A Review of Lithium-Air Battery Modeling Studies
Abstract
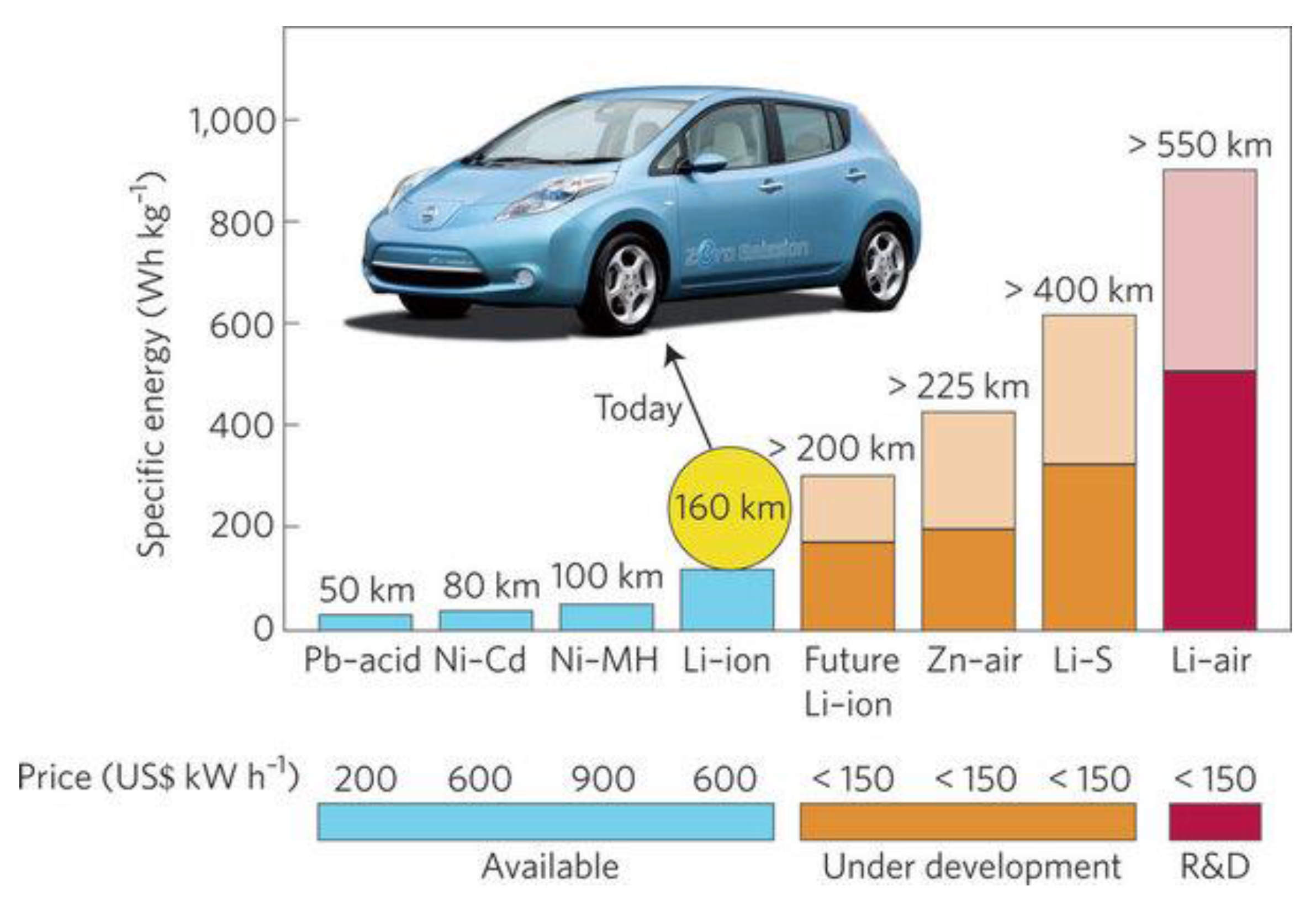
:1. Background
2. Continuum-Based Models
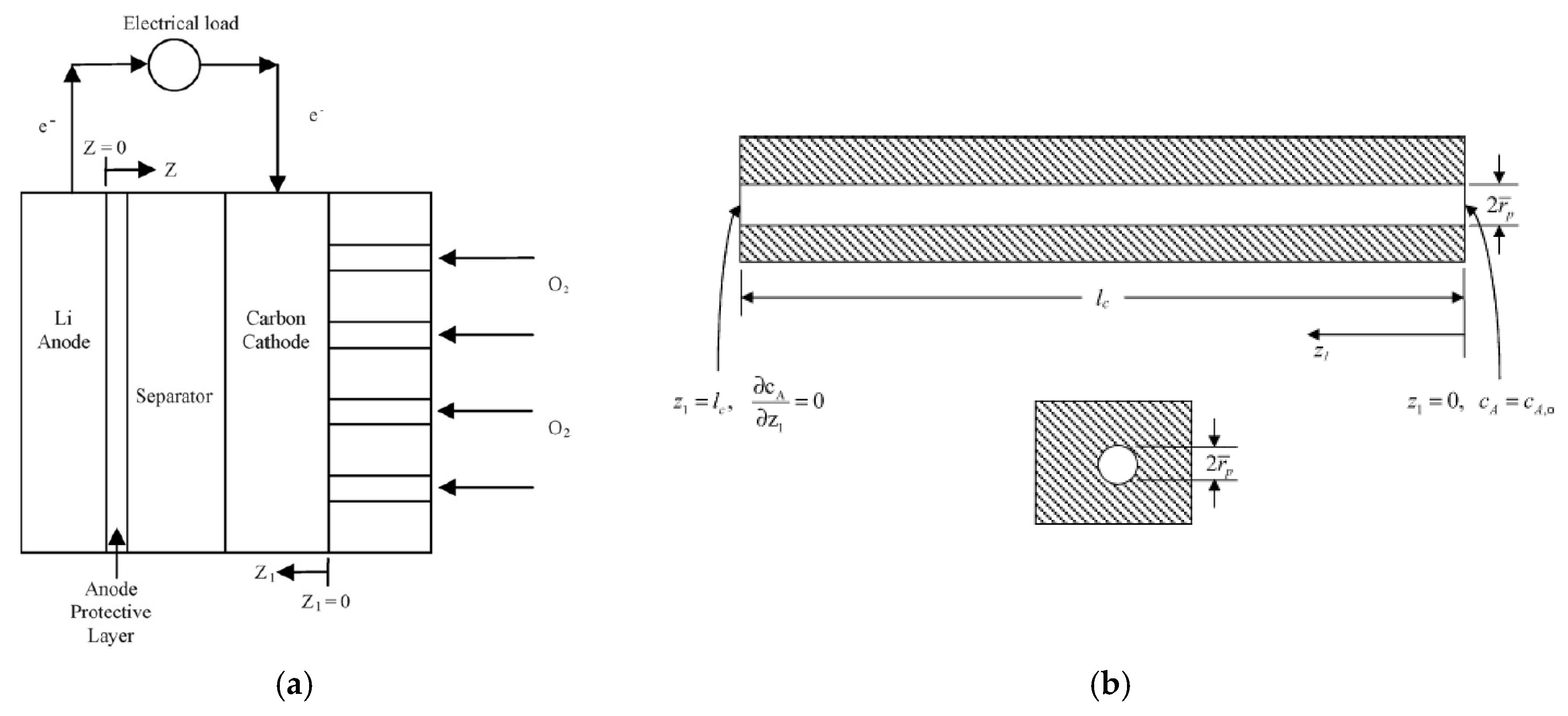
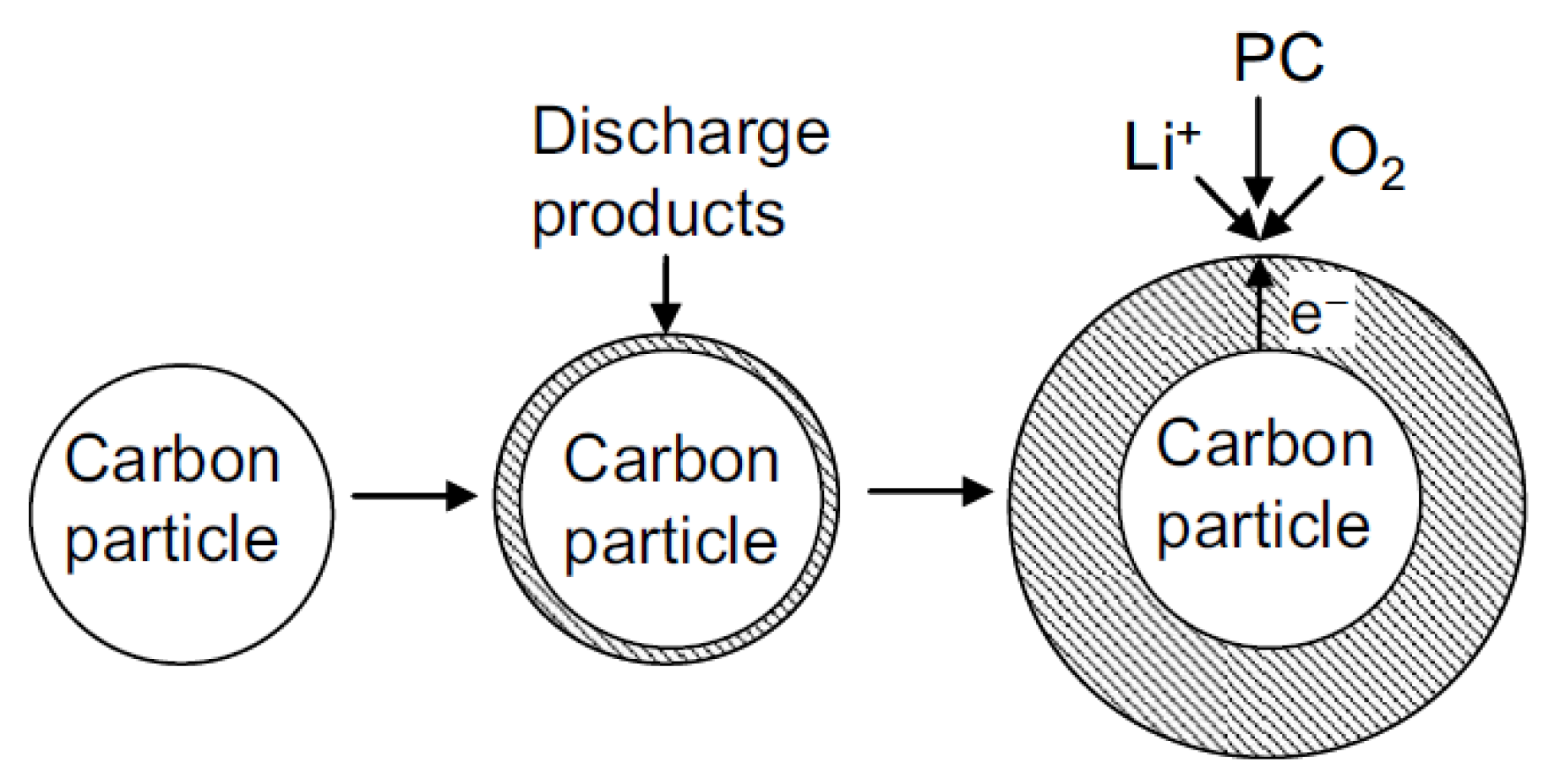
2.1. Microstructure of the Air Cathode
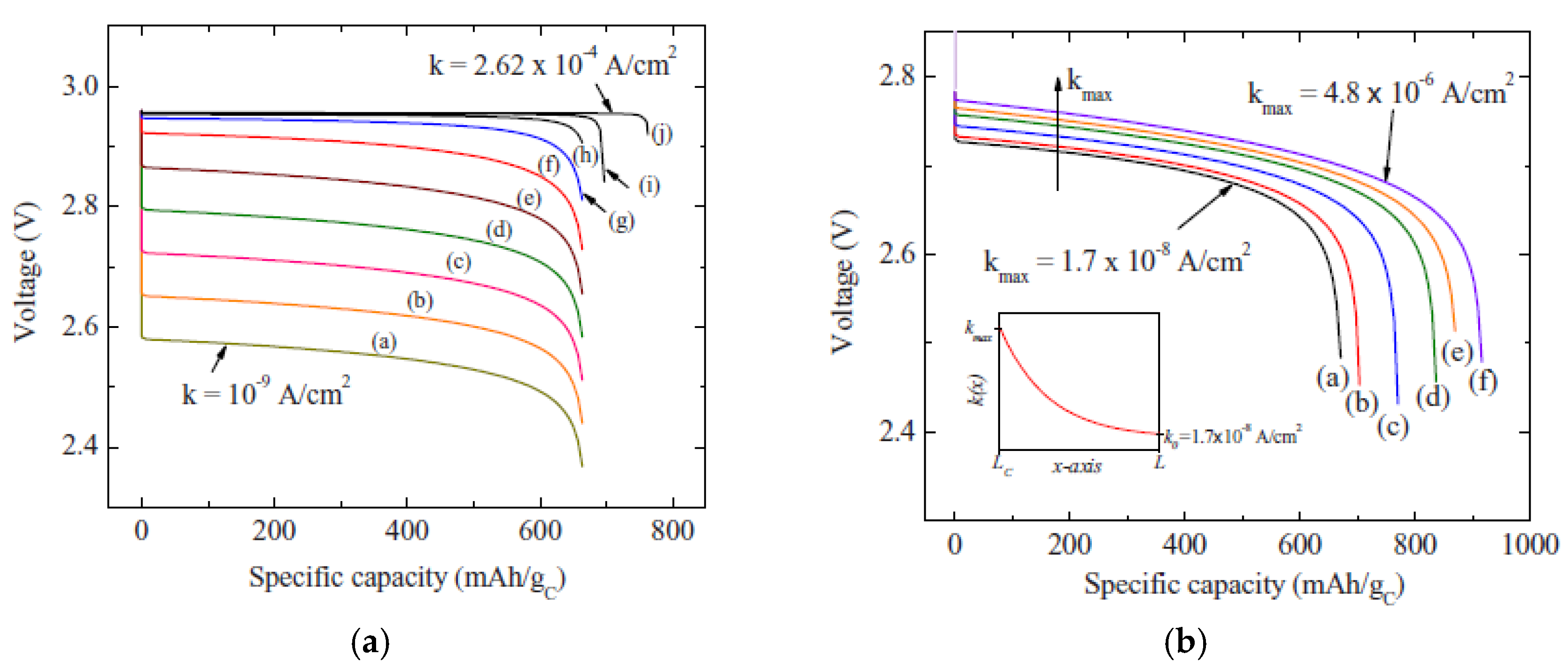
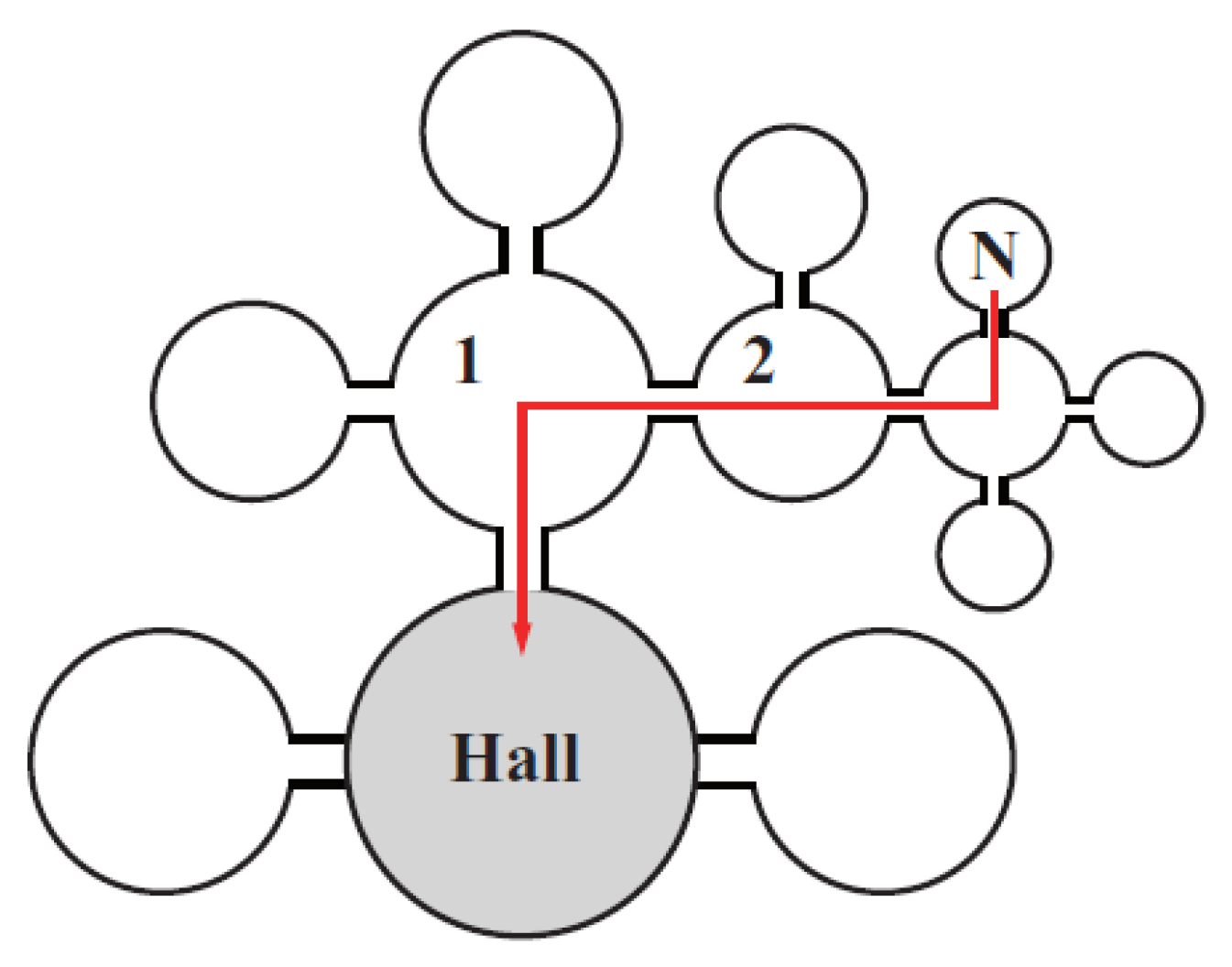
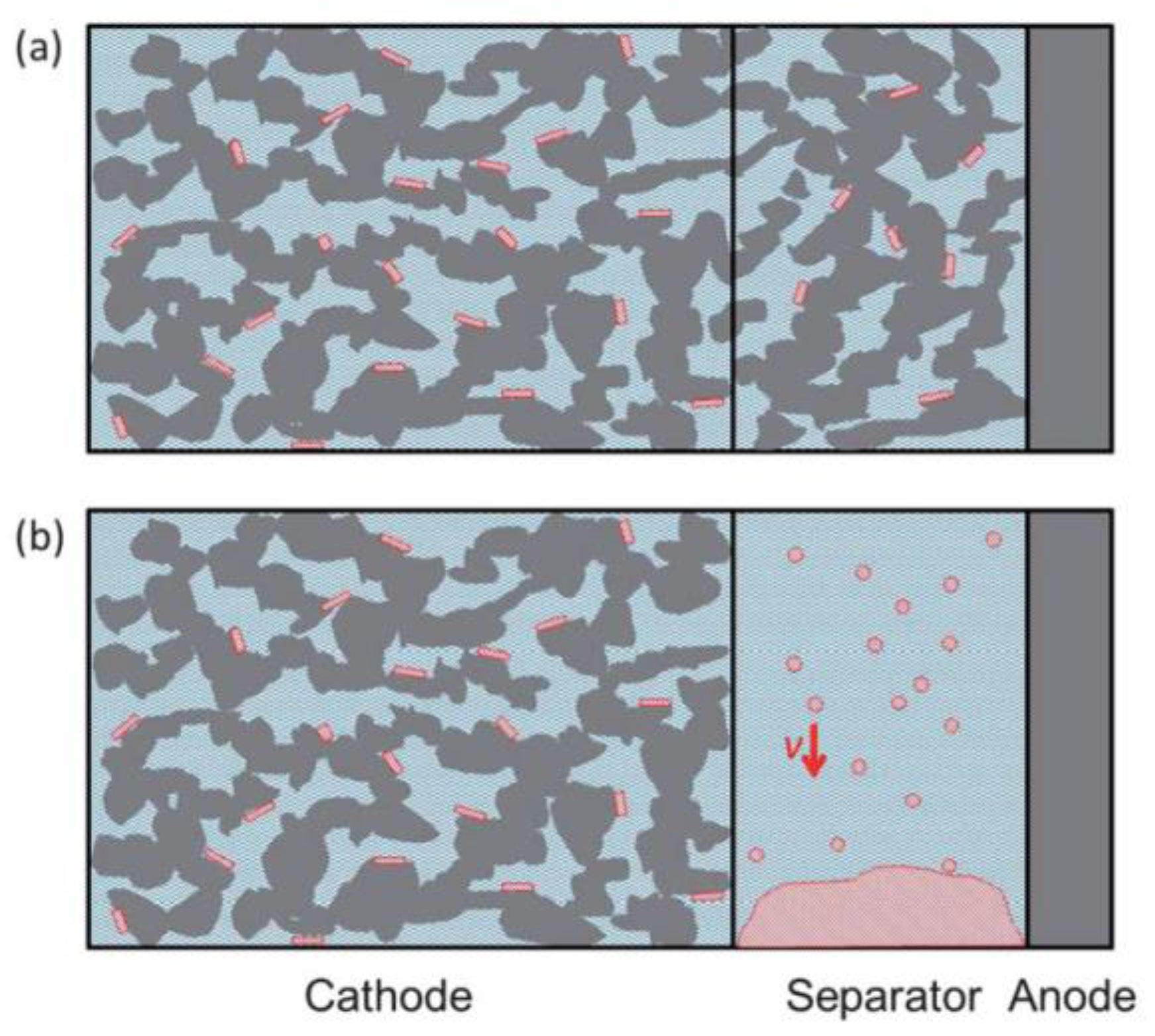
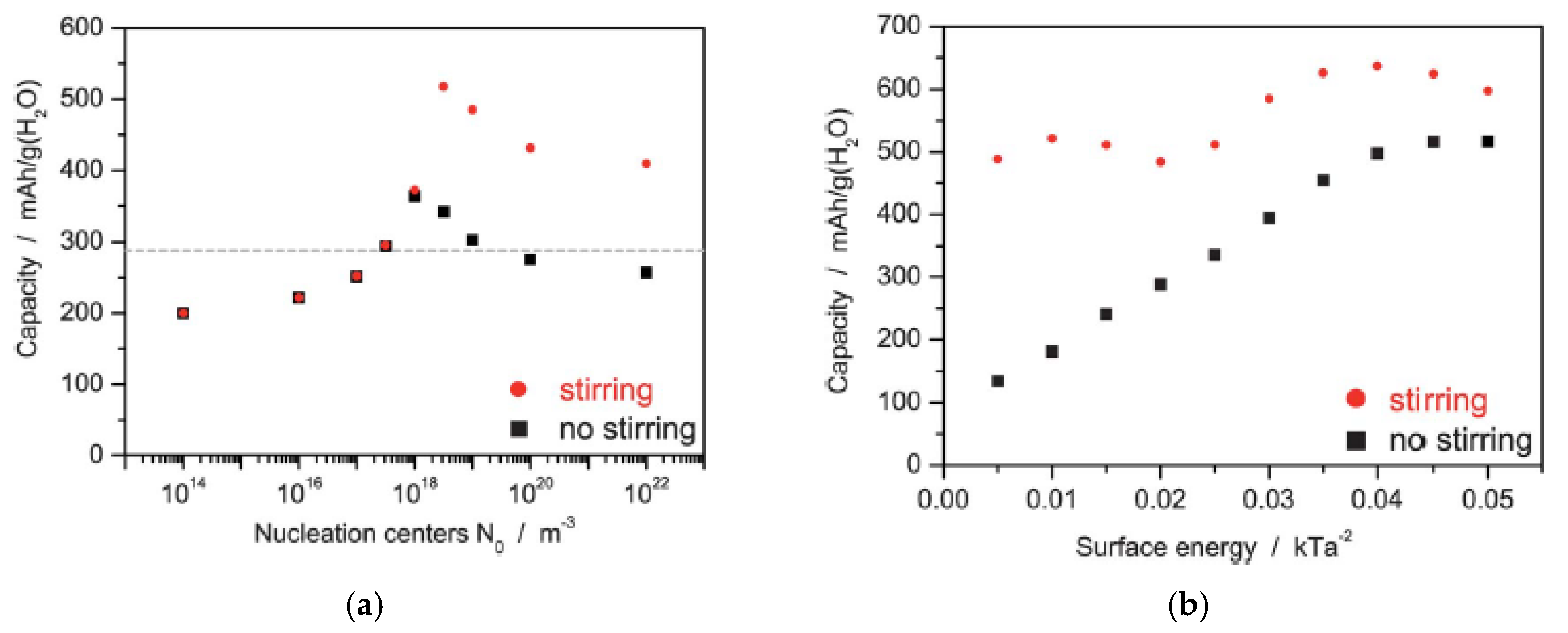
2.1.1. Shape of Cathode Materials
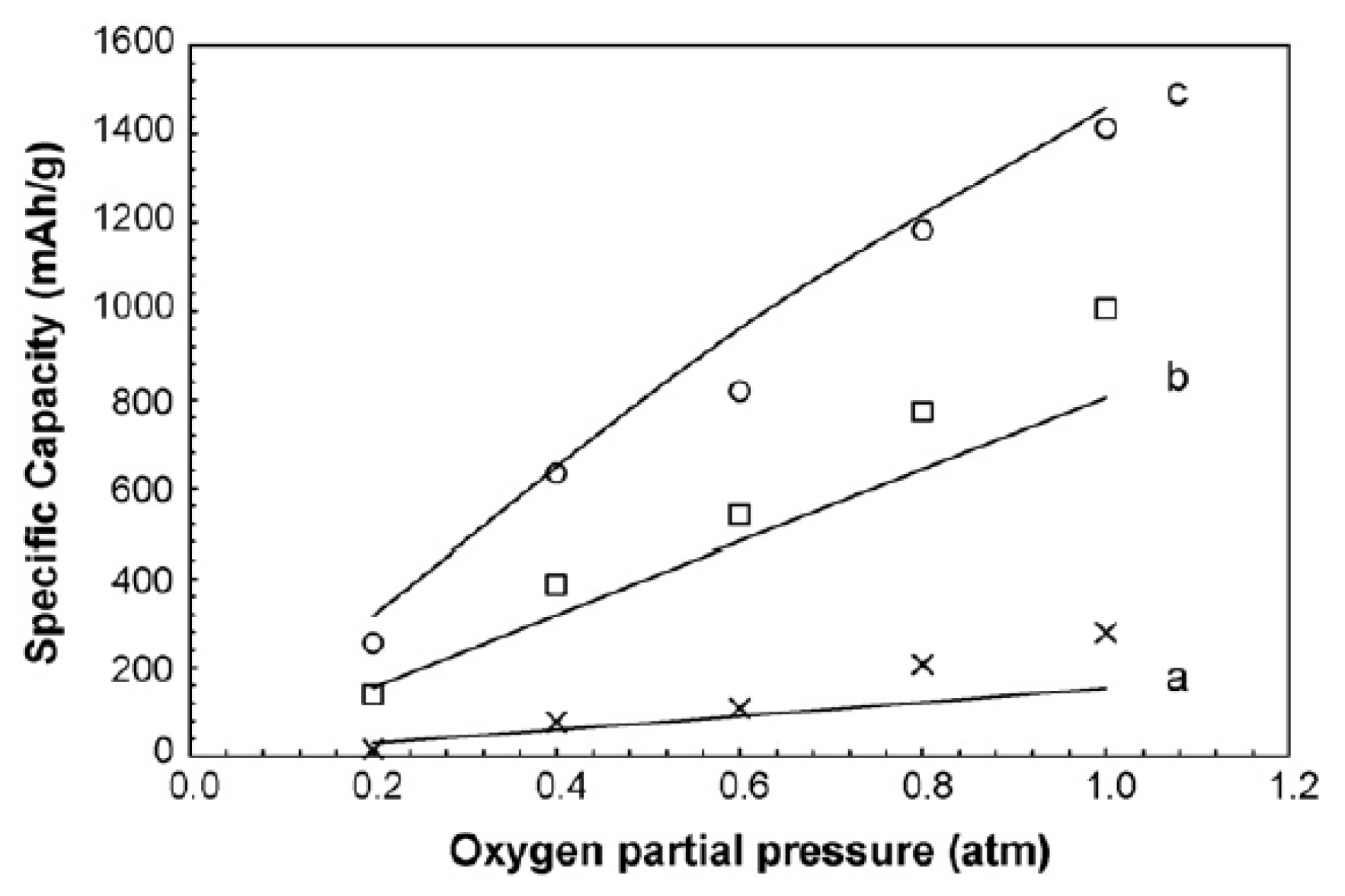
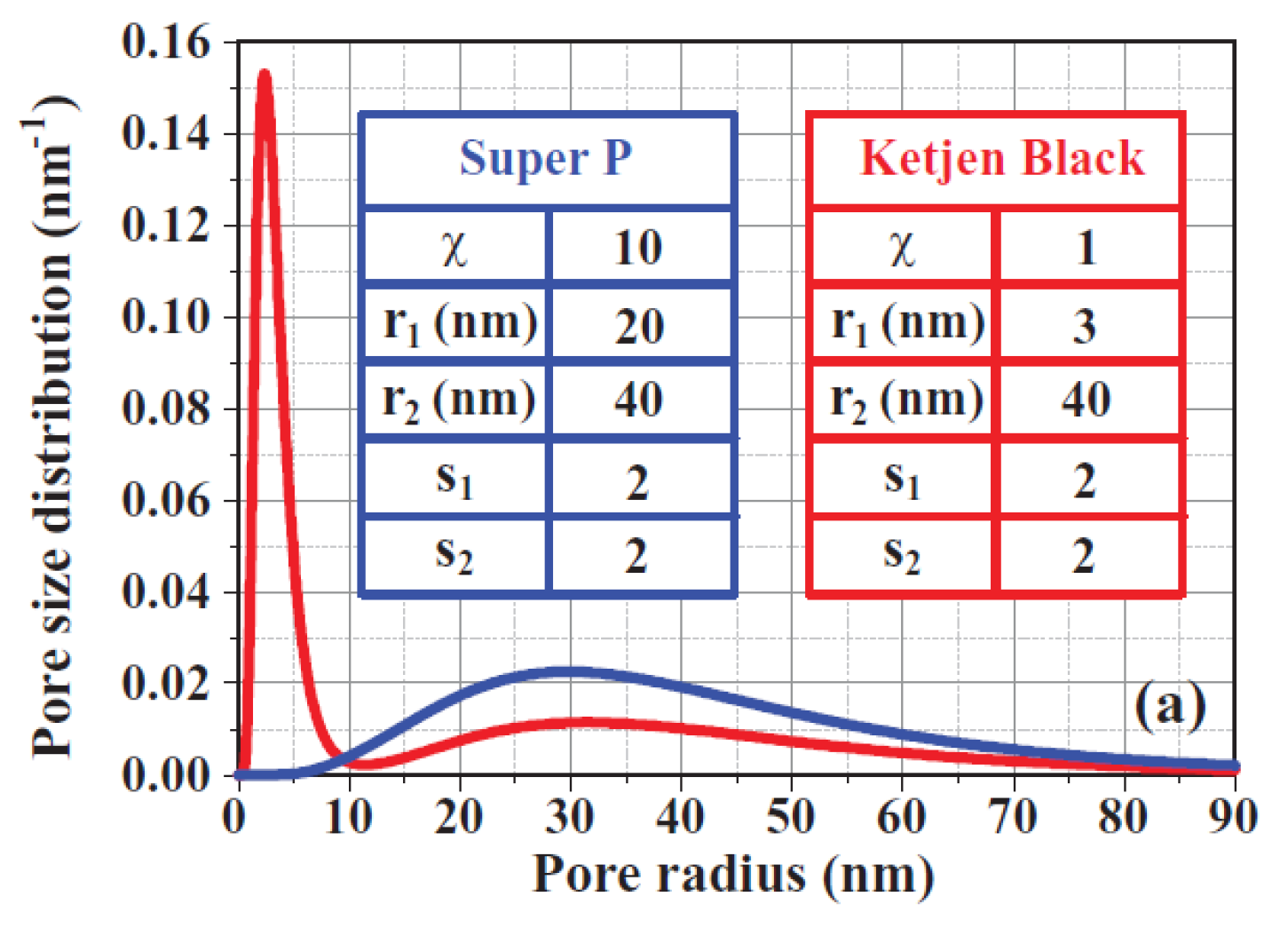
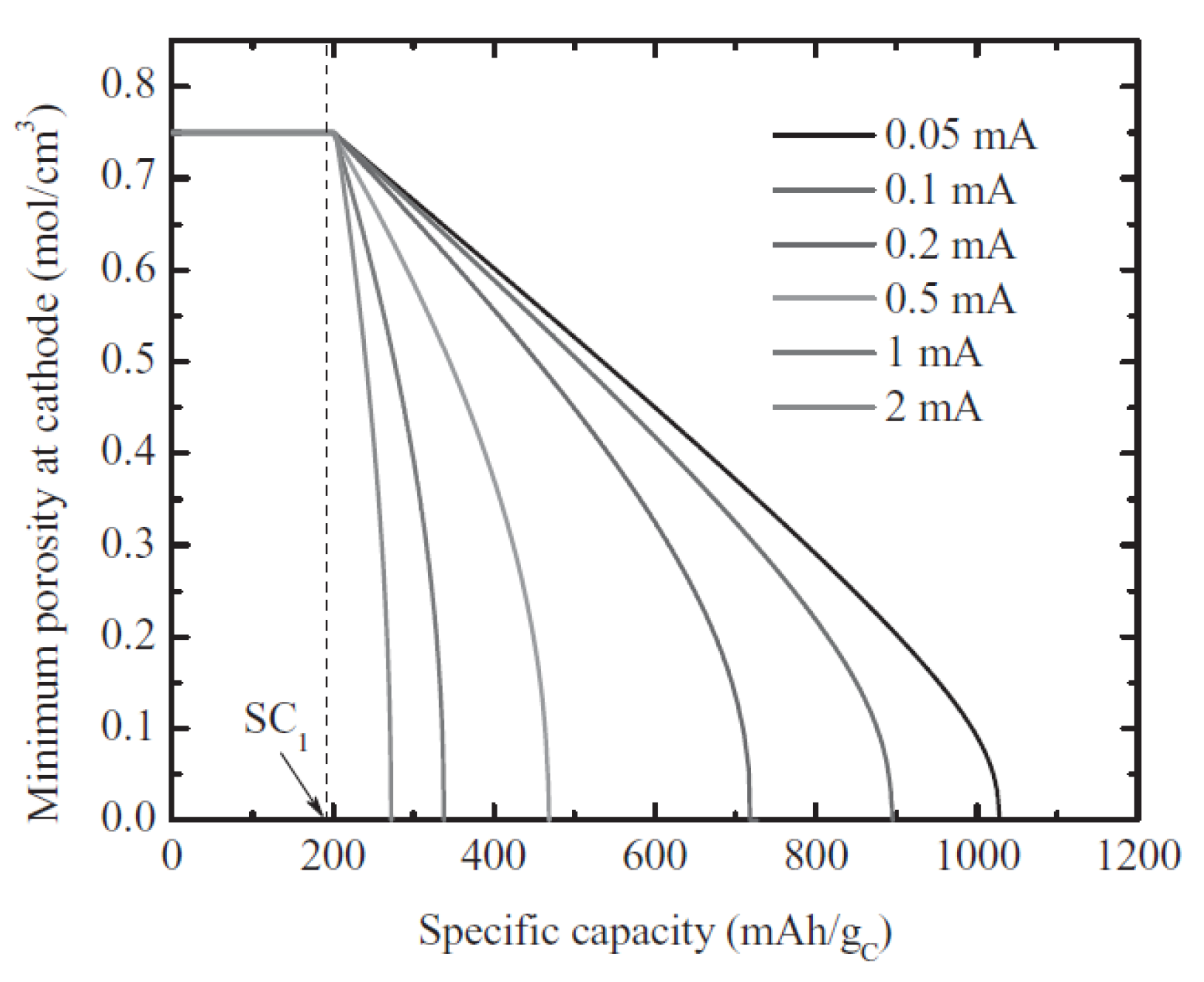
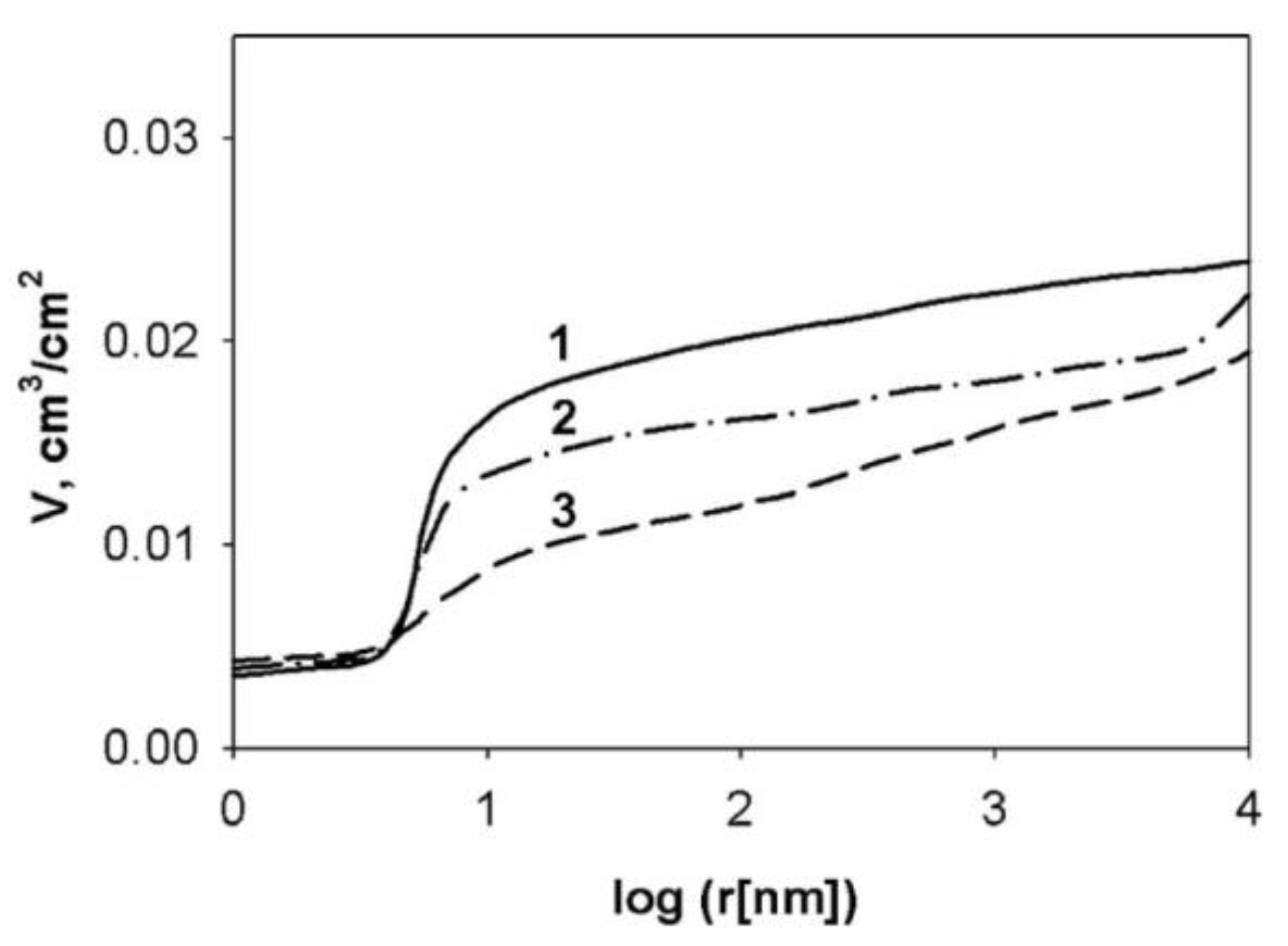
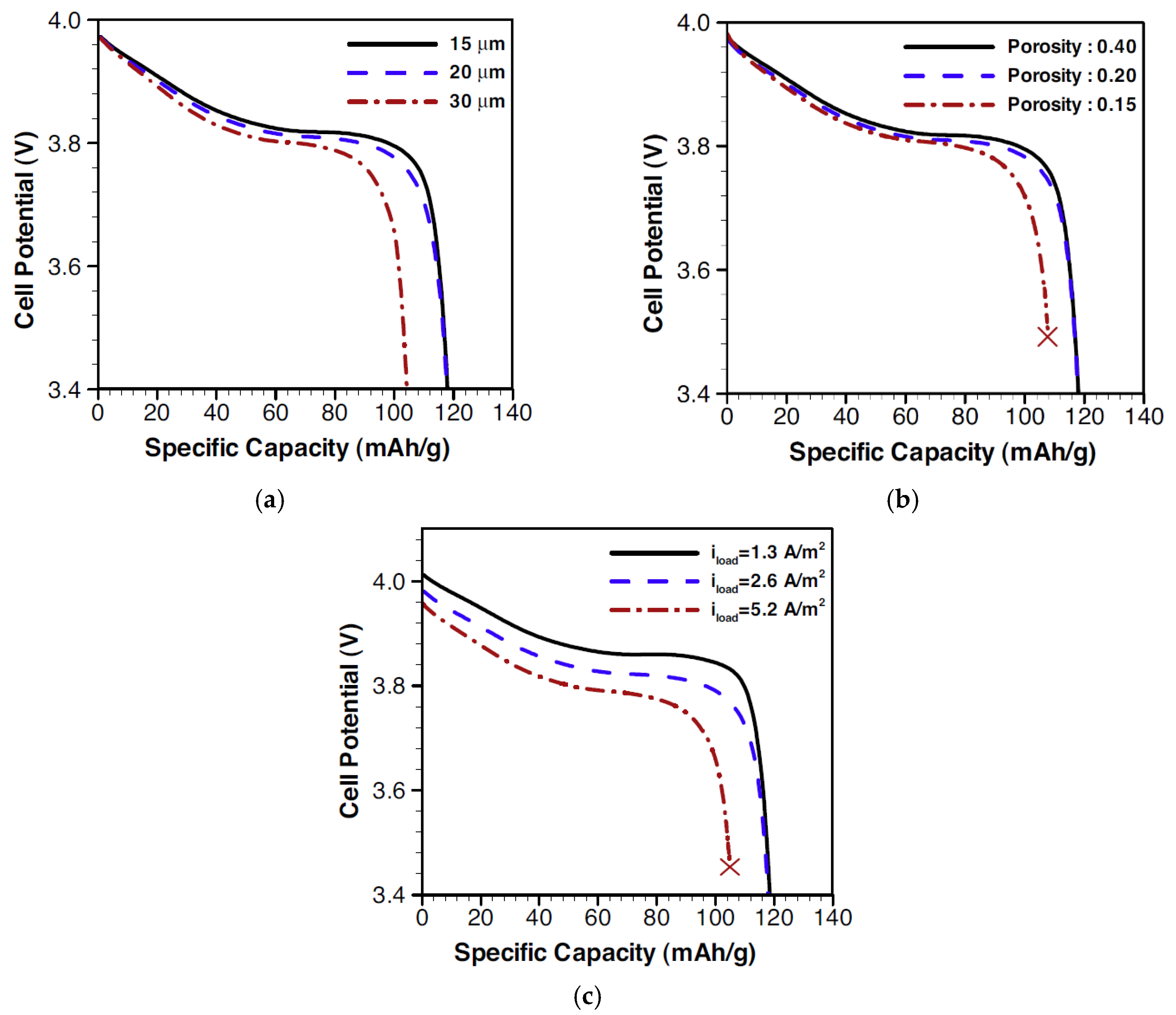
2.1.2. Size of Cathode Materials
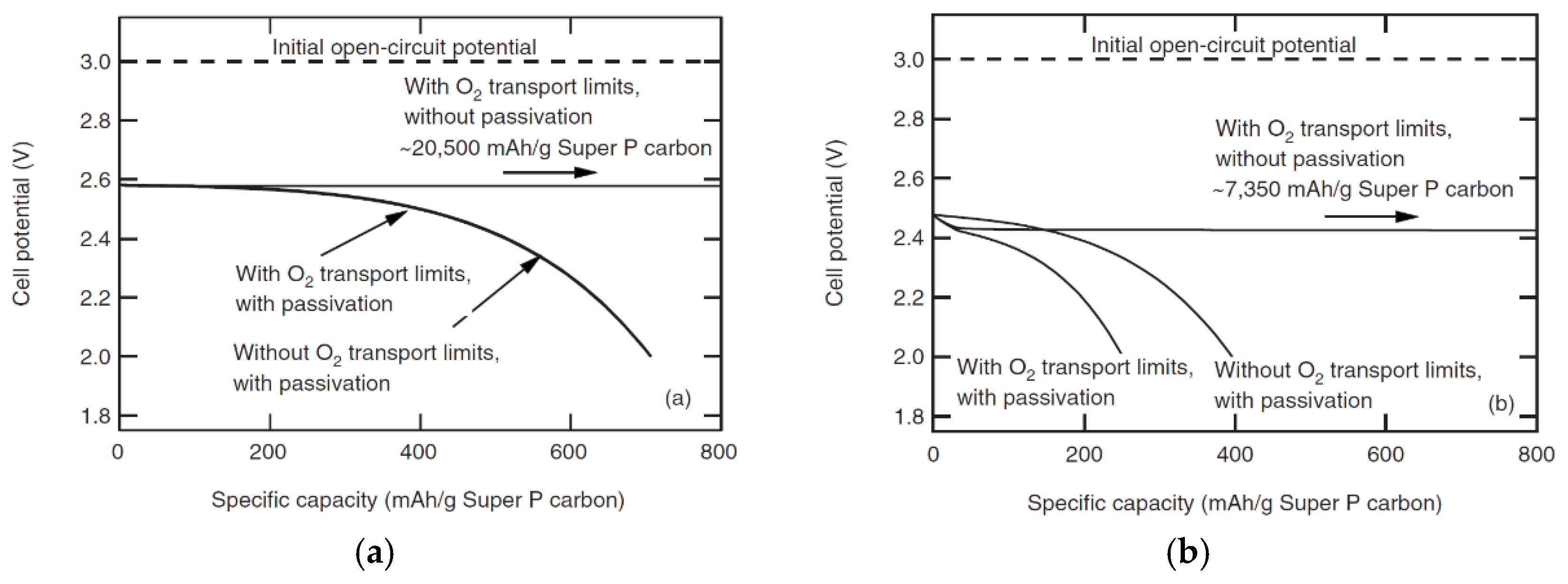
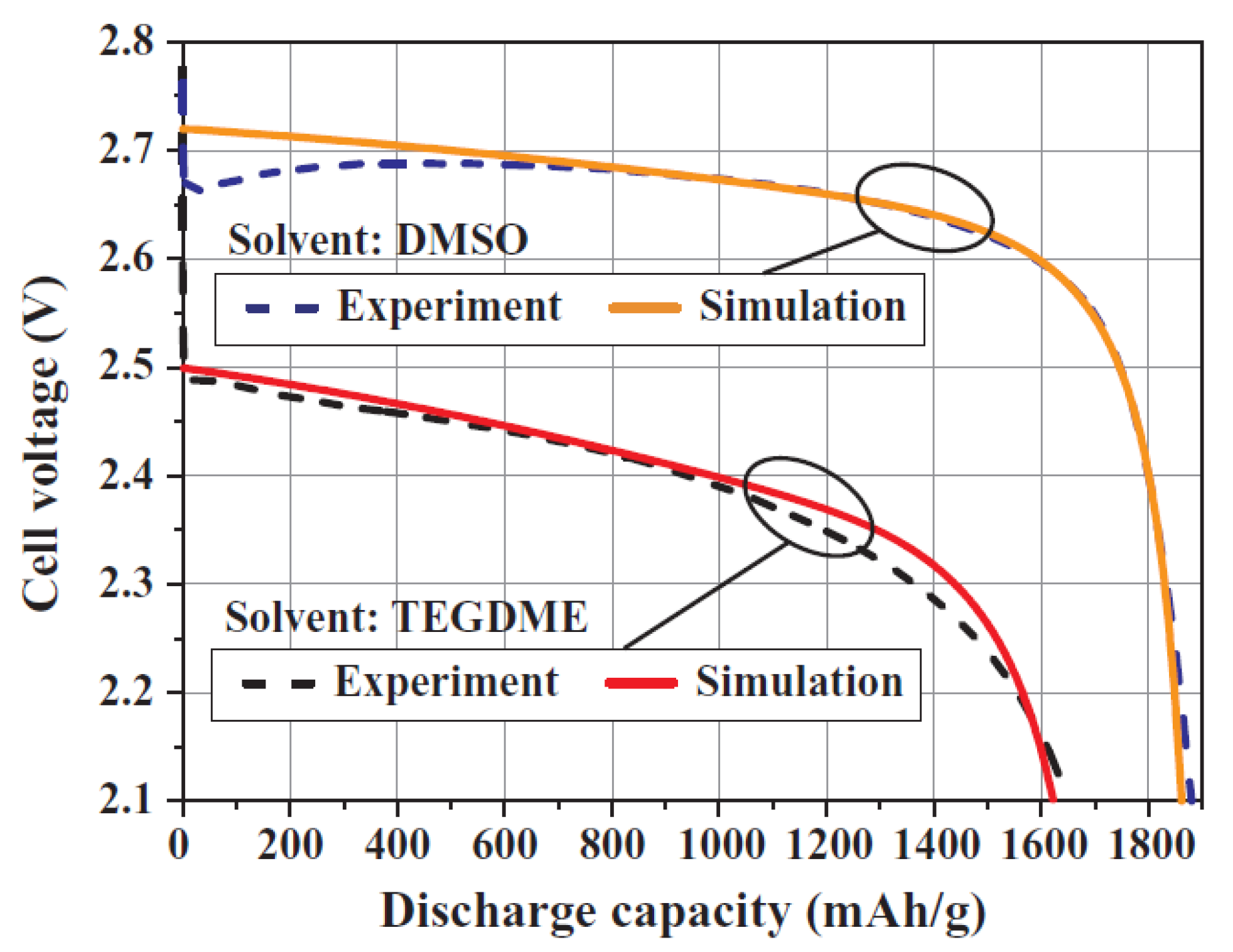
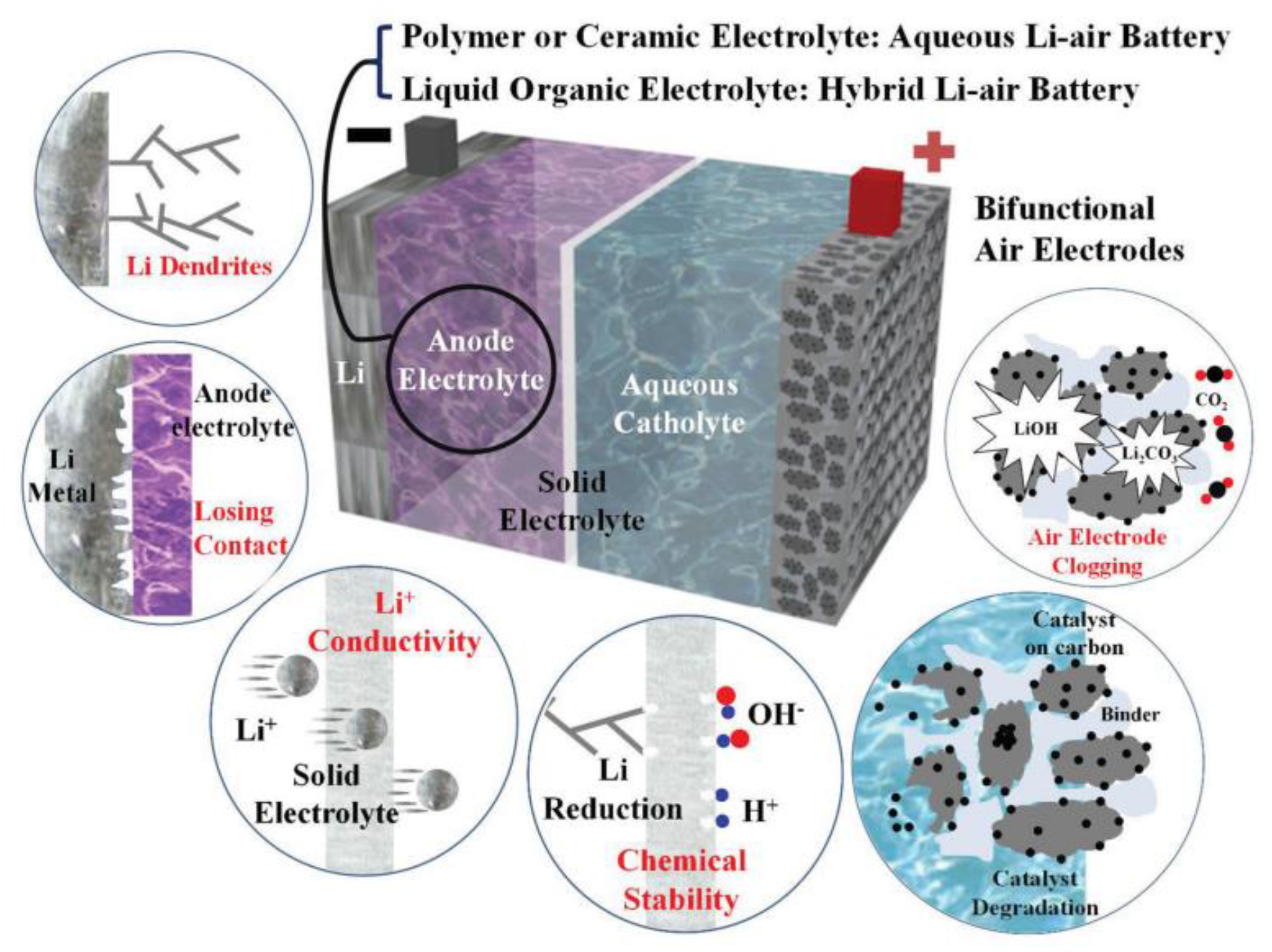
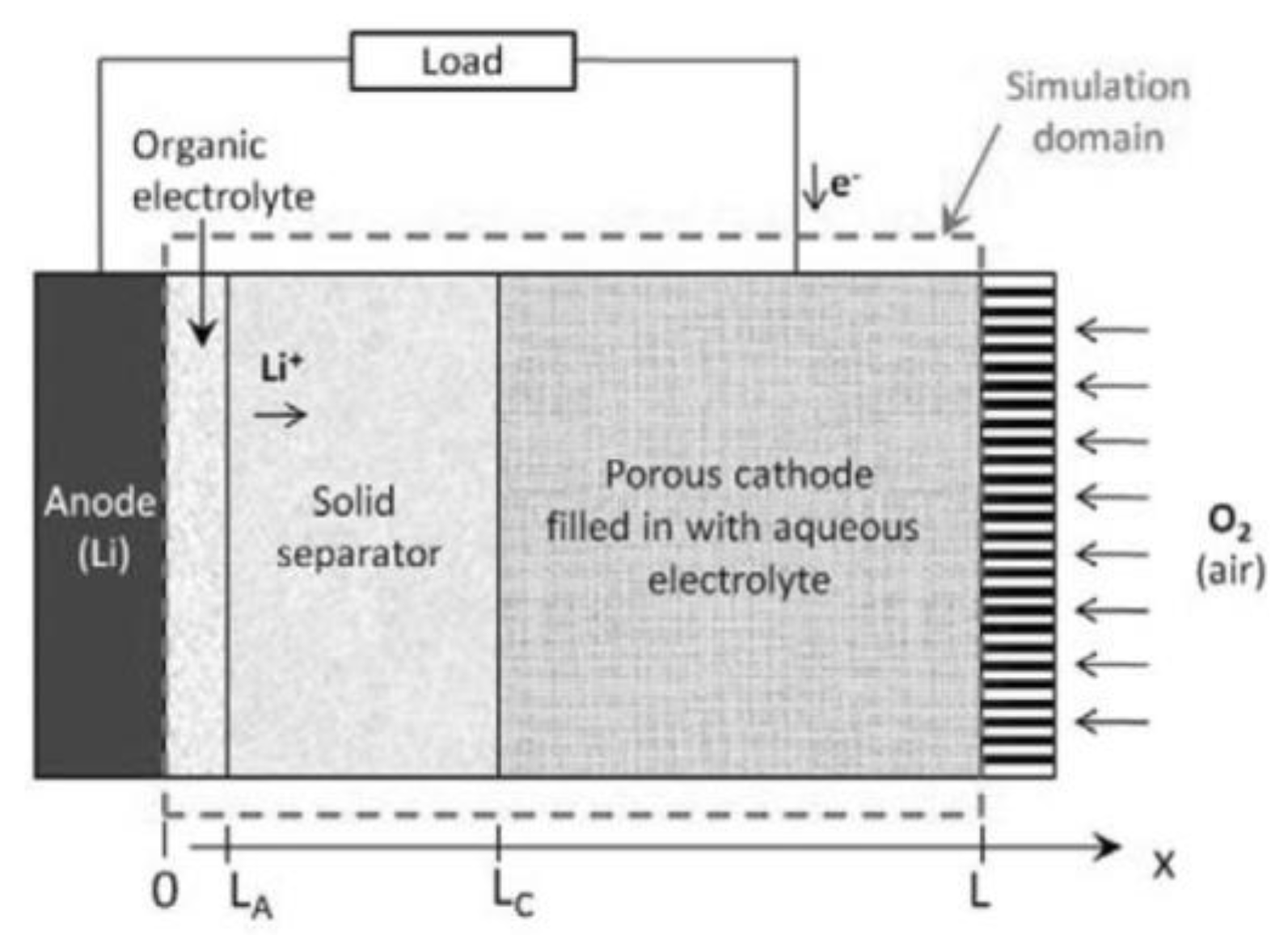
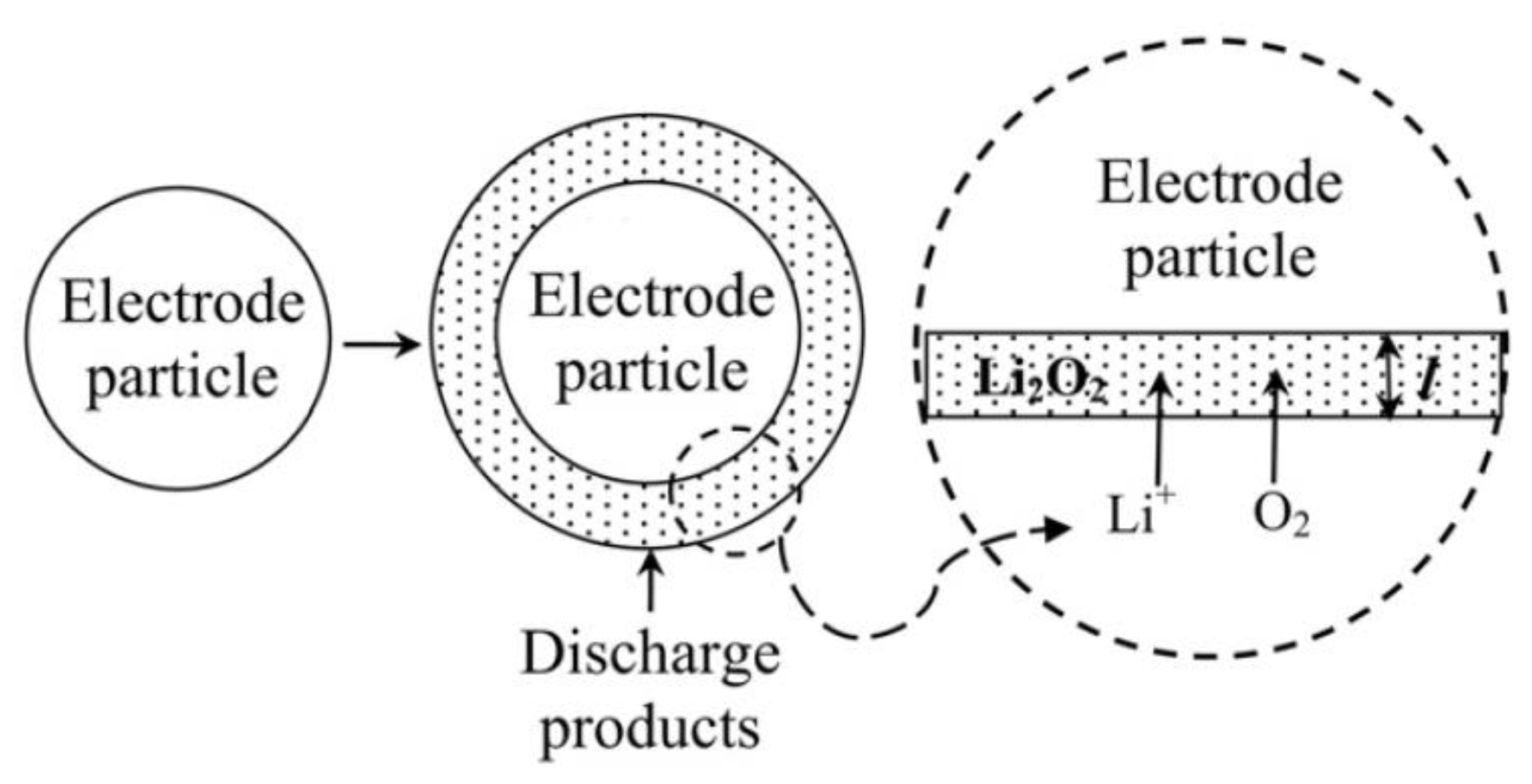
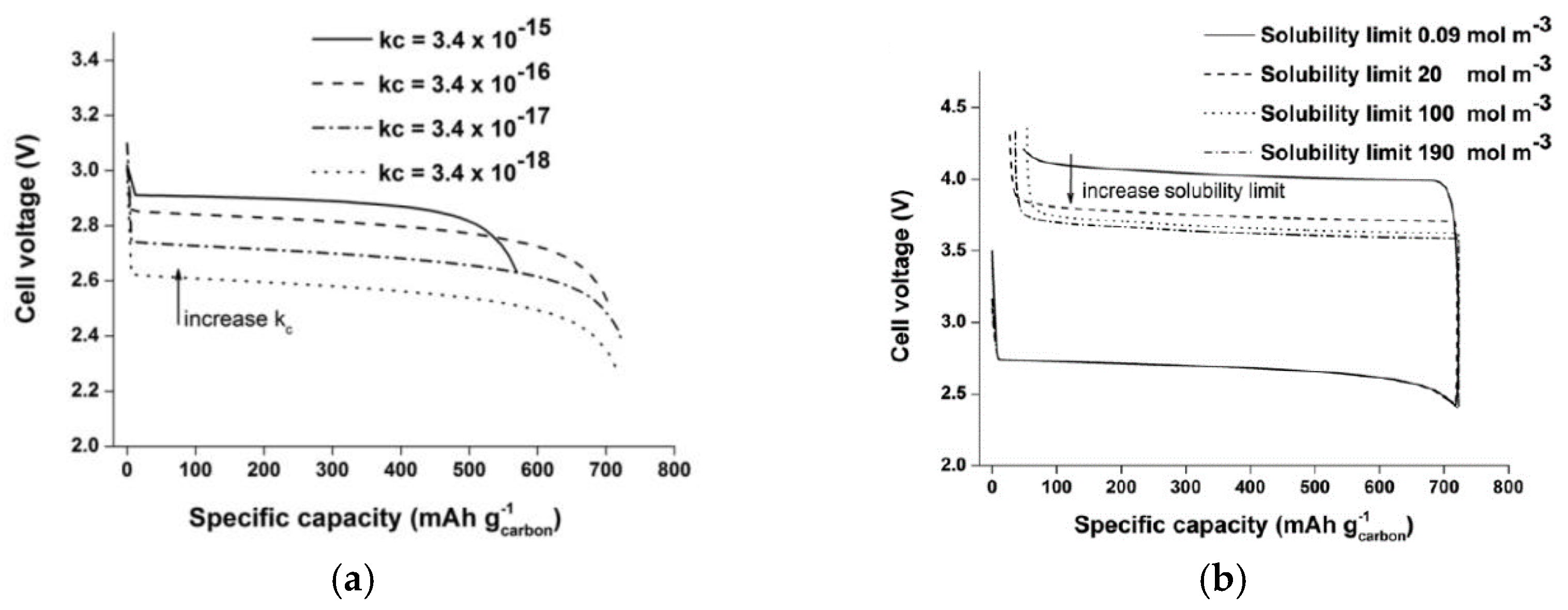
2.2. Aqueous Electrolyte
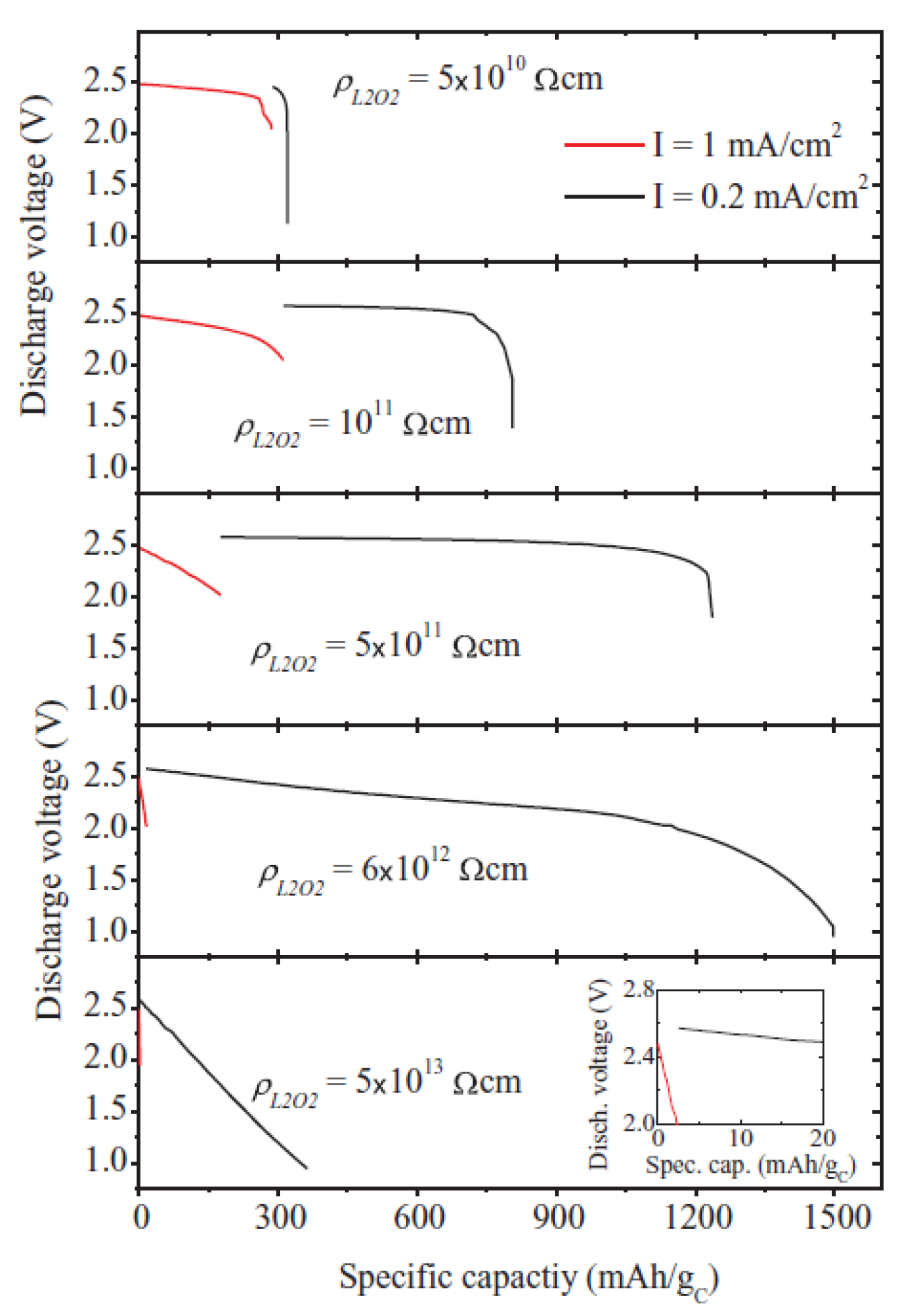
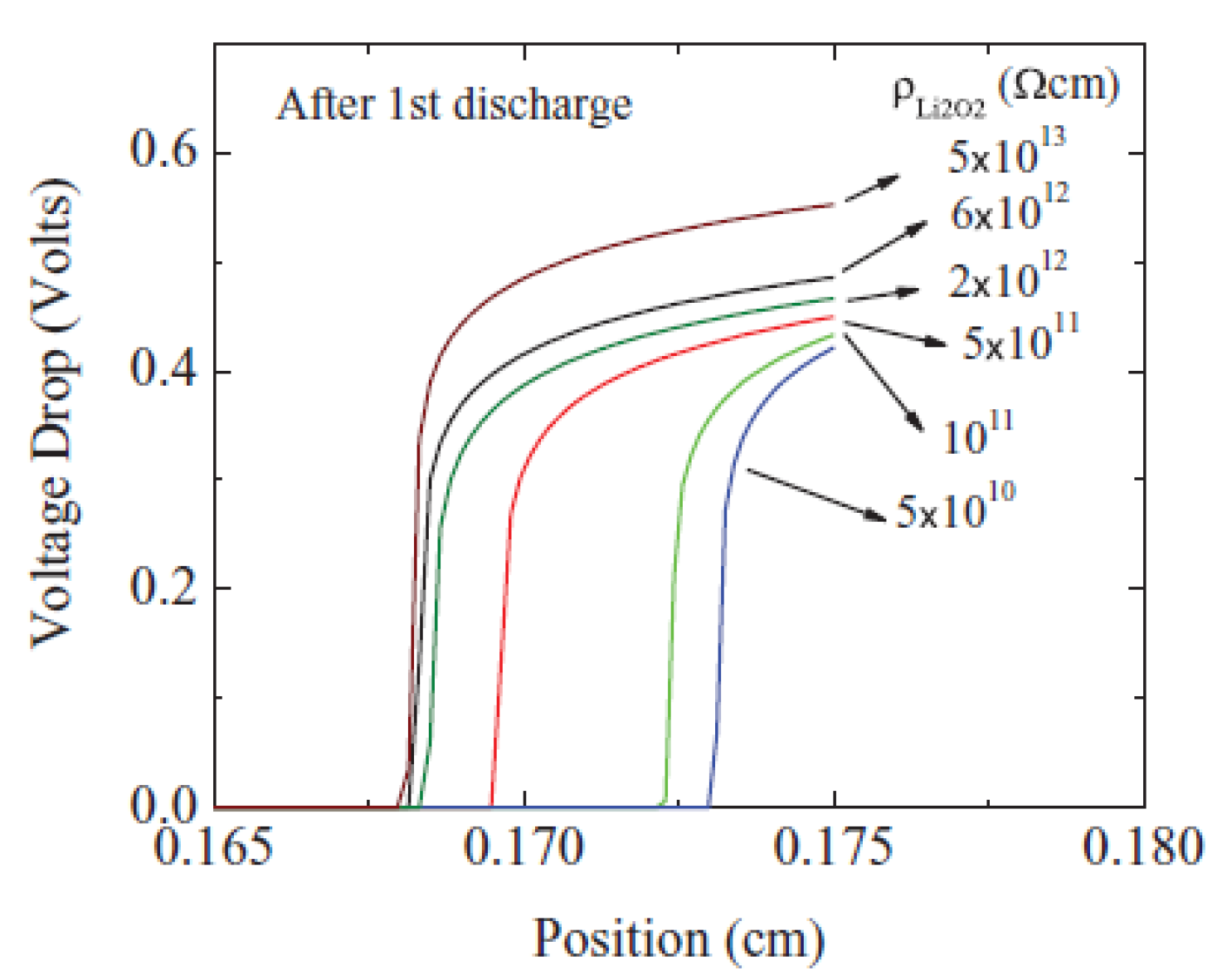
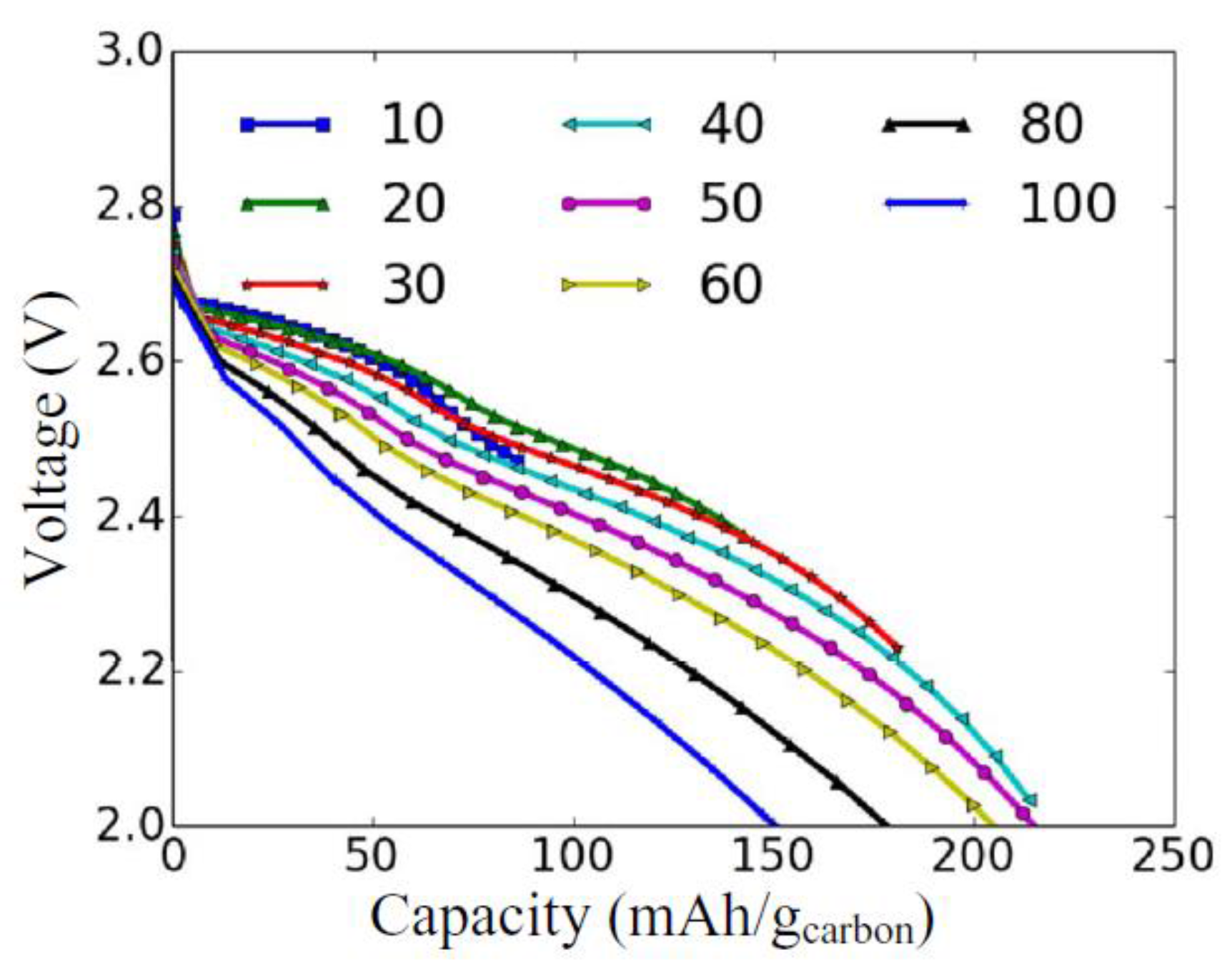
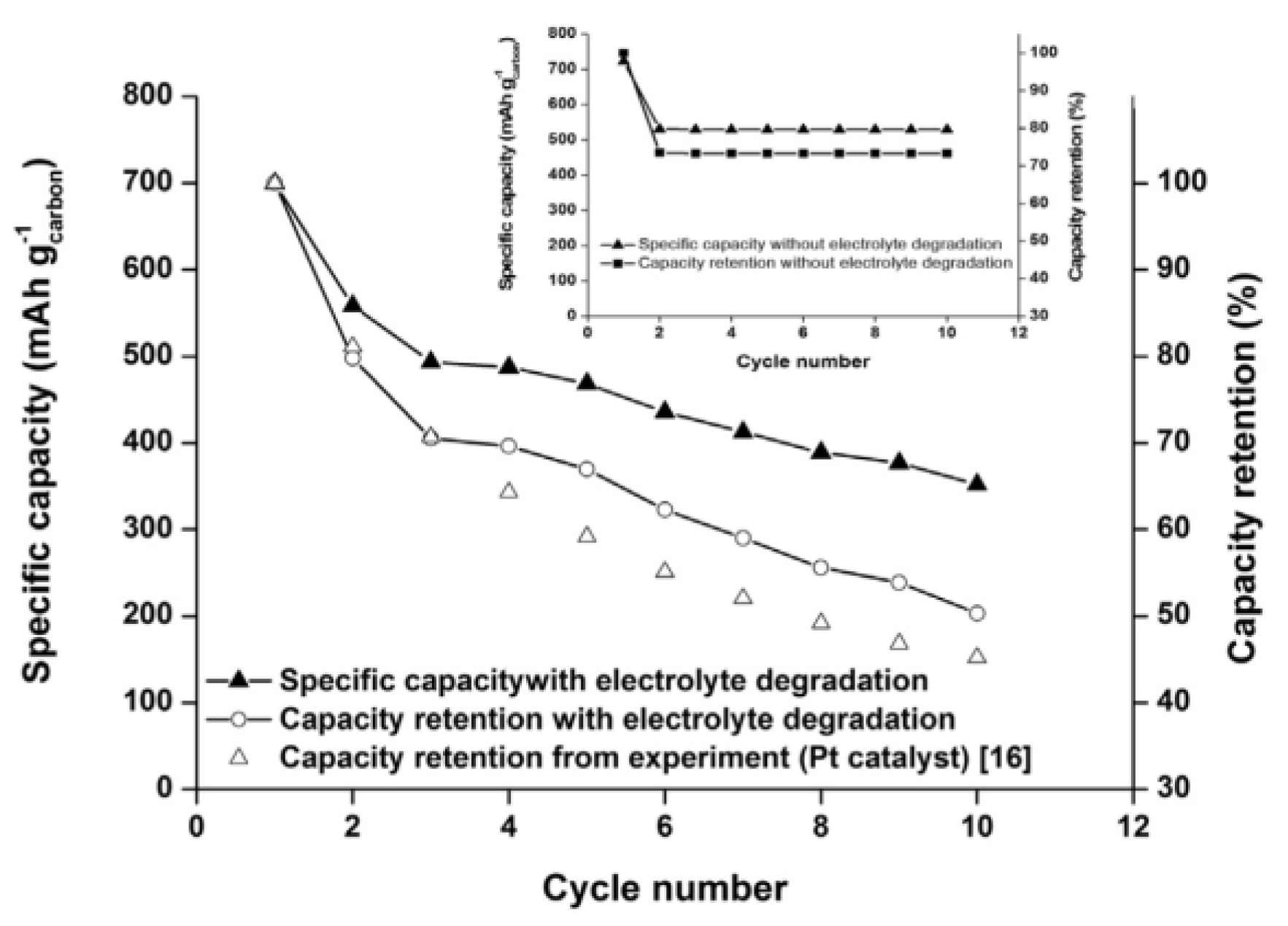
2.3. Modeling of Cycle Performance
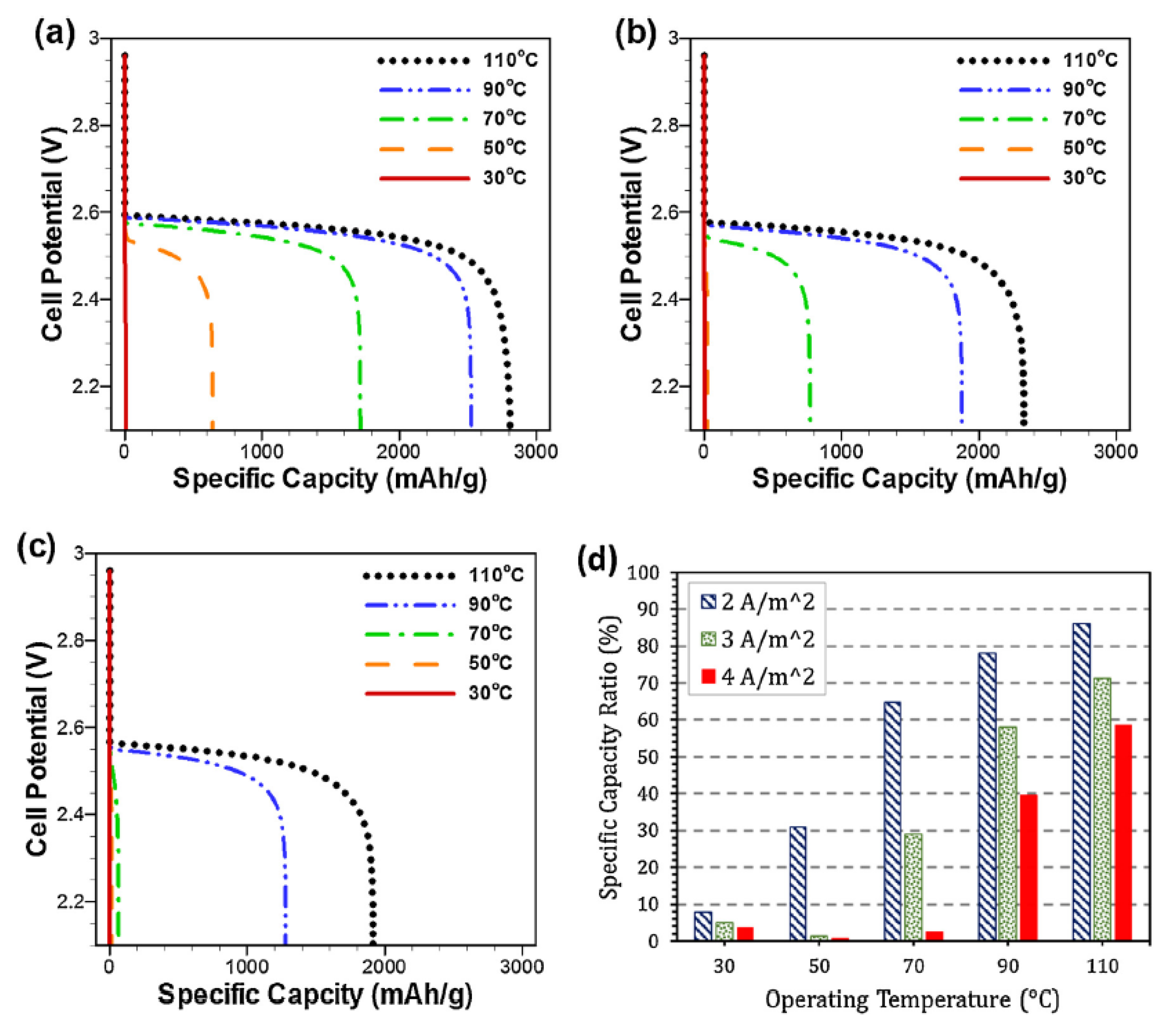
2.4. IL-Based Electrolyte
2.5. Two-Dimensional (2-D) Modeling
3. Atomistic and Molecular Models
3.1. Developing Appropriate Electrolyte
3.2. Identifying Suitable Cathode Structure and Catalysts
3.3. Providing Insights into Formation and Charge Transport Properties of Lithium Peroxide
4. Conclusions and Future Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cairns, E.J.; Albertus, P. Batteries for Electric and Hybrid-Electric Vehicles. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, S.T.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal-Air Batteries with High Energy Density: Li-Air versus Zn-Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium—Air Battery: Promise and Challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Grande, L.; Paillard, E.; Hassoun, J.; Park, J.-B.; Lee, Y.-J.; Sun, Y.-K.; Passerini, S.; Scrosati, B. The Lithium/Air Battery: Still an Emerging System or a Practical Reality? Adv. Mater. 2015, 27, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.N.; Kim, J.; Yamauchi, Y.; Park, M.S.; Lee, J.W.; Kim, J.H. Rechargeable lithium-air batteries: A perspective on the development of oxygen electrodes. J. Mater. Chem. A 2016, 4, 14050–14068. [Google Scholar] [CrossRef]
- Lu, J.; Lau, K.C.; Sun, Y.K.; Curtiss, L.A.; Amine, K. Review-Understanding and Mitigating Some of the Key Factors that Limit Non-Aqueous Lithium-Air Battery Performance. J. Electrochem. Soc. 2015, 162, A2439–A2446. [Google Scholar] [CrossRef]
- Albertus, P.; Girishkumar, G.; McCloskey, B.; Sanchez-Carrera, R.S.; Kozinsky, B.; Christensen, J.; Luntz, A.C. Identifying Capacity Limitations in the Li/Oxygen Battery Using Experiments and Modeling. J. Electrochem. Soc. 2011, 158, A343–A351. [Google Scholar] [CrossRef]
- Sahapatsombut, U.; Cheng, H.; Scott, K. Modelling the micro-macro homogeneous cycling behaviour of a lithium-air battery. J. Power Sources 2013, 227, 243–253. [Google Scholar] [CrossRef]
