and ORZ. The velocity in the CRZ, ORZ, and the annular jet flow was enhanced by improving the combustor inlet temperature from 384 K to 484 K.
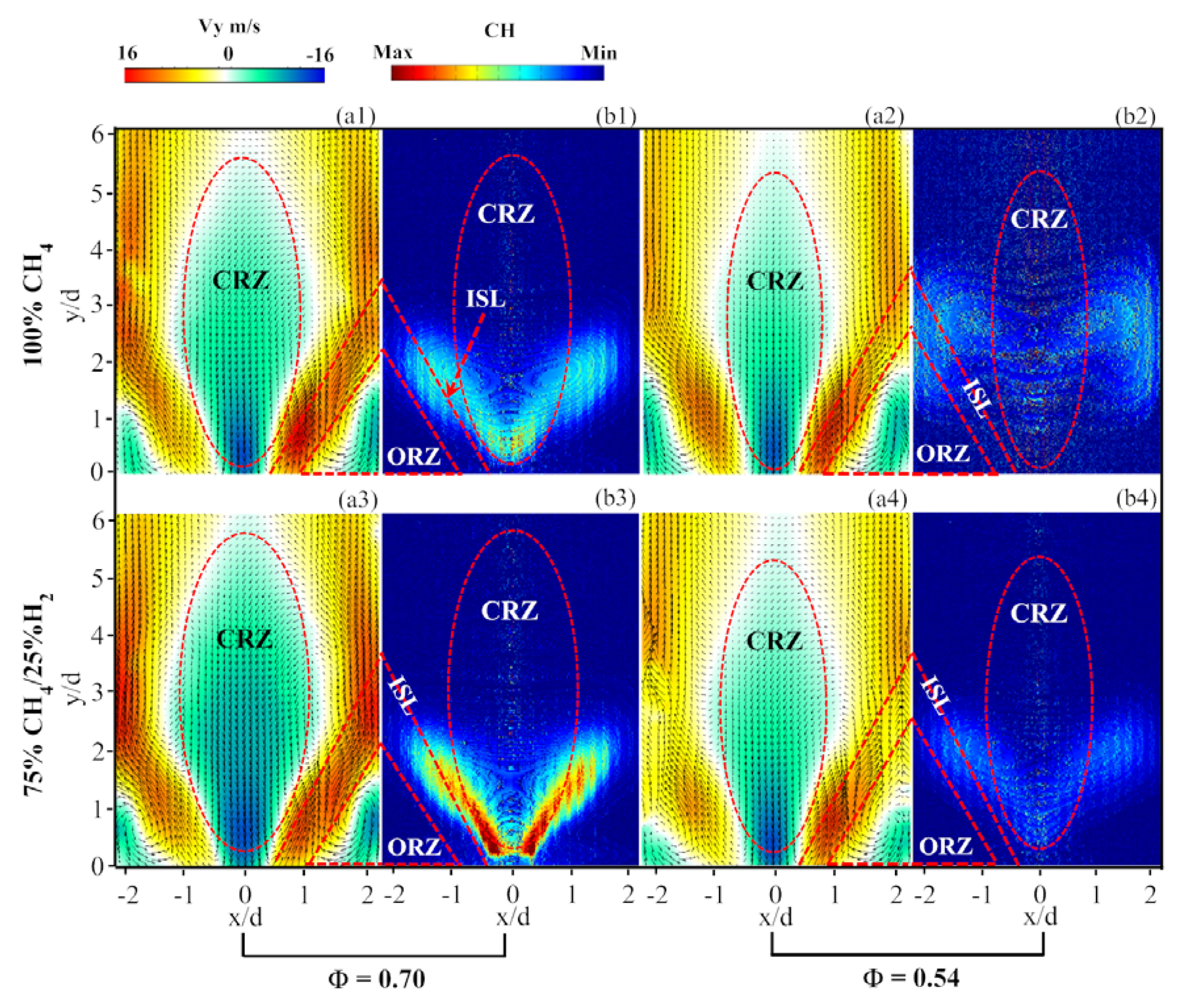
- The time-average velocity field of reacting flow indicated that the H2 addition could lead to a velocity increase in the flow field at equivalence ratio 0.7. However, at a lower equivalence ratio 0.54, this effect was not observed. Reducing equivalence ratio resulted in a size reduction of CRZ for both the CH4 flame and 75%CH4/25%H2 flame. By using inverse Abel transform, it could be observed that the flame with 25% H2 addition was able to stabilize at the ISL at a lower equivalence ratio when compared to the CH4 flame.