- Xue, K.-H.; McTurk, E.; Johnson, L.; Bruce, P.G.; Franco, A.A. A comprehensive model for non-aqueous lithium air batteries involving different reaction mechanisms. J. Electrochem. Soc. 2015, 162, A614–A621. [Google Scholar] [CrossRef]
- Mehta, M.; Mixon, G.; Zheng, J.P.; Andrei, P. Analytical electrochemical impedance modeling of Li-air batteries under DC discharge. J. Electrochem. Soc. 2013, 160, A2033–A2045. [Google Scholar] [CrossRef]
- Read, J. Characterization of the lithium/oxygen organic electrolyte battery. J. Electrochem. Soc. 2002, 149, A1190–A1195. [Google Scholar] [CrossRef]
- Read, J.; Mutolo, K.; Ervin, M.; Behl, W.; Wolfenstine, J.; Driedger, A.; Forster, D. Oxygen transport properties of organic electrolytes and performance of lithium/oxygen battery. J. Electrochem. Soc. 2003, 150, A1351–A1356. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Fellner, J.P.; Brutchen, G.W. Diffusion-limited model for a lithium/air battery with an organic electrolyte. J. Power Sources 2007, 164, 365–371. [Google Scholar] [CrossRef]
- Andrei, P.; Zheng, J.P.; Hendrickson, M.; Plichta, E.J. Some Possible Approaches for Improving the Energy Density of Li-Air Batteries. J. Electrochem. Soc. 2010, 157, A1287–A1295. [Google Scholar] [CrossRef]
- Newman, J.S. Electrochemical Systems; Prentice-Hall: Englewood Cliffs, NJ, USA, 1972. [Google Scholar]
- Yoo, K.; Banerjee, S.; Dutta, P. Modeling of volume change phenomena in a Li-air battery. J. Power Sources 2014, 258, 340–350. [Google Scholar] [CrossRef]
- Chen, X.J.; Bevara, V.V.; Andrei, P.; Hendrickson, M.; Plichta, E.J.; Zheng, J.P. Combined effects of oxygen diffusion and electronic resistance in Li-air batteries with carbon nanofiber cathodes. J. Electrochem. Soc. 2014, 161, A1877–A1883. [Google Scholar] [CrossRef]
- Wang, Y. Modeling discharge deposit formation and its effect on lithium-air battery performance. Electrochim. Acta 2012, 75, 239–246. [Google Scholar] [CrossRef]
- Jung, C.Y.; Zhao, T.S.; An, L. Modeling of lithium–oxygen batteries with the discharge product treated as a discontinuous deposit layer. J. Power Sources 2015, 273, 440–447. [Google Scholar] [CrossRef]
- Wang, Y.; Cho, S.C. Analysis of air cathode perfomance for lithium-air batteries. J. Electrochem. Soc. 2013, 160, A1847–A1855. [Google Scholar] [CrossRef]
- Xue, K.-H.; Nguyen, T.-K.; Franco, A.A. Impact of the cathode microstructure on the discharge performance of lithium air batteries: A multiscale model. J. Electrochem. Soc. 2014, 161, E3028–E3035. [Google Scholar] [CrossRef]
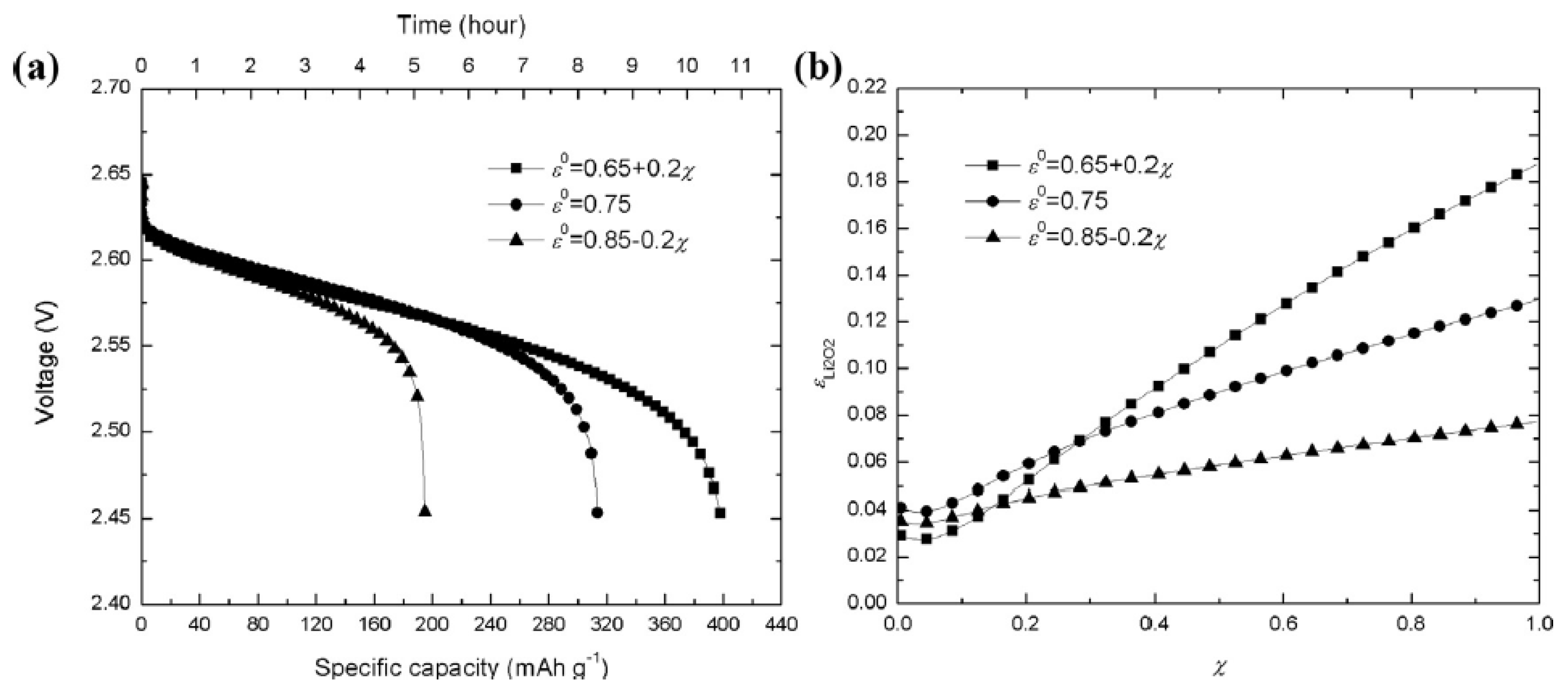
- Li, X. A modeling study of the pore size evolution in lithium-oxygen battery electrodes. J. Electrochem. Soc. 2015, 162, A1636–A1645. [Google Scholar] [CrossRef]
- He, P.; Wang, Y.; Zhou, H. A Li-air fuel cell with recycle aqueous electrolyte for improved stability. Electrochem. Commun. 2010, 12, 1686–1689. [Google Scholar] [CrossRef]
- Zhang, T.; Imanishi, N.; Shimonishi, Y.; Hirano, A.; Xie, J.; Takeda, Y.; Yamamoto, O.; Sammes, N. Stability of a water-stable lithium metal anode for a lithium–air battery with acetic acid–water solutions. J. Electrochem. Soc. 2010, 157, A214–A218. [Google Scholar] [CrossRef]
- Manthiram, A.; Li, L. Hybrid and Aqueous Lithium-Air Batteries. Adv. Energy Mater. 2015, 5, 1401302. [Google Scholar] [CrossRef]
- Andrei, P.; Zheng, J.P.; Hendrickson, M.; Plichta, E.J. Modeling of Li-air batteries with dual electrolyte. J. Electrochem. Soc. 2012, 159, A770–A780. [Google Scholar] [CrossRef]
- Horstmann, B.; Danner, T.; Bessler, W.G. Precipitation in aqueous lithium–oxygen batteries: A model-based analysis. Energy Environ. Sci. 2013, 6, 1299–1314. [Google Scholar] [CrossRef] [Green Version]
- Nimon, V.Y.; Visco, S.J.; de Jonghe, L.C.; Volfkovich, Y.M.; Bograchev, D.A. Modeling and experimental study of porous carbon cathodes in Li-O2 cells with non-aqueous electrolyte. ECS Electrochem. Lett. 2013, 2, A33–A35. [Google Scholar] [CrossRef]
- Imanishi, N.; Yamamoto, O. Rechargeable lithium–air batteries: Characteristics and prospects. Mater. Today 2014, 17, 24–30. [Google Scholar] [CrossRef]
- Lim, H.D.; Park, K.Y.; Song, H.; Jang, E.Y.; Gwon, H.; Kim, J.; Kim, Y.H.; Lima, M.D.; Robles, R.O.; Lepró, X.; et al. Enhanced Power and Rechargeability of a Li-O2 Battery Based on a Hierarchical-Fibril CNT Electrode. Adv. Mater. 2013, 25, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Qiao, Y.; Yang, S.; Ishida, M.; He, P.; Zhou, H. Organic hydrogen peroxide-driven low charge potentials for high-performance lithium-oxygen batteries with carbon cathodes. Nat. Commun. 2017, 8, 15607. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-G.; Hassoun, J.; Park, J.-B.; Sun, Y.-K.; Scrosati, B. An improved high-performance lithium-air battery. Nat. Chem. 2012, 4, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Leskes, M.; Yu, W.; Moore, A.J.; Zhou, L.; Bayley, P.M.; Kim, G.; Grey, C.P. Cycling Li-O2 batteries via LiOH formation and decomposition. Science 2015, 350, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Elia, G.A.; Hassoun, J.; Kwak, W.J.; Sun, Y.K.; Scrosati, B.; Mueller, F.; Bresser, D.; Passerini, S.; Oberhumer, P.; Tsiouvaras, N.; et al. An advanced lithium–air battery exploiting an ionic liquid-based electrolyte. Nano Lett. 2014, 14, 6572–6577. [Google Scholar] [CrossRef] [PubMed]
- Sahapatsombut, U.; Cheng, H.; Scott, K. Modelling of electrolyte degradation and cycling behaviour in a lithium-air battery. J. Power Sources 2013, 243, 409–418. [Google Scholar] [CrossRef]
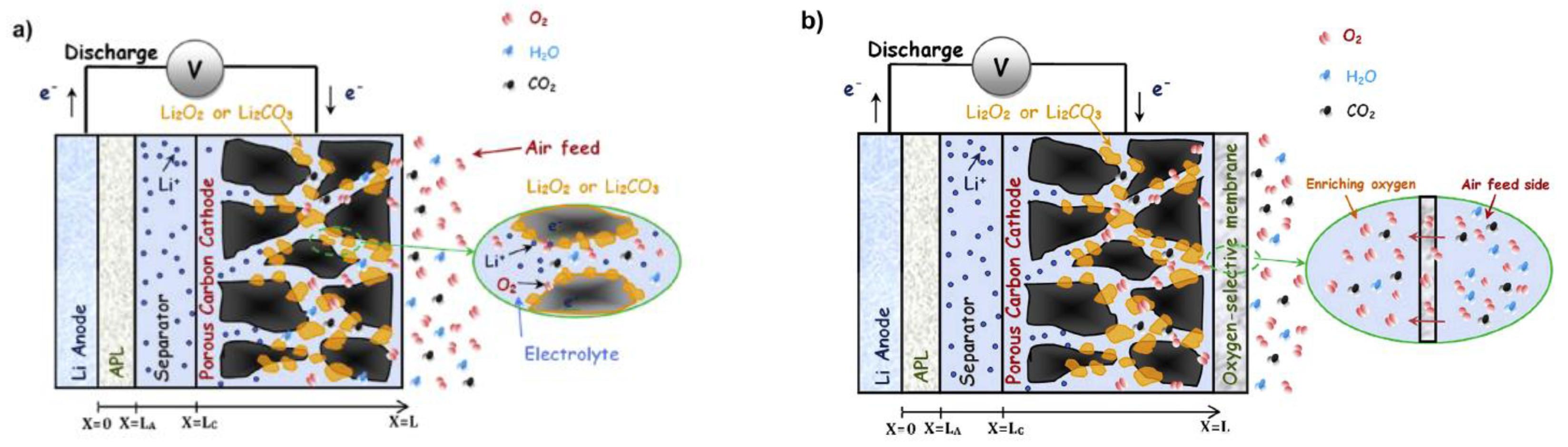
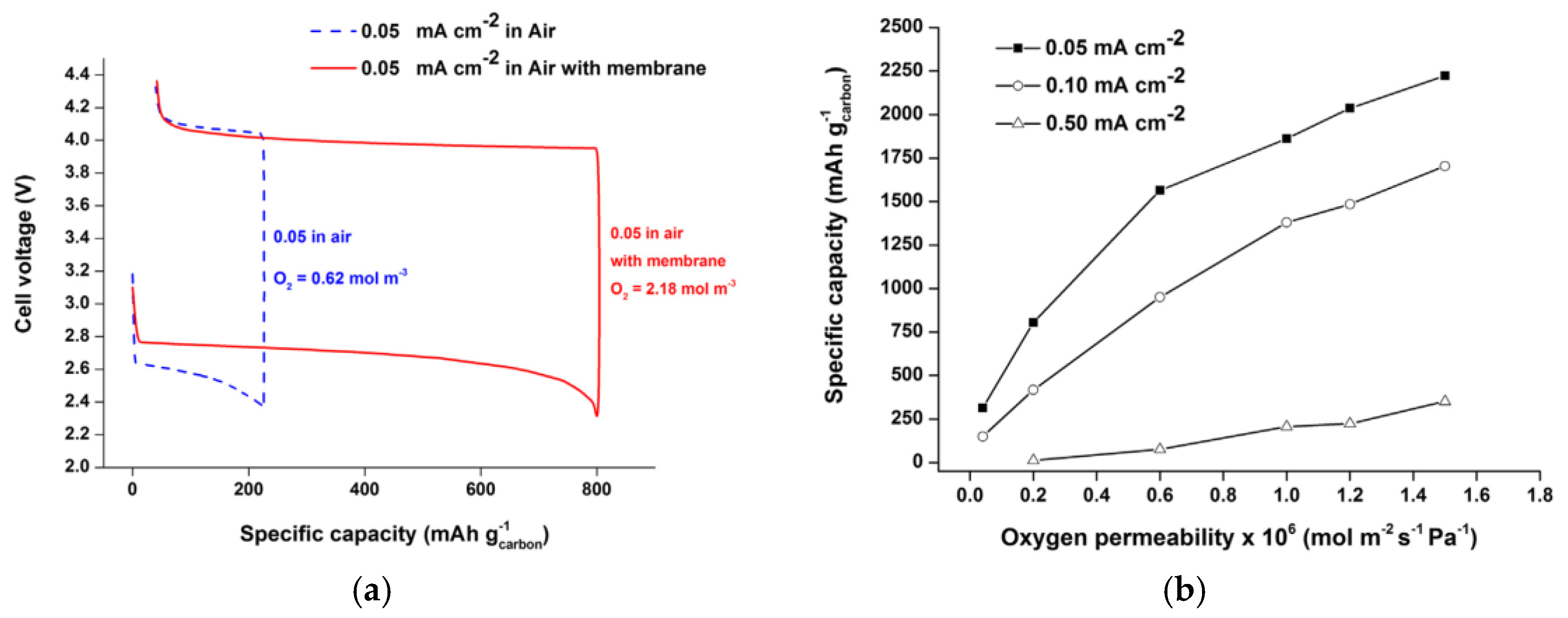
- Sahapatsombut, U.; Cheng, H.; Scott, K. Modelling of operation of a lithium-air battery with ambient air and oxygen-selective membrane. J. Power Sources 2014, 249, 418–430. [Google Scholar] [CrossRef]
- Yoo, K.; Deshpande, A.; Banerjee, S.; Dutta, P. Electrochemical Model for Ionic Liquid Electrolytes in Lithium Batteries. Electrochim. Acta 2015, 176, 301–310. [Google Scholar] [CrossRef]
- Saint, J.; Best, A.S.; Hollenkamp, A.F.; Kerr, J.; Shin, J.H.; Doeff, M.M. Compatibility of LixTiyMn1−yO2 (y = 0, 0.11) electrode materials with pyrrolidinium-based ionic liquid electrolyte systems. J. Electrochem. Soc. 2008, 155, A172–A180. [Google Scholar] [CrossRef]
- Sakaebe, H.; Matsumoto, H. N-Methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide (PP 13-TFSI)—Novel electrolyte base for Li battery. Electrochem. Commun. 2003, 5, 594–598. [Google Scholar] [CrossRef]