- Unlike the average flow field, the instantaneous velocity field of the reacting flow revealed the unsteady vortices structures, which indicated the presence of a coherent helical vortex. Such a helical vortex often appears in swirling flow and it is commonly referred to as the precessing vortex core (PVC).
- In the non-reacting flow, the structure of POD spatial mode and their relevant time coefficients indicated that the first two POD modes were associated with a self-excited single helical instability which features a PVC. In the reacting flow, the flame of CH4 and 75%CH4/25%H2 were studied at the equivalence ratio of 0.70 and 0.54. Fast Fourier transform showed that the PVC was suppressed by the combustion.
- The CO emission measurement showed that the equivalence ratio operation range with low CO (under the UDL) was extended by H2 addition and improving the combustor inlet temperature. In addition, adding H2 made it possible to operate a low CO combustion at lower adiabatic flame temperatures. However, increasing H2 molar fraction from 25% to 50% did not significantly extend the margin of adiabatic flame temperature for low CO operation.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Correa, S.M. Power generation and aeropropulsion gas turbines: From combustion science to combustion technology. Symp. (Int.) Combust. 1998, 27, 1793–1807. [Google Scholar] [CrossRef]
- Lefebvre, A.H. The role of fuel preparation in low-emission combustion. J. Eng. Gas Turbines Power 1995, 117, 617–654. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lim, A.-H.; Ann, K.-Y.; Lee, S.-M. Study on the Combustion Characteristics of a Lean-Premixed Combustor. J. Korean Soc. Combust. 2004, 9, 25–31. [Google Scholar]
- Nguyen, O.; Samuelsen, S. The effect of discrete pilot hydrogen dopant injection on the lean blowout performance of a model gas turbine combustor. In Proceedings of the ASME 1999 International Gas Turbine and Aeroengine Congress and Exhation, Indianapolis, IN, USA, 7–10 June 1999. [Google Scholar]
- Phillips, J.N.; Roby, R.J. Enhanced gas turbine combustor performance using H2-enriched natural gas. In Proceedings of the ASME 1999 International Gas Turbine and Aeroengine Congress and Exhation, Indianapolis, IN, USA, 7–10 June 1999. [Google Scholar]
- Di Sarli, V. Stability and emissions of a lean pre-mixed combustor with rich catalytic/lean-burn pilot. Int. J. Chem. Reactor Eng. 2014, 12, 77–89. [Google Scholar] [CrossRef]
- Ilbas, M.; Crayford, A.; Yılmaz, I.; Bowen, P.; Syred, N. Laminar-burning velocities of hydrogen-air and hydrogen-methane-air mixtures: An experimental study. Int. J. Hydrog. Energy 2006, 31, 1768–1779. [Google Scholar] [CrossRef]
- Halter, F.; Chauveau, C.; Djebaili-Chaumeix, N.; Gökalp, I. Characterization of the effects of pressure and hydrogen concentration on laminar burning velocities of methane-hydrogen-air mixtures. Proc. Combust. Inst. 2005, 30, 201–208. [Google Scholar] [CrossRef]
- Yu, G.; Law, C.; Wu, C. Laminar flame speeds of hydrocarbon + air mixtures with hydrogen addition. Combust. Flame 1986, 63, 339–347. [Google Scholar] [CrossRef]
- Scholte, T.; Vaags, P. Burning velocities of mixtures of hydrogen, carbon monoxide and methane with air. Combust. Flame 1959, 3, 511–524. [Google Scholar] [CrossRef]
- Di Sarli, V.; Di Benedetto, A. Laminar burning velocity of hydrogen-methane/air premixed flames. Int. J. Hydrog. Energy 2007, 32, 637–646. [Google Scholar] [CrossRef]
- Di Sarli, V.; Di Benedetto, A. Effects of non-equidiffusion on unsteady propagation of hydrogen-enriched methane/air premixed flames. Int. J. Hydrog. Energy 2013, 38, 7510–7518. [Google Scholar] [CrossRef]
- Di Sarli, V.; Di Benedetto, A.; Long, E.J.; Hargrave, G.K. Time-Resolved Particle Image Velocimetry of dynamic interactions between hydrogen-enriched methane/air premixed flames and toroidal vortex structures. Int. J. Hydrog. Energy 2012, 37, 16201–16213. [Google Scholar] [CrossRef] [Green Version]