- Kuboki, T.; Okuyama, T.; Ohsaki, T.; Takami, N. Lithium-air batteries using hydrophobic room temperature ionic liquid electrolyte. J. Power Sources 2005, 146, 766–769. [Google Scholar] [CrossRef]
- Higashi, S.; Kato, Y.; Takechi, K.; Nakamoto, H.; Mizuno, F.; Nishikoori, H.; Iba, H.; Asaoka, T. Evaluation and analysis of Li-air battery using ether-functionalized ionic liquid. J. Power Sources 2013, 240, 14–17. [Google Scholar] [CrossRef]
- Yoo, K.; Dive, A.M.; Kazemiabnavi, S.; Banerjee, S.; Dutta, P. Effects of Operating Temperature on the Electrical Performance of a Li-air Battery operated with Ionic Liquid Electrolyte. Electrochim. Acta 2016, 194, 317–329. [Google Scholar] [CrossRef]
- Li, X.; Faghri, A. Optimization of the cathode structure of lithium-air batteries based on a two-dimensional, transient, non-isothermal model. J. Electrochem. Soc. 2012, 159, A1747–A1754. [Google Scholar] [CrossRef]
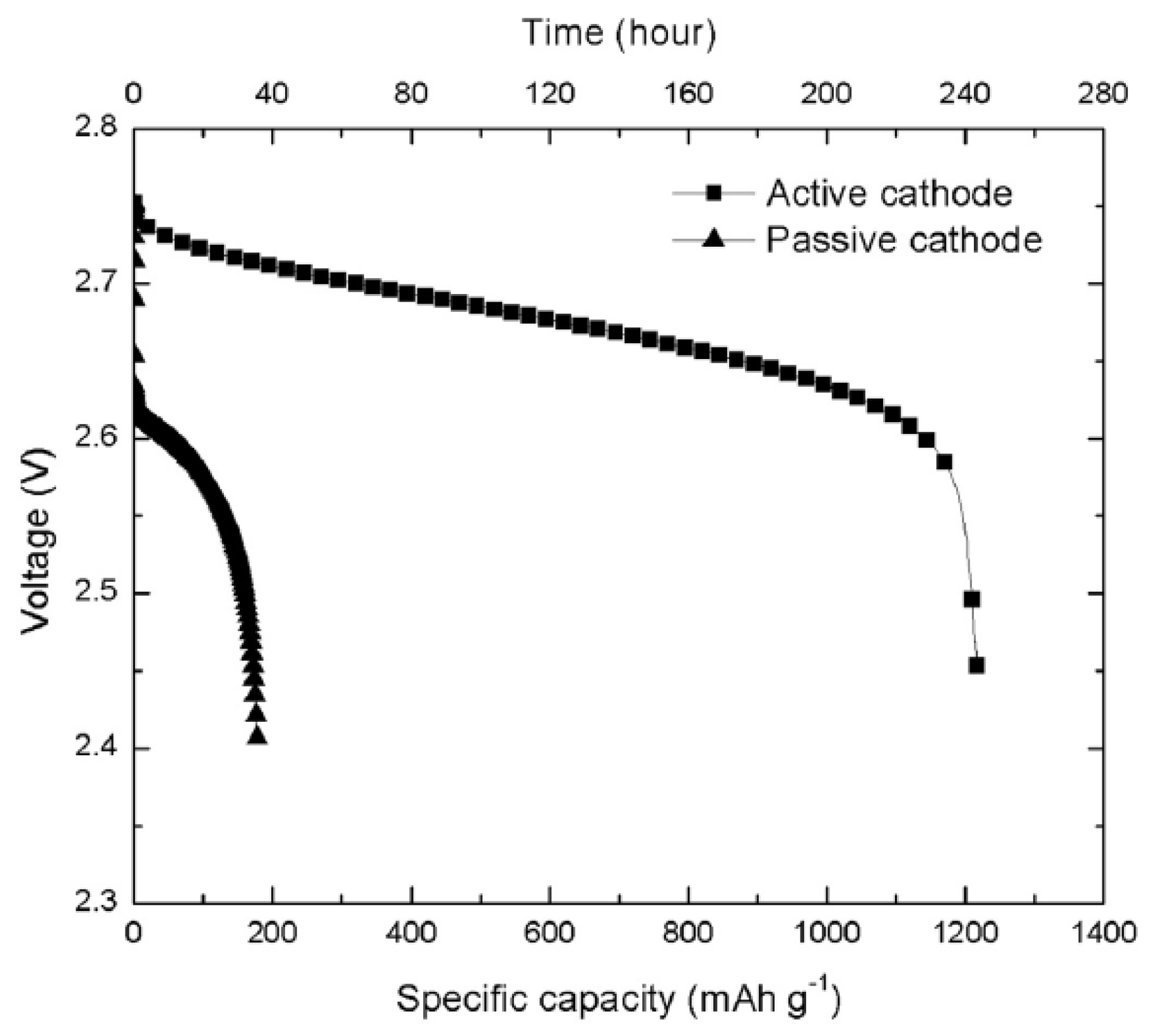
- Li, X.; Huang, J.; Faghri, A. Modeling study of a Li-O2 battery with an active cathode. Energy 2015, 81, 489–500. [Google Scholar] [CrossRef]
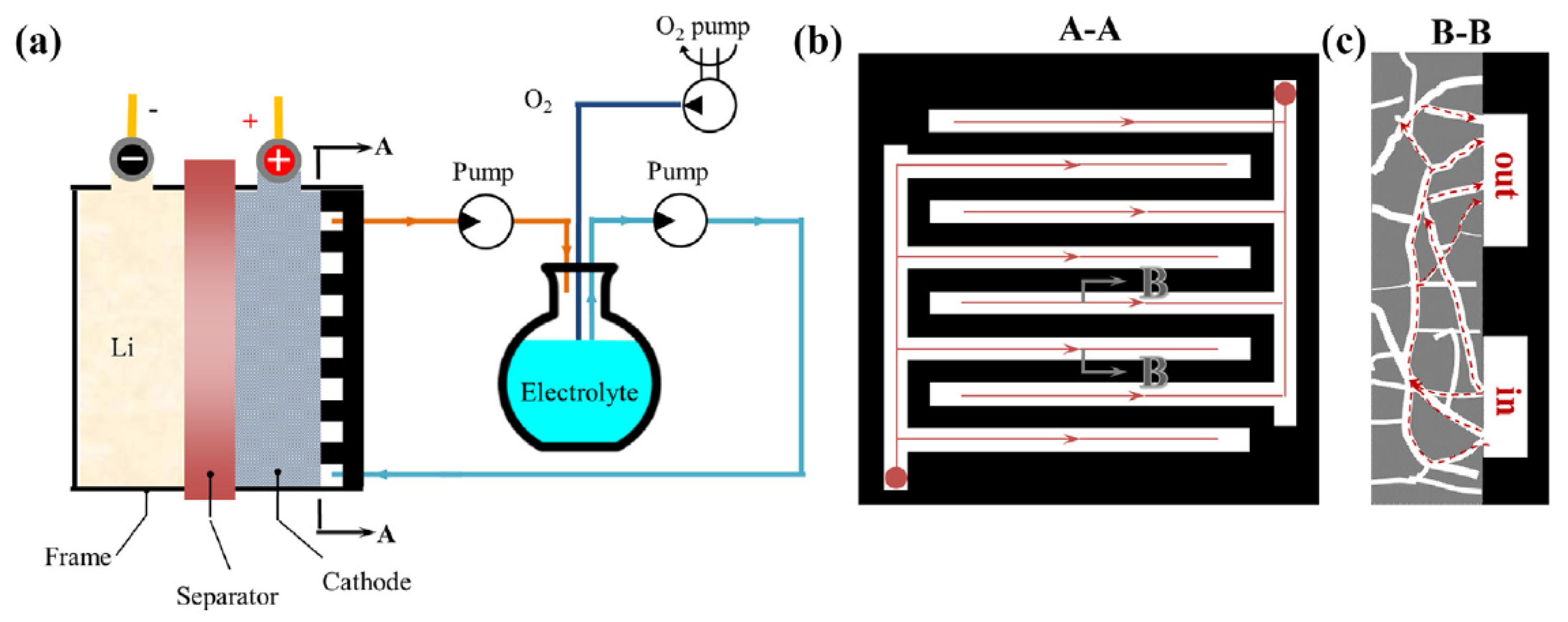
- Huang, J.; Faghri, A. Capacity enhancement of a lithium oxygen flow battery. Electrochim. Acta 2015, 174, 908–918. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Zheng, W.T.; Cui, X.Q.; Rojo, T.; Zhang, Q. Towards High-Safe Lithium Metal Anodes: Suppressing Lithium Dendrites via Tuning Surface Energy. Adv. Sci. 2017, 4, 1600168. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Ely, D.R.; Garcia, R.E. Dendrite-separator interactions in lithium-based batteries. J. Power Sources 2015, 275, 912–921. [Google Scholar] [CrossRef]
- Welland, M.J.; Lau, K.C.; Redfern, P.C.; Liang, L.; Zhai, D.; Wolf, D.; Curtiss, L.A. An atomistically informed mesoscale model for growth and coarsening during discharge in lithium-oxygen batteries. J. Chem. Phys. 2015, 143, 224113. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.P.; Andreussi, O.; Wu, Y.; Marzari, N.; Ceder, G. Electrochemical Windows of Room-Temperature Ionic Liquids from Molecular Dynamics and Density Functional Theory Calculations. Chem. Mater. 2011, 23, 2979–2986. [Google Scholar] [CrossRef]
- Borodin, O.; Olguin, M.; Spear, C.E.; Leiter, K.W.; Knap, J. Towards high throughput screening of electrochemical stability of battery electrolytes. Nanotechnology 2015, 26, 354003. [Google Scholar] [CrossRef] [PubMed]
- Knap, J.; Spear, C.E.; Borodin, O.; Leiter, K.W. Advancing a distributed multi-scale computing framework for large-scale high-throughput discovery in materials science. Nanotechnology 2015, 26, 434004. [Google Scholar] [CrossRef] [PubMed]
- Parker, V.D. Energetics of Electrode Reactions. II. The Relationship Between Redox Potentials, Ionization Potentials, Electron Affinities, and Solvation Energies of Aromatic Hydrocarbons. J. Am. Chem. Soc. 1976, 98, 98–103. [Google Scholar] [CrossRef]
- Shao, N.; Sun, X.-G.; Dai, S.; Jiang, D.-E. Electrochemical Windows of Sulfone-Based Electrolytes for High-Voltage Li-Ion Batteries. J. Phys. Chem. B 2011, 115, 12120–12125. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Sun, X.-G.; Dai, S.; Jiang, D.-E. Oxidation Potentials of Functionalized Sulfone Solvents for High-Voltage Li-Ion Batteries: A Computational Study. J. Phys. Chem. B 2012, 116, 3235–3238. [Google Scholar] [CrossRef] [PubMed]
- Borodin, O.; Behl, W.; Jow, T.R. Oxidative Stability and Initial Decomposition Reactions of Carbonate, Sulfone, and Alkyl Phosphate-Based Electrolytes. J. Phys. Chem. C 2013, 117, 8661–8682. [Google Scholar] [CrossRef]
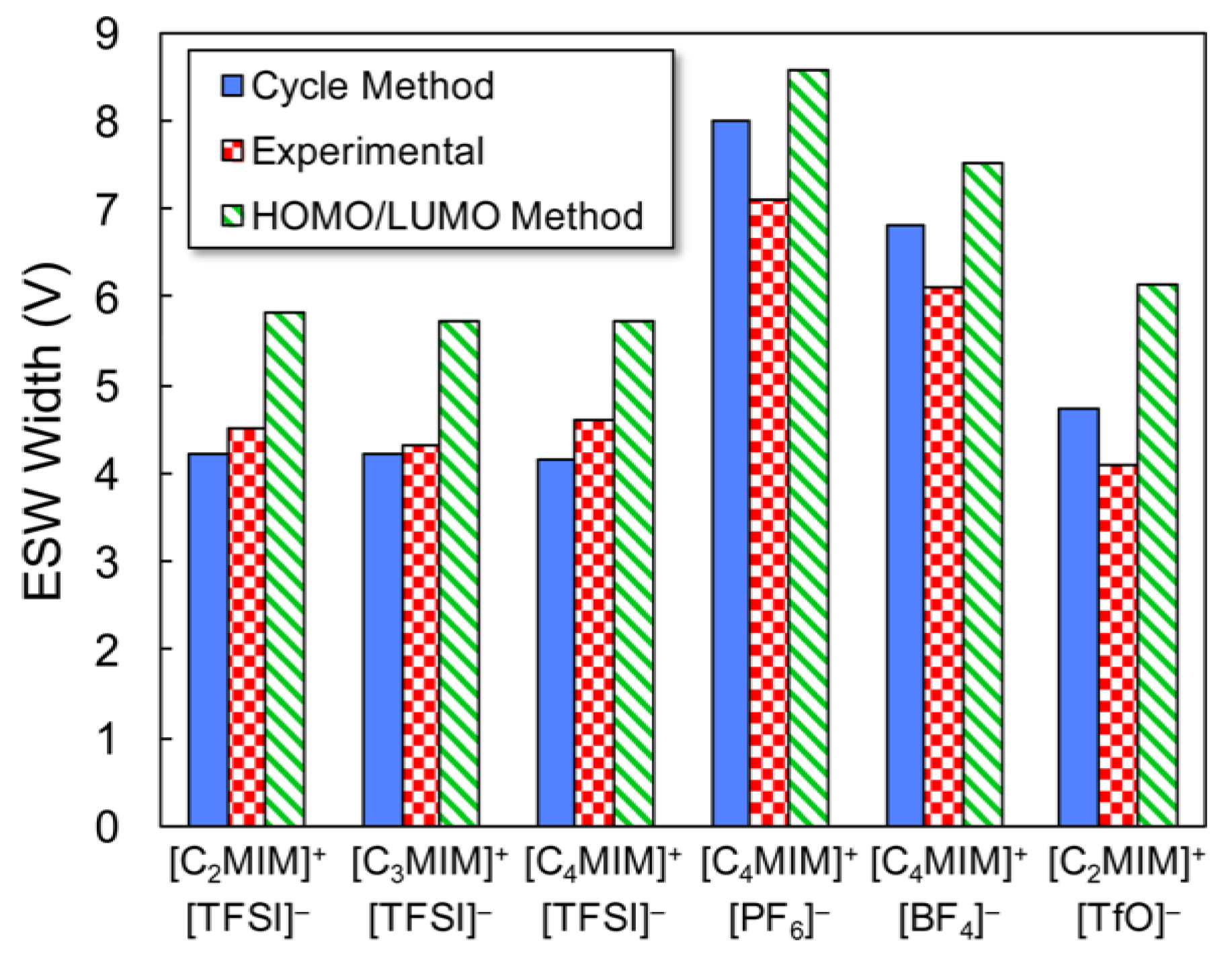
- Kazemiabnavi, S.; Zhang, Z.; Thornton, K.; Banerjee, S. Electrochemical Stability Window of Imidazolium-Based Ionic Liquids as Electrolytes for Lithium Batteries. J. Phys. Chem. B 2016, 120, 5691–5702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Pugh, J.K.; Ross, P.N. Computation of Thermodynamic Oxidation Potentials of Organic Solvents Using Density Functional Theory. J. Electrochem. Soc. 2001, 148, E183–E188. [Google Scholar] [CrossRef]
- Galinski, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Bryantsev, V.S.; Faglioni, F. Predicting Autoxidation Stability of Ether- and Amide-Based Electrolyte Solvents for Li-Air Batteries. J. Phys. Chem. A 2012, 116, 7128–7138. [Google Scholar] [CrossRef] [PubMed]
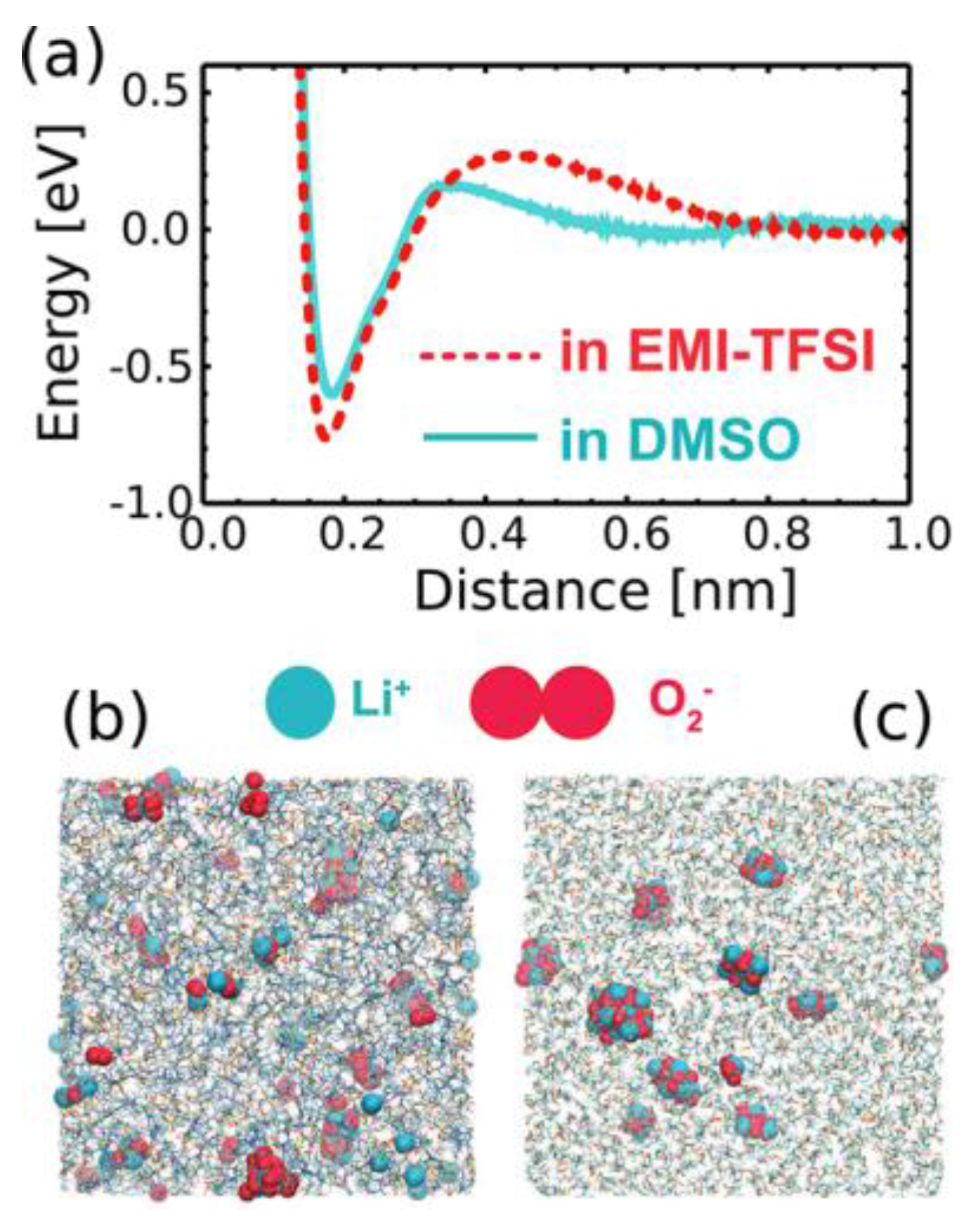
- Jung, S.; Canova, F.F.; Akagi, K. Characteristics of Lithium Ions and Superoxide Anions in EMI-TFSI and Dimethyl Sulfoxide. J. Phys. Chem. A 2016, 120, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Scheers, J.; Lidberg, D.; Sodeyama, K.; Futera, Z.; Tateyama, Y. Life of superoxide in aprotic Li-O2 battery electrolytes: Simulated solvent and counter-ion effects. Phys. Chem. Chem. Phys. 2016, 18, 9961–9968. [Google Scholar] [CrossRef] [PubMed]
- Chau, V.K.C.; Chen, Z.N.; Hu, H.; Chan, K.Y. Exploring Solvent Stability against Nucleophilic Attack by Solvated LiO2- in an Aprotic Li-O2 Battery. J. Electrochem. Soc. 2017, 164, A284–A289. [Google Scholar] [CrossRef]
- Liu, B.; Xu, W.; Yan, P.; Sun, X.; Bowden, M.E.; Read, J.; Qian, J.; Mei, D.; Wang, C.-M.; Zhang, J.-G. Enhanced Cycling Stability of Rechargeable Li-O2 Batteries Using High-Concentration Electrolytes. Adv. Funct. Mater. 2016, 26, 605–613. [Google Scholar] [CrossRef]
- Deshpande, A.; Dutta, P.; Banerjee, S. Solubility of oxygen in lithium-air battery electrolytes: A molecular dynamics study. In Proceedings of the ASME 2014 International Mechanical Engineering Congress and Exposition (IMECE 2014), Montreal, QC, Canada, 14–20 November 2014. [Google Scholar]
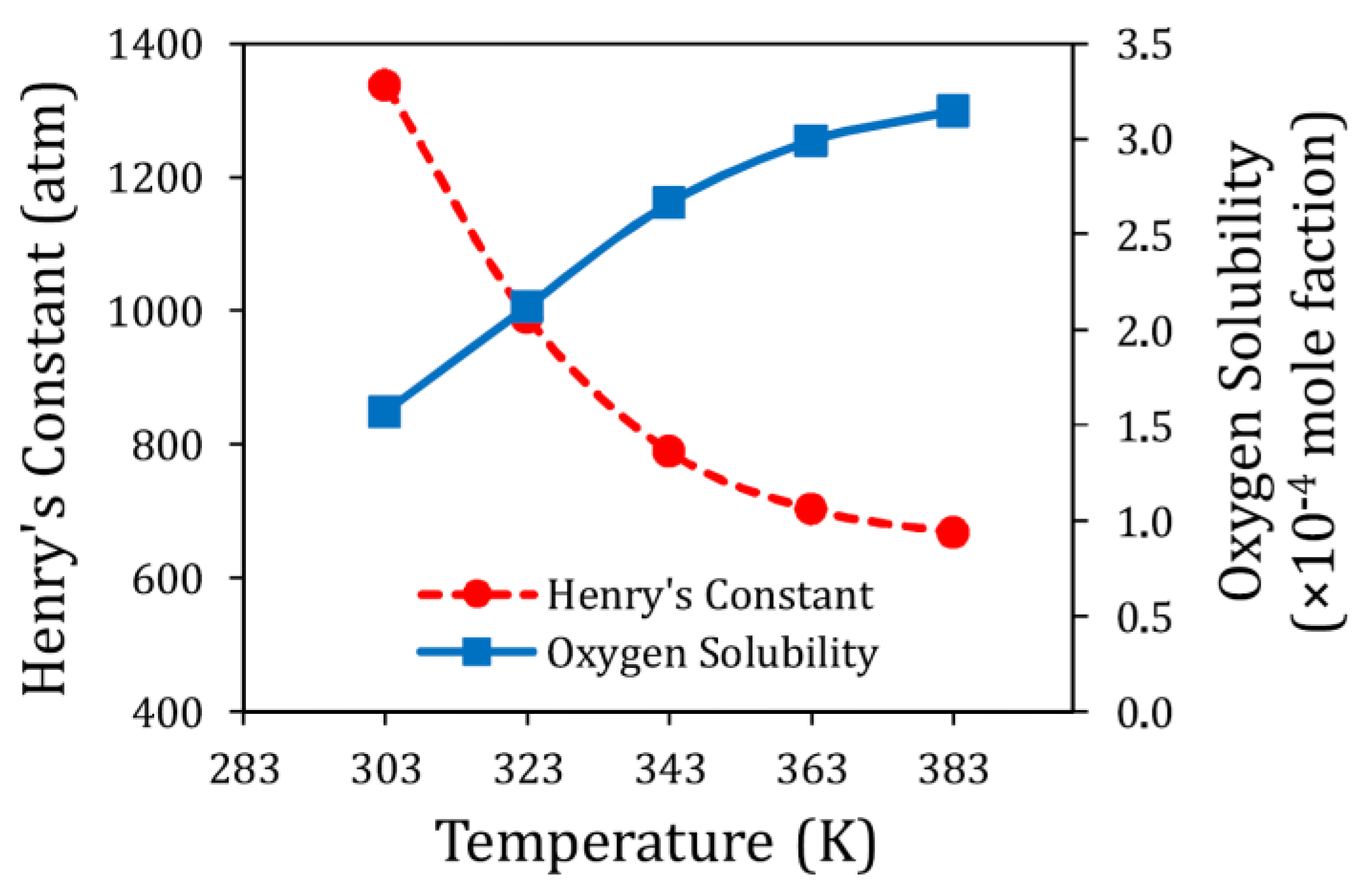
- Gittleson, F.S.; Jones, R.E.; Ward, D.K.; Foster, M.E. Oxygen solubility and transport in Li-air battery electrolytes: Establishing criteria and strategies for electrolyte design. Energy Environ. Sci. 2017, 10, 1167–1179. [Google Scholar] [CrossRef]
- Borodin, O.; Smith, G.D. Structure and dynamics of N-methyl-N-propylpyrrolidinium bis(trifluoromethanesulfonyl)imide ionic liquid from molecular dynamics simulations. J. Phys. Chem. B 2006, 110, 11481–11490. (In English) [Google Scholar] [CrossRef] [PubMed]