- İlbaş, M.; Yılmaz, İ. Experimental analysis of the effects of hydrogen addition on methane combustion. Int. J. Energy Res. 2012, 36, 643–647. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Li, G.; Li, P. Effects of Hydrogen Addition on the Performance of a Pilot-Ignition Direct-Injection Natural Gas Engine: A Numerical Study. Energy Fuels 2017, 31, 4407–4423. [Google Scholar] [CrossRef]
- Guo, H.; Smallwood, G.J.; Liu, F.; Ju, Y.; Gülder, Ö.L. The effect of hydrogen addition on flammability limit and NOx emission in ultra-lean counterflow CH 4/air premixed flames. Proc. Combust. Inst. 2005, 30, 303–311. [Google Scholar] [CrossRef]
- Cho, E.-S.; Chung, S.H. Improvement of flame stability and NOx reduction in hydrogen-added ultra lean premixed combustion. J. Mech. Sci. Technol. 2009, 23, 650–658. [Google Scholar] [CrossRef]
- Lieuwen, T.; McDonell, V.; Petersen, E.; Santavicca, D. Fuel flexibility influences on premixed combustor blowout, flashback, autoignition, and stability. J. Eng. Gas Turbines Power 2008, 130, 011506. [Google Scholar] [CrossRef]
- Kim, K.T.; Lee, J.G.; Lee, H.J.; Quay, B.D.; Santavicca, D.A. Characterization of forced flame response of swirl-stabilized turbulent lean-premixed flames in a gas turbine combustor. J. Eng. Gas Turbines Power 2010, 132, 041502. [Google Scholar] [CrossRef]
- Davis, D.; Therkelsen, P.; Littlejohn, D.; Cheng, R. Effects of hydrogen on the thermo-acoustics coupling mechanisms of low-swirl injector flames in a model gas turbine combustor. Proc. Combust. Inst. 2013, 34, 3135–3143. [Google Scholar] [CrossRef]
- Yilmaz, I.; Ratner, A.; Ilbas, M.; Huang, Y. Experimental investigation of thermoacoustic coupling using blended hydrogen–methane fuels in a low swirl burner. Int. J. Hydrog. Energy 2010, 35, 329–336. [Google Scholar] [CrossRef]
- Kee, R.; Rupley, F.; Miller, J. CHEMKIN-PRO 15112; Reaction Design: San Diego, CA, USA, 2011. [Google Scholar]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C., Jr. GRI-Mech 3.0. 1999. Available online: http://combustion.berkeley.edu/gri-mech/ (accessed on 22 August 2017).
- Sayad, P.; Schönborn, A.; Li, M.; Klingmann, J. Visualization of different flashback mechanisms for H2/CH4 mixtures in a variable-swirl burner. J. Eng. Gas Turbines Power 2015, 137, 031507. [Google Scholar] [CrossRef]
- Lumley, J.L. The structure of inhomogeneous turbulent flows. Atmos. Turbul. Radio Wave Propag. 1967, 166–178. [Google Scholar]
- Cavar, D.; Meyer, K.E. LES of turbulent jet in cross flow: Part 2—POD analysis and identification of coherent structures. Int. J. Heat Fluid Flow 2012, 36, 35–46. [Google Scholar] [CrossRef]
- Roux, A.; Gicquel, L.; Sommerer, Y.; Poinsot, T. Large eddy simulation of mean and oscillating flow in a side-dump ramjet combustor. Combust. Flame 2008, 152, 154–176. [Google Scholar] [CrossRef]
- Holmes, P. Turbulence, Coherent Structures, Dynamical Systems and Symmetry; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Sirovich, L. Turbulence and the dynamics of coherent structures. I. Coherent structures. Q. Appl. Math. 1987, 45, 561–571. [Google Scholar] [CrossRef]
- Driscoll, J.; Temme, J. Role of swirl in flame stabilization. In Proceedings of the 49th AIAA Aerospace Sciences Meeting including the New Horizons Forum and Aerospace Exposition, Orlando, FL, USA, 4–7 January 2011. [Google Scholar]
- Syred, N.; Beer, J. Combustion in swirling flows: A review. Combust. Flame 1974, 23, 143–201. [Google Scholar] [CrossRef]
- Weigand, P.; Meier, W.; Duan, X.; Stricker, W.; Aigner, M. Investigations of swirl flames in a gas turbine model combustor: I. Flow field, structures, temperature, and species distributions. Combust. Flame 2006, 144, 205–224. [Google Scholar] [CrossRef]