- Mozhzhukhina, N.; Longinotti, M.P.; Corti, H.R.; Calvo, E.J. A conductivity study of preferential solvation of lithium ion in acetonitrile-dimethyl sulfoxide mixtures. Electrochim. Acta 2015, 154, 456–461. [Google Scholar] [CrossRef]
- Semino, R.; Zaldivar, G.; Calvo, E.J.; Laria, D. Lithium solvation in dimethyl sulfoxide-acetonitrile mixtures. J. Chem. Phys. 2014, 141, 214509. [Google Scholar] [CrossRef] [PubMed]
- Iliksu, M.; Khetan, A.; Yang, S.; Simon, U.; Pitsch, H.; Sauer, D.U. Elucidation and Comparison of the Effect of LiTFSI and LiNO3 Salts on Discharge Chemistry in Nonaqueous Li-O2 Batteries. ACS Appl. Mater. Interfaces 2017, 9, 19319–19325. [Google Scholar] [CrossRef] [PubMed]
- Korth, M. Large-scale virtual high-throughput screening for the identification of new battery electrolyte solvents: Evaluation of electronic structure theory methods. Phys. Chem. Chem. Phys. 2014, 16, 7919–7926. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Assary, R.S.; Qu, X.; Jain, A.; Ong, S.P.; Rajput, N.N.; Persson, K.; Curtiss, L.A. Accelerating Electrolyte Discovery for Energy Storage with High-Throughput Screening. J. Phys. Chem. Lett. 2015, 6, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Jain, A.; Rajput, N.N.; Cheng, L.; Zhang, Y.; Ong, S.P.; Brafman, M.; Maginn, E.; Curtiss, L.A.; Persson, K.A. The Electrolyte Genome project: A big data approach in battery materials discovery. Comput. Mater. Sci. 2015, 103, 56–67. [Google Scholar] [CrossRef]
- Shan, S.; Luo, J.; Wu, J.; Kang, N.; Zhao, W.; Cronk, H.; Zhao, Y.; Joseph, P.; Petkov, V.; Zhong, C.-J. Nanoalloy catalysts for electrochemical energy conversion and storage reactions. RSC Adv. 2014, 4, 42654–42669. [Google Scholar] [CrossRef]
- Dabrowski, T.; Ciacchi, L.C. Atomistic Modeling of the Charge Process in Lithium/Air Batteries. J. Phys. Chem. C 2015, 119, 25807–25817. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, H.S. Facilitating the Oxygen Evolution Reaction of Lithium Peroxide via Molecular Adsorption. J. Phys. Chem. C 2016, 120, 10237–10243. [Google Scholar] [CrossRef]
- Kang, J.; Yu, J.S.; Han, B. First-Principles Design of Graphene-Based Active Catalysts for Oxygen Reduction and Evolution Reactions in the Aprotic Li-O2 Battery. J. Phys. Chem. Lett. 2016, 7, 2803–2808. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.H.; Hwang, Y.; Chung, Y.C. Effective catalytic media using graphitic nitrogen-doped site in graphene for a non-aqueous Li-O2 battery: A density functional theory study. J. Power Sources 2015, 277, 222–227. [Google Scholar] [CrossRef]
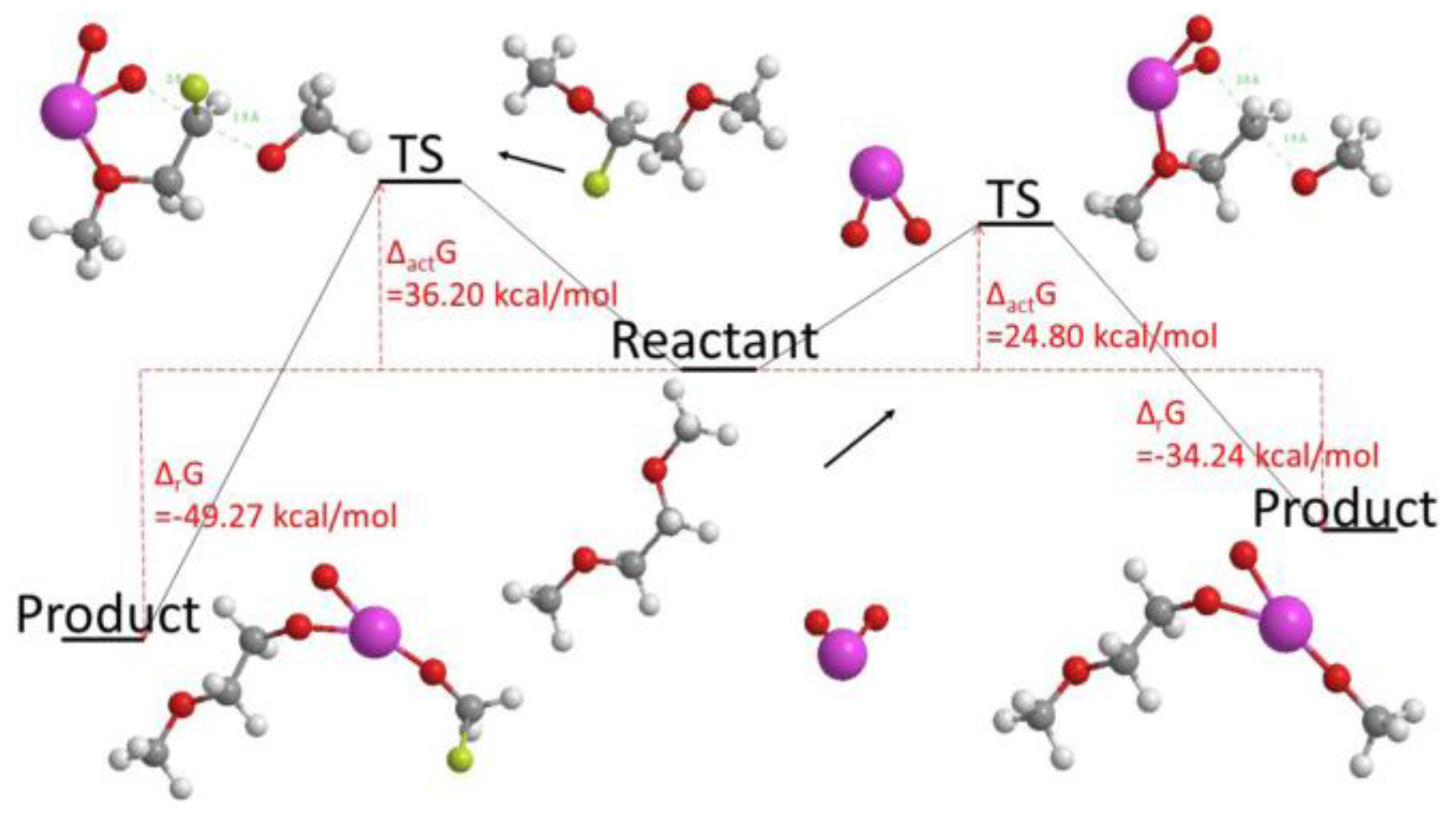
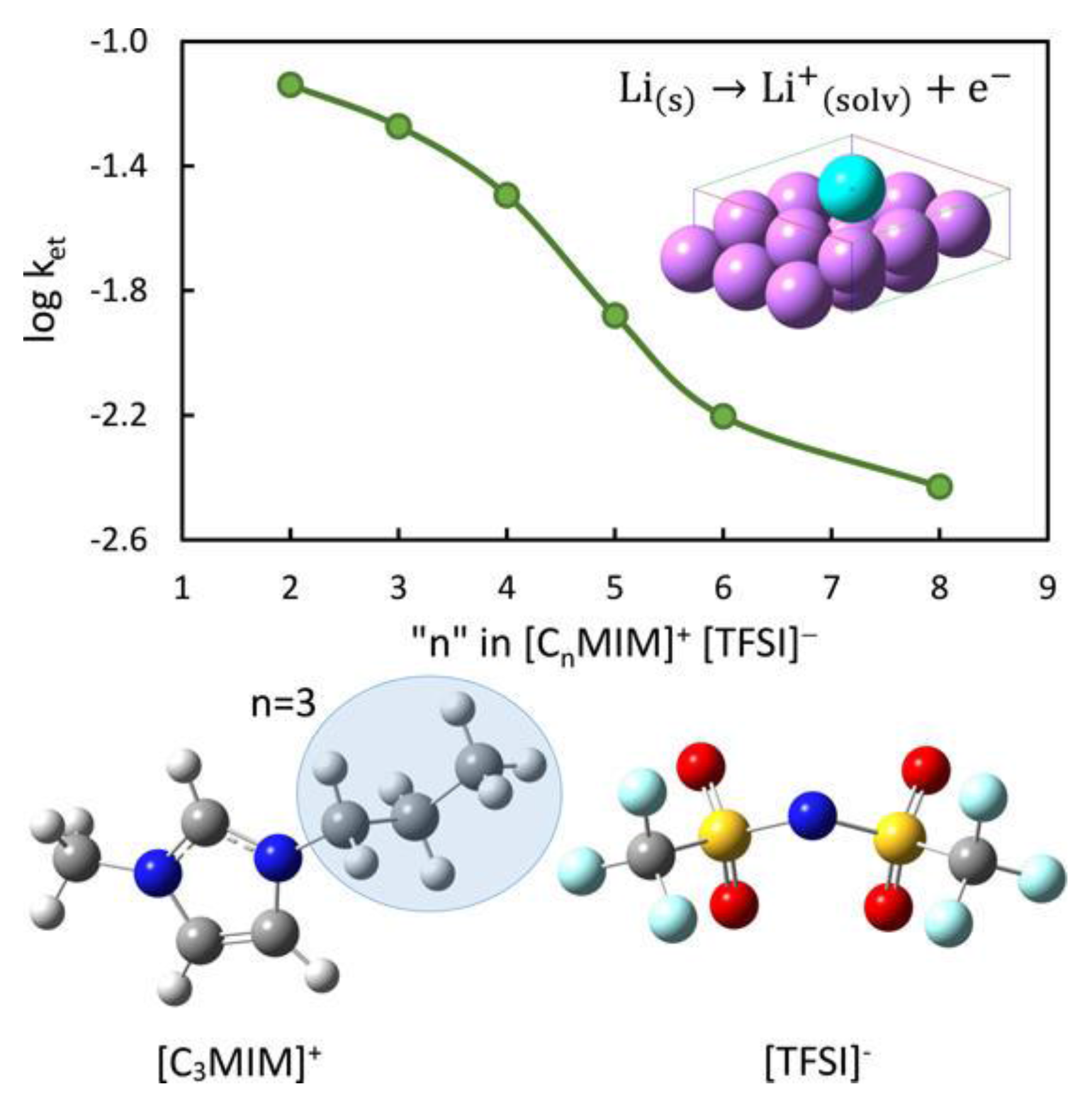
- Kazemiabnavi, S.; Dutta, P.; Banerjee, S. Density Functional Theory Based Study of the Electron Transfer Reaction at the Lithium Metal Anode in a Lithium–Air Battery with Ionic Liquid Electrolytes. J. Phys. Chem. C 2014, 118, 27183–27192. [Google Scholar] [CrossRef]
- Kazemiabnavi, S.; Dutta, P.; Banerjee, S. Ab Initio Modeling of the Electron Transfer Reaction Rate at the Electrode-Electrolyte Interface in Lithium-Air Batteries. In Proceedings of the ASME 2014 International Mechanical Engineering Congress and Exposition (IMECE 2014), Montreal, QC, Canada, 14–20 November 2014; Volume 6A, p. V06AT07A037. [Google Scholar]
- Kazemiabnavi, S.; Dutta, P.; Banerjee, S. A density functional theory based study of the electron transfer reaction at the cathode-electrolyte interface in lithium-air batteries. Phys. Chem. Chem. Phys. 2015, 17, 11740–11751. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.D.; Lee, J.S. Density Functional Theory (DFT) Study for Role of Ion-Conducting Lithium Salts Regarding the Oxygen Reduction Reaction (ORR) Kinetics in Li-air (O2) Batteries. Electrochim. Acta 2015, 182, 1124–1131. [Google Scholar] [CrossRef]
- Lee, M.; Hwang, Y.; Yun, K.H.; Chung, Y.C. Cathode reaction mechanism on the h-BN/Ni (111) heterostructure for the lithium-oxygen battery. J. Power Sources 2016, 307, 379–384. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Yang, X.; Jia, L.; Fu, Y.Q.; Chi, B.; Pu, J.; Li, J. First-principles study of the initial oxygen reduction reaction on stoichiometric and reduced CeO2 (111) surfaces as a cathode catalyst for lithium-oxygen batteries. J. Mater. Chem. A 2017, 5, 3320–3329. [Google Scholar] [CrossRef]
- Sankarasubramanian, S.; Singh, N.; Mizuno, F.; Prakash, J. Ab initio investigation of the oxygen reduction reaction activity on noble metal (Pt, Au, Pd), Pt3M (M = Fe, Co, Ni, Cu) and Pd3M (M = Fe, Co, Ni, Cu) alloy surfaces, for Li-O2 cells. J. Power Sources 2016, 319, 202–209. [Google Scholar] [CrossRef]
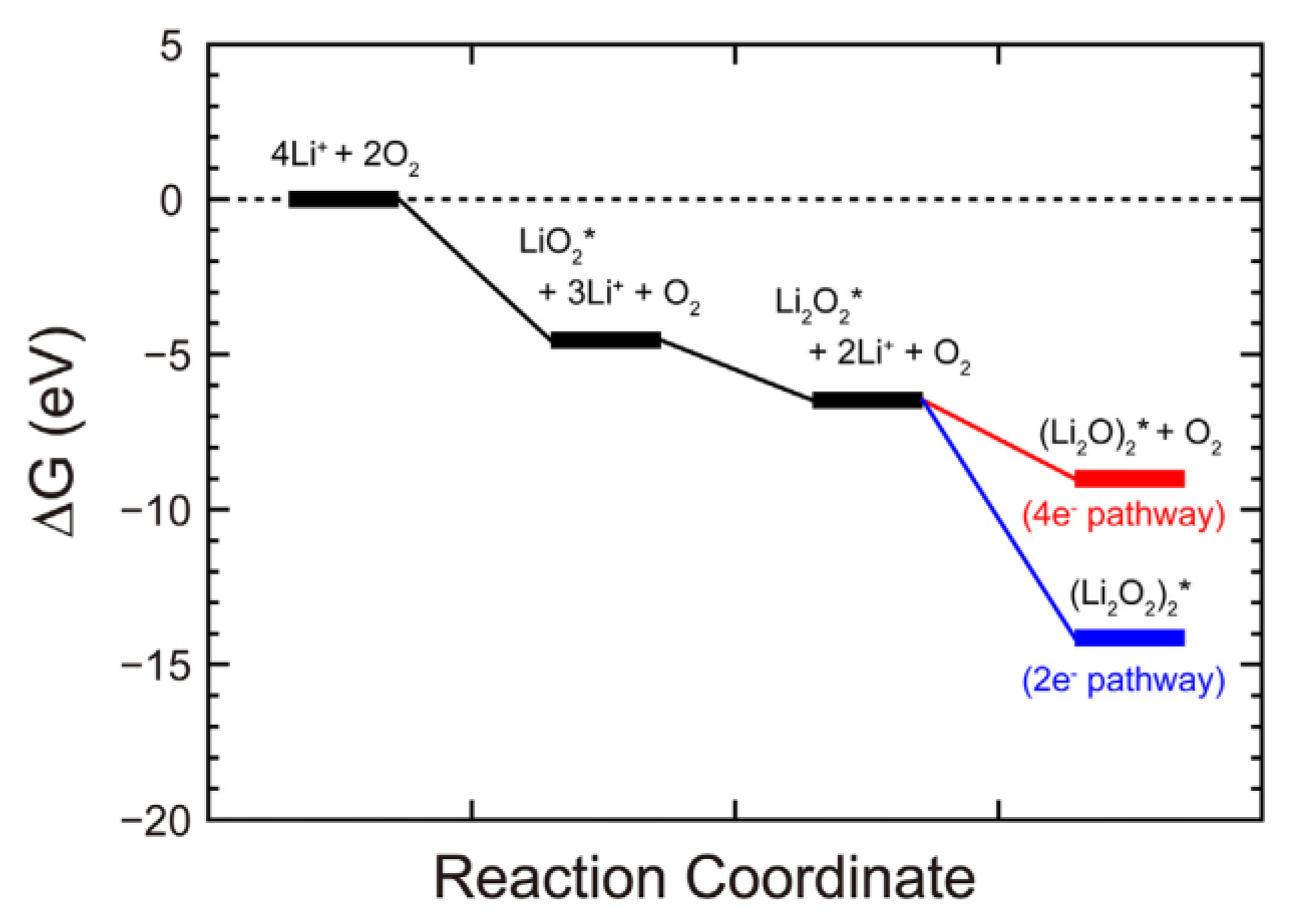
- Lau, K.C.; Assary, R.S.; Redfern, P.; Greeley, J.; Curtiss, L.A. Electronic Structure of Lithium Peroxide Clusters and Relevance to Lithium-Air Batteries. J. Phys. Chem. C 2012, 116, 23890–23896. [Google Scholar] [CrossRef]
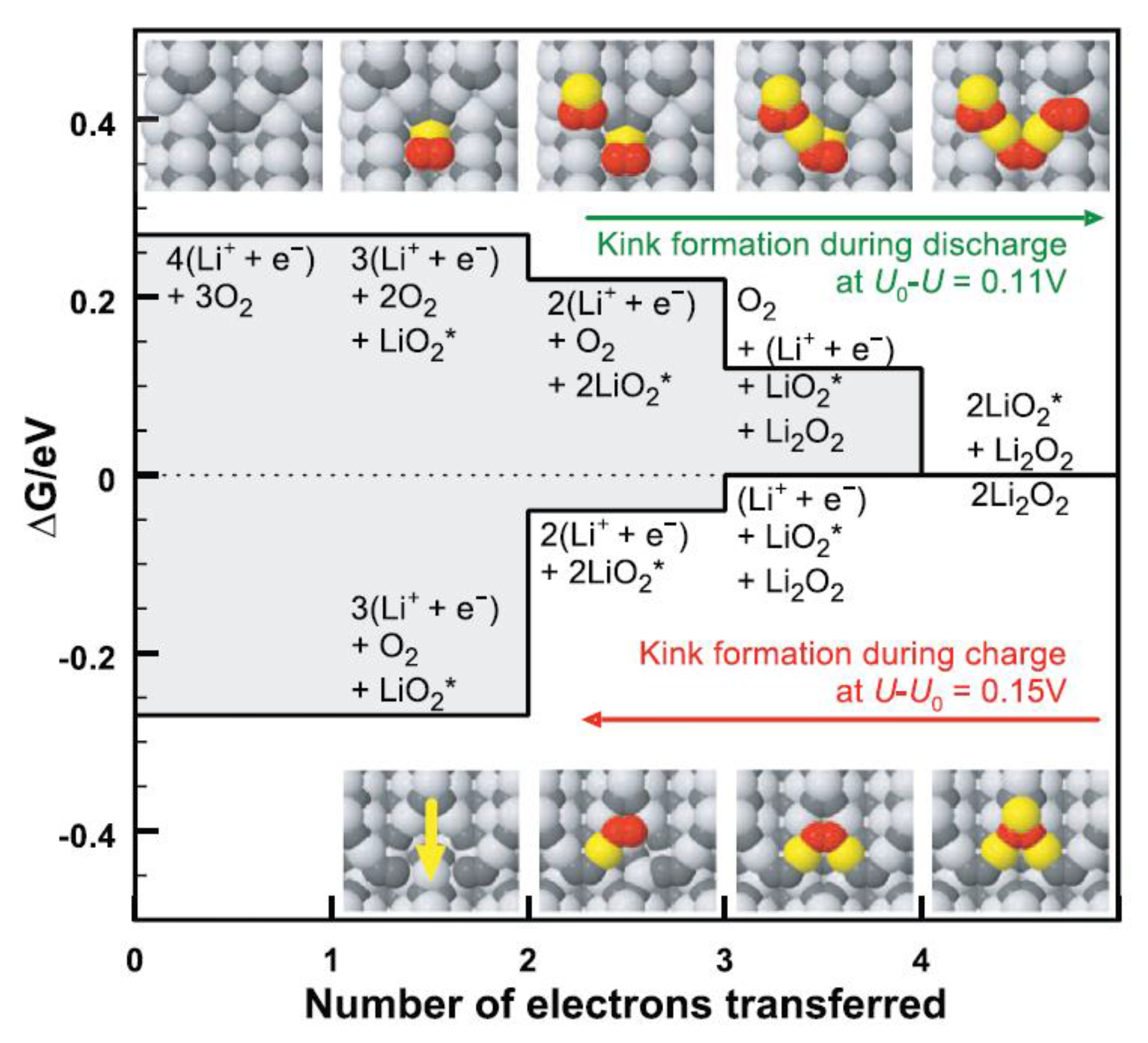
- Hummelshoj, J.S.; Luntz, A.C.; Norskov, J.K. Theoretical evidence for low kinetic overpotentials in Li-O2 electrochemistry. J. Chem. Phys. 2013, 138, 034703. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, Y.S.; Garcia-Lastra, J.M.; Hummelshoj, J.S.; Jin, C.J.; Vegge, T. Role of Li2O2@Li2CO3 Interfaces on Charge Transport in Nonaqueous Li-Air Batteries. J. Phys. Chem. C 2015, 119, 18066–18073. [Google Scholar] [CrossRef] [Green Version]
- Radin, M.D.; Monroe, C.W.; Siegel, D.J. How Dopants Can Enhance Charge Transport in Li2O2. Chem. Mater. 2015, 27, 839–847. [Google Scholar] [CrossRef]
- Kumar, N.; Radin, M.D.; Wood, B.C.; Ogitsu, T.; Siegel, D.J. Surface-Mediated Solvent Decomposition in Li-Air Batteries: Impact of Peroxide and Superoxide Surface Terminations. J. Phys. Chem. C 2015, 119, 9050–9060. [Google Scholar] [CrossRef]
- Tu, F.; Hu, J.; Xie, J.; Cao, G.; Zhang, S.; Yang, S.A.; Zhao, X.; Yang, H.Y. Au-Decorated Cracked Carbon Tube Arrays as Binder-Free Catalytic Cathode Enabling Guided Li2O2 Inner Growth for High-Performance Li-O2 Batteries. Adv. Funct. Mater. 2016, 26, 7725–7732. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.G.; Moon, H.S.; Park, H.; Kim, I.T.; Lee, S.G. Adsorption mechanisms of lithium oxides (LixO2) on a graphene-based electrode: A density functional theory approach. Appl. Surf. Sci. 2015, 351, 193–202. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.G.; Kim, I.T.; Kwon, S.; Lee, I.; Lee, S.G. Adsorption mechanisms of lithium oxides (LixO2) on N-doped graphene: A density functional theory study with implications for lithium-air batteries. Theor. Chem. Acc. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Tompsett, D.A.; Parker, S.C.; Islam, M.S. Rutile (beta-)MnO2 Surfaces and Vacancy Formation for High Electrochemical and Catalytic Performance. J. Am. Chem. Soc. 2014, 136, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; de Jesus, L.R.; Banerjee, S.; Mukherjee, P.P. Mechanistic Evaluation of LixOy Formation on delta-MnO2 in Nonaqueous Li-Air Batteries. ACS Appl. Mater. Interfaces 2016, 8, 23028–23036. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Besenhard, J.O. Electrochemical lithiation of tin and tin-based intermetallics and composites. Electrochim. Acta 1999, 45, 31–50. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, D.; Xu, W.; Wang, D.; Williford, R.E.; Liu, J.; Zhang, J.-G. Optimization of air electrode for Li/air batteries. J. Electrochem. Soc. 2010, 157, A487–A492. [Google Scholar] [CrossRef]
- Jithin, M.; Das, M.K.; De, A. Lattice Boltzmann Simulation of Lithium Peroxide Formation in Lithium–Oxygen Battery. J. Electrochem. Energy Convers. Storage 2016, 13, 031003. [Google Scholar] [CrossRef]
- Blanquer, G.; Yin, Y.; Quiroga, M.A.; Franco, A.A. Modeling Investigation of the Local Electrochemistry in Lithium-O2 Batteries: A Kinetic Monte Carlo Approach. J. Electrochem. Soc. 2016, 163, A329–A337. [Google Scholar] [CrossRef]
- Andersen, C.P.; Hu, H.; Qiu, G.; Kalra, V.; Sun, Y. Pore-Scale Transport Resolved Model Incorporating Cathode Microstructure and Peroxide Growth in Lithium-Air Batteries. J. Electrochem. Soc. 2015, 162, A1135–A1145. [Google Scholar] [CrossRef]

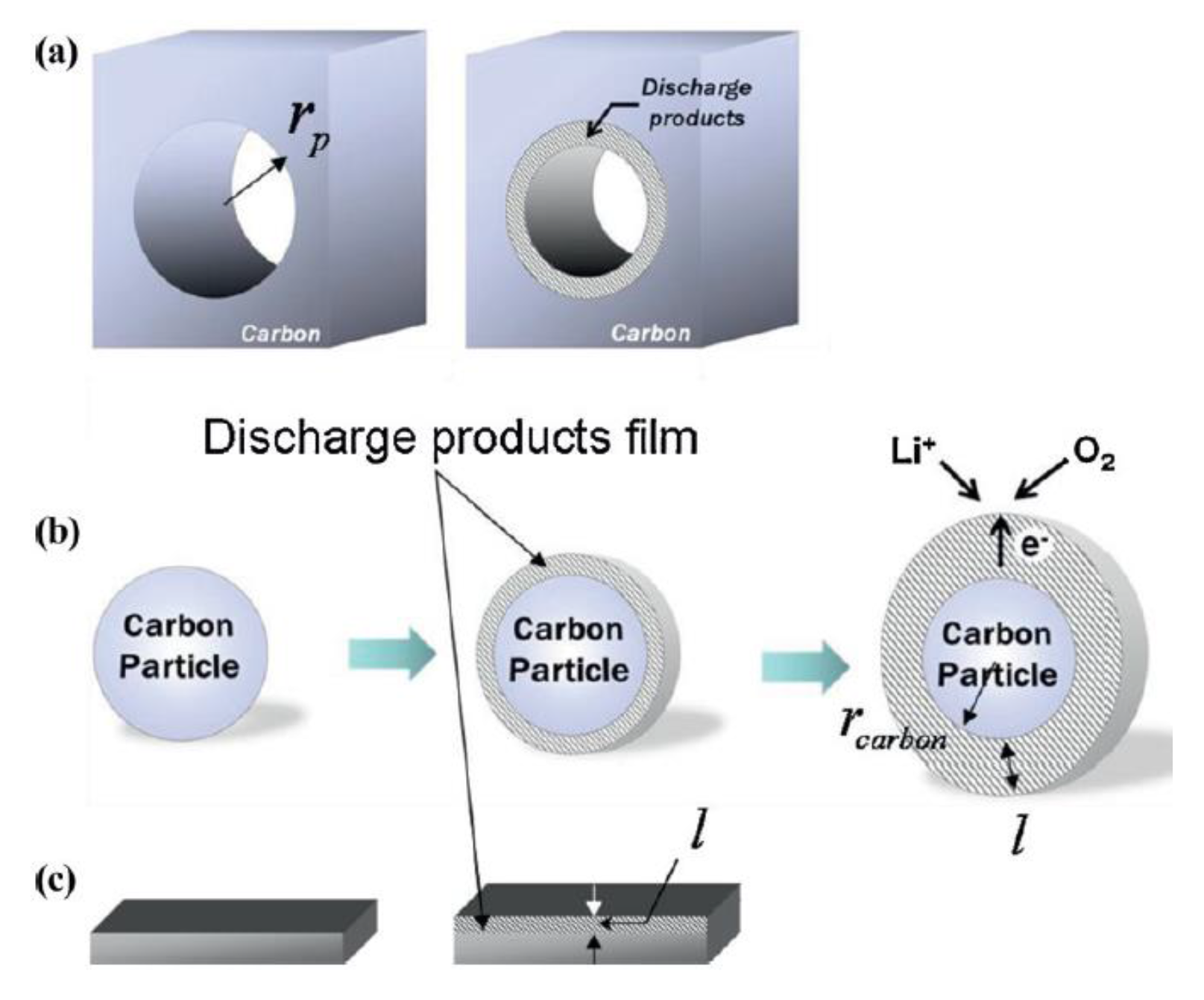
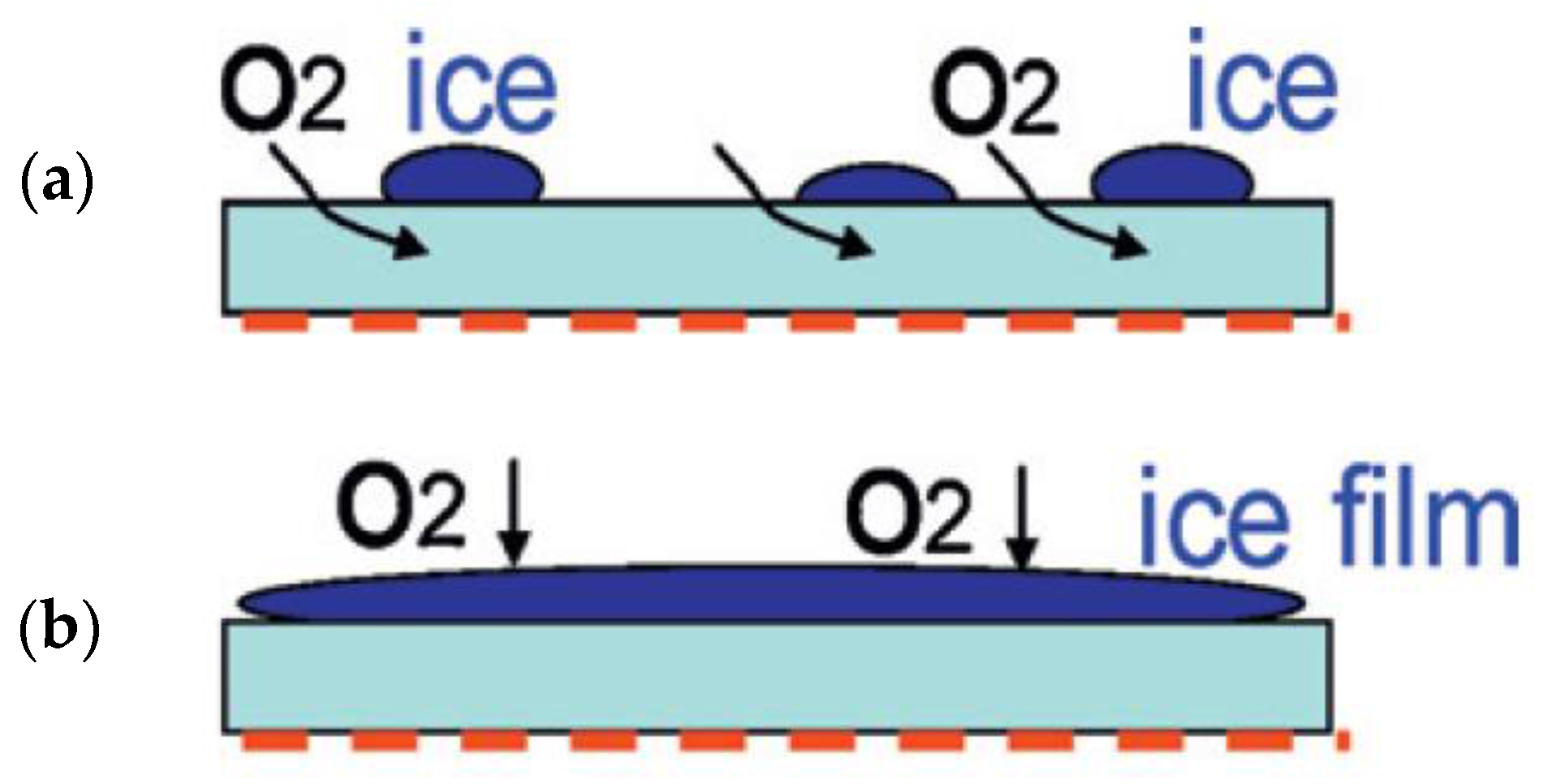
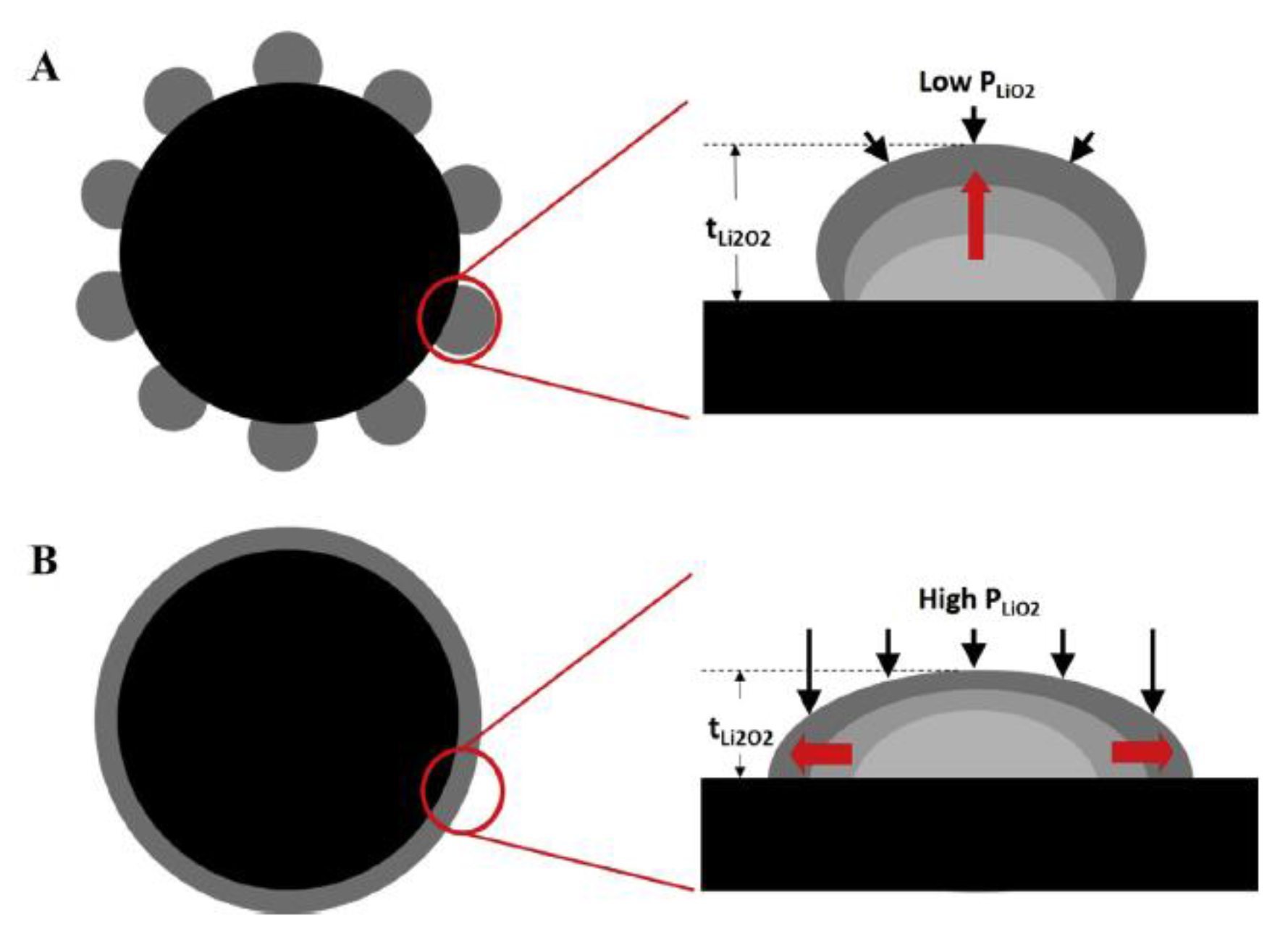

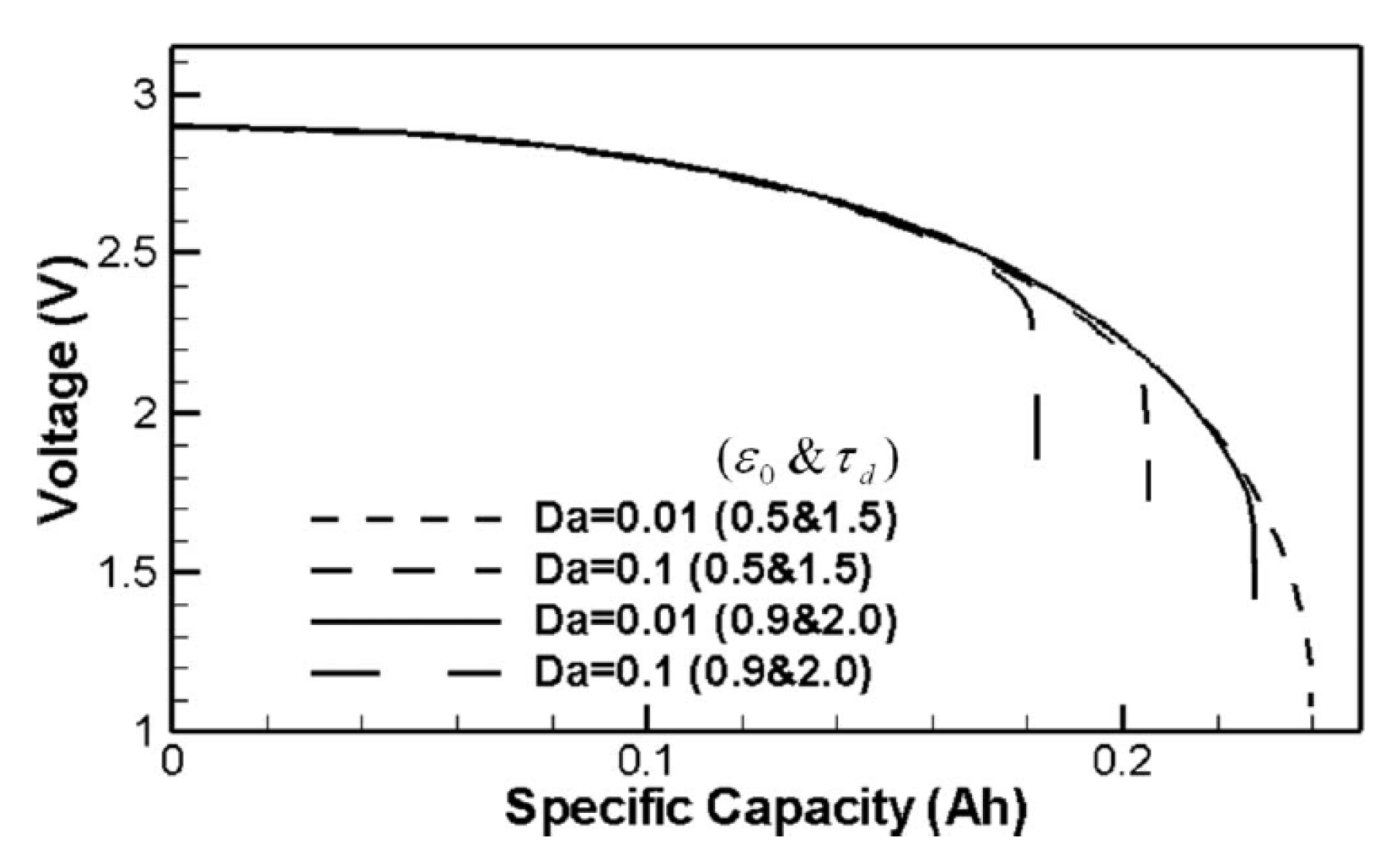



| Solvent | Salt | Electrolyte | Ions | System |
|---|---|---|---|---|
| Organic (propylene carbonate) | LiPF6 | Organic Electrolyte | Li+, PF6− | Binary |
| Ionic Liquid (mppy-TFSI) | LiTFSI | Ionic Liquid Electrolyte | Li+, mppy+, TFSI− | Ternary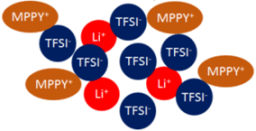 |
| Parameter | Temperature (oC) | Unit | |||||
|---|---|---|---|---|---|---|---|
| 30 | 50 | 70 | 90 | 110 | |||
| Maxwell-Stefan Diffusivities | D12 a | 2.6625 | 4.1332 | 4.3976 | 10.1566 | 26.9723 | ×10−12 (m2/s) |
| D13 a | 4.6936 | 6.6999 | 7.5526 | 16.5441 | 44.812 | ||
| D23 a | 5.2847 | 5.2847 | 15.814 | 21.352 | 27.7031 | ||
| Heny’s constant of Oxygen | 1337.35 | 989.48 | 788.61 | 702.56 | 668.01 | atm | |
| Diffusivity of Oxygen | 1.213 | 3.416 | 5.412 | 6.732 | 7.526 | ×10−10 (m2/s) | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, K.; Banerjee, S.; Kim, J.; Dutta, P. A Review of Lithium-Air Battery Modeling Studies. Energies 2017, 10, 1748. https://doi.org/10.3390/en10111748
Yoo K, Banerjee S, Kim J, Dutta P. A Review of Lithium-Air Battery Modeling Studies. Energies. 2017; 10(11):1748. https://doi.org/10.3390/en10111748
Chicago/Turabian StyleYoo, Kisoo, Soumik Banerjee, Jonghoon Kim, and Prashanta Dutta. 2017. "A Review of Lithium-Air Battery Modeling Studies" Energies 10, no. 11: 1748. https://doi.org/10.3390/en10111748