- Hardalupas, Y.; Orain, M. Local measurements of the time-dependent heat release rate and equivalence ratio using chemiluminescent emission from a flame. Combust. Flame 2004, 139, 188–207. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, V. Dynamics and stability of lean-premixed swirl-stabilized combustion. Prog. Energy Combust. Sci. 2009, 35, 293–364. [Google Scholar] [CrossRef]
- Syred, N. A review of oscillation mechanisms and the role of the precessing vortex core (PVC) in swirl combustion systems. Prog. Energy Combust. Sci. 2006, 32, 93–161. [Google Scholar] [CrossRef]
- Berkooz, G.; Holmes, P.; Lumley, J.L. The proper orthogonal decomposition in the analysis of turbulent flows. Annu. Rev. Fluid Mech. 1993, 25, 539–575. [Google Scholar] [CrossRef]
- Chen, H.; Reuss, D.L.; Sick, V. On the use and interpretation of proper orthogonal decomposition of in-cylinder engine flows. Meas. Sci. Technol. 2012, 23, 085302. [Google Scholar] [CrossRef]
- Oberleithner, K.; Sieber, M.; Nayeri, C.; Paschereit, C.; Petz, C.; Hege, H.-C.; Noack, B.; Wygnanski, I. Three-dimensional coherent structures in a swirling jet undergoing vortex breakdown: Stability analysis and empirical mode construction. J. Fluid Mech. 2011, 679, 383–414. [Google Scholar] [CrossRef]
- Stöhr, M.; Sadanandan, R.; Meier, W. Phase-resolved characterization of vortex–flame interaction in a turbulent swirl flame. Exp. Fluids 2011, 51, 1153–1167. [Google Scholar] [CrossRef] [Green Version]
- Galley, D.; Ducruix, S.; Lacas, F.; Veynante, D. Mixing and stabilization study of a partially premixed swirling flame using laser induced fluorescence. Combust. Flame 2011, 158, 155–171. [Google Scholar] [CrossRef]
- Kuenne, G.; Ketelheun, A.; Janicka, J. LES modeling of premixed combustion using a thickened flame approach coupled with FGM tabulated chemistry. Combust. Flame 2011, 158, 1750–1767. [Google Scholar] [CrossRef]
- Roux, S.; Lartigue, G.; Poinsot, T.; Meier, U.; Bérat, C. Studies of mean and unsteady flow in a swirled combustor using experiments, acoustic analysis, and large eddy simulations. Combust. Flame 2005, 141, 40–54. [Google Scholar] [CrossRef]
- Giauque, A.; Selle, L.; Gicquel, L.; Poinsot, T.; Buechner, H.; Kaufmann, P.; Krebs, W. System identification of a large-scale swirled partially premixed combustor using LES and measurements. J. Turbul. 2005, 6, N21. [Google Scholar] [CrossRef]
- Boxx, I.; Arndt, C.M.; Carter, C.D.; Meier, W. High-speed laser diagnostics for the study of flame dynamics in a lean premixed gas turbine model combustor. Exp. Fluids 2012, 52, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Terhaar, S.; Oberleithner, K.; Paschereit, C.O. Impact of steam-dilution on the flame shape and coherent structures in swirl-stabilized combustors. Combust. Sci. Technol. 2014, 186, 889–911. [Google Scholar] [CrossRef]

| Items | Units | Operating Parameter | Uncertainty |
|---|---|---|---|
| Fuel | - | CH4/H2 blends | - |
| H2 molar fraction | - | 0–50% | - |
| Air flow rate | g/s | 2.96 | ±0.02 |
| Equivalence ratio | - | 1–LBO (lean blowout) limits | 0.01 |
| Swirl number | - | 0.58 | ±0.01 |
| Preheated temperature | K | 384/484 | ±2 |
| PIV System | Lavision |
|---|---|
| Camera | Phantom V611 |
| Recording resolution | 1280 × 800 pixels |
| Recording frequency | 2 kHz |
| Laser | ND:YLF |
| Laser sheet thickness | ≈1 mm |
| Laser wavelength | 527 nm |
| Laser pulse interval | 50 µs |
| Seeding particles | TiO2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Tong, Y.; Klingmann, J.; Thern, M. Experimental Study of Hydrogen Addition Effects on a Swirl-Stabilized Methane-Air Flame. Energies 2017, 10, 1769. https://doi.org/10.3390/en10111769
Li M, Tong Y, Klingmann J, Thern M. Experimental Study of Hydrogen Addition Effects on a Swirl-Stabilized Methane-Air Flame. Energies. 2017; 10(11):1769. https://doi.org/10.3390/en10111769
Chicago/Turabian StyleLi, Mao, Yiheng Tong, Jens Klingmann, and Marcus Thern. 2017. "Experimental Study of Hydrogen Addition Effects on a Swirl-Stabilized Methane-Air Flame" Energies 10, no. 11: 1769. https://doi.org/10.3390/en10111769





