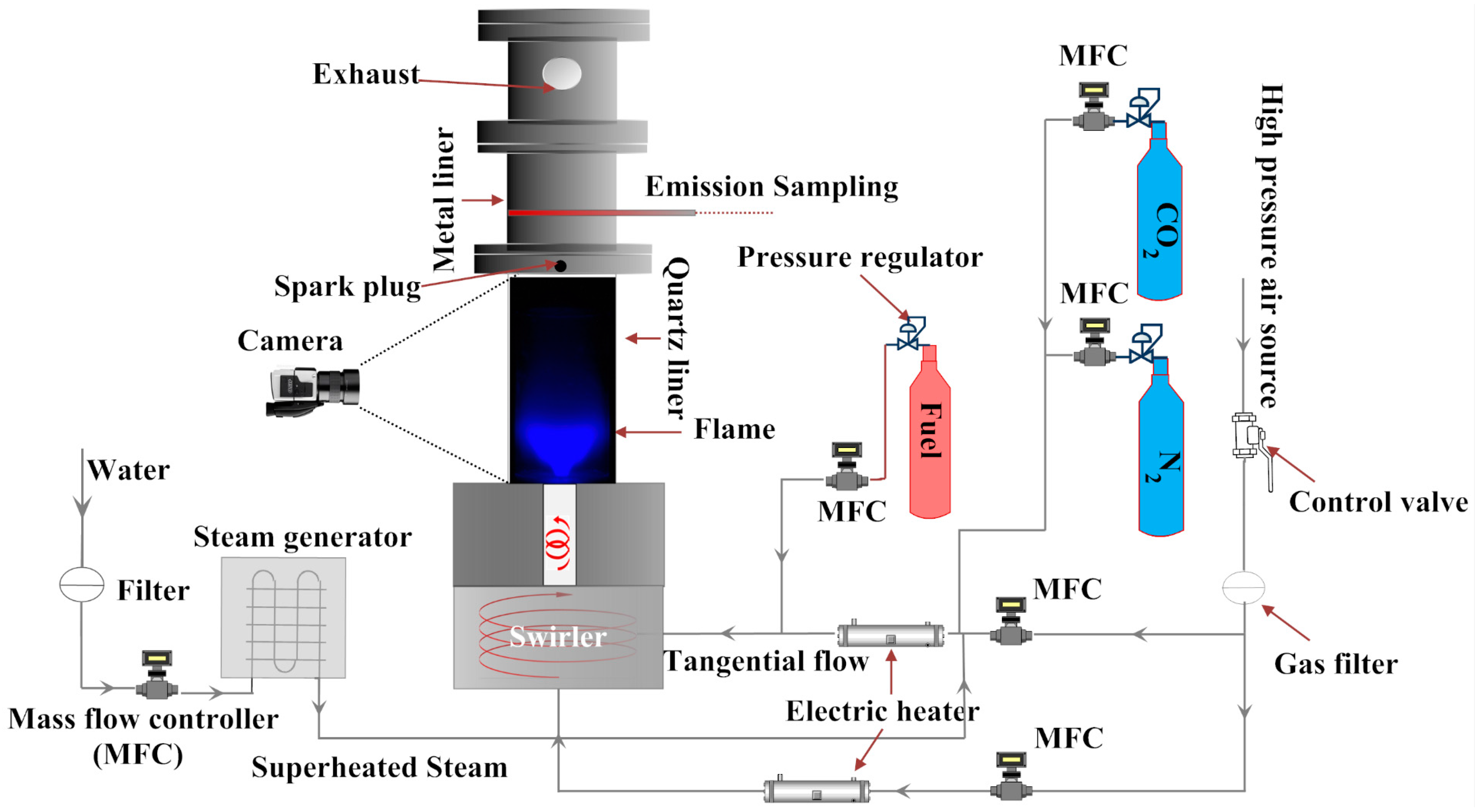
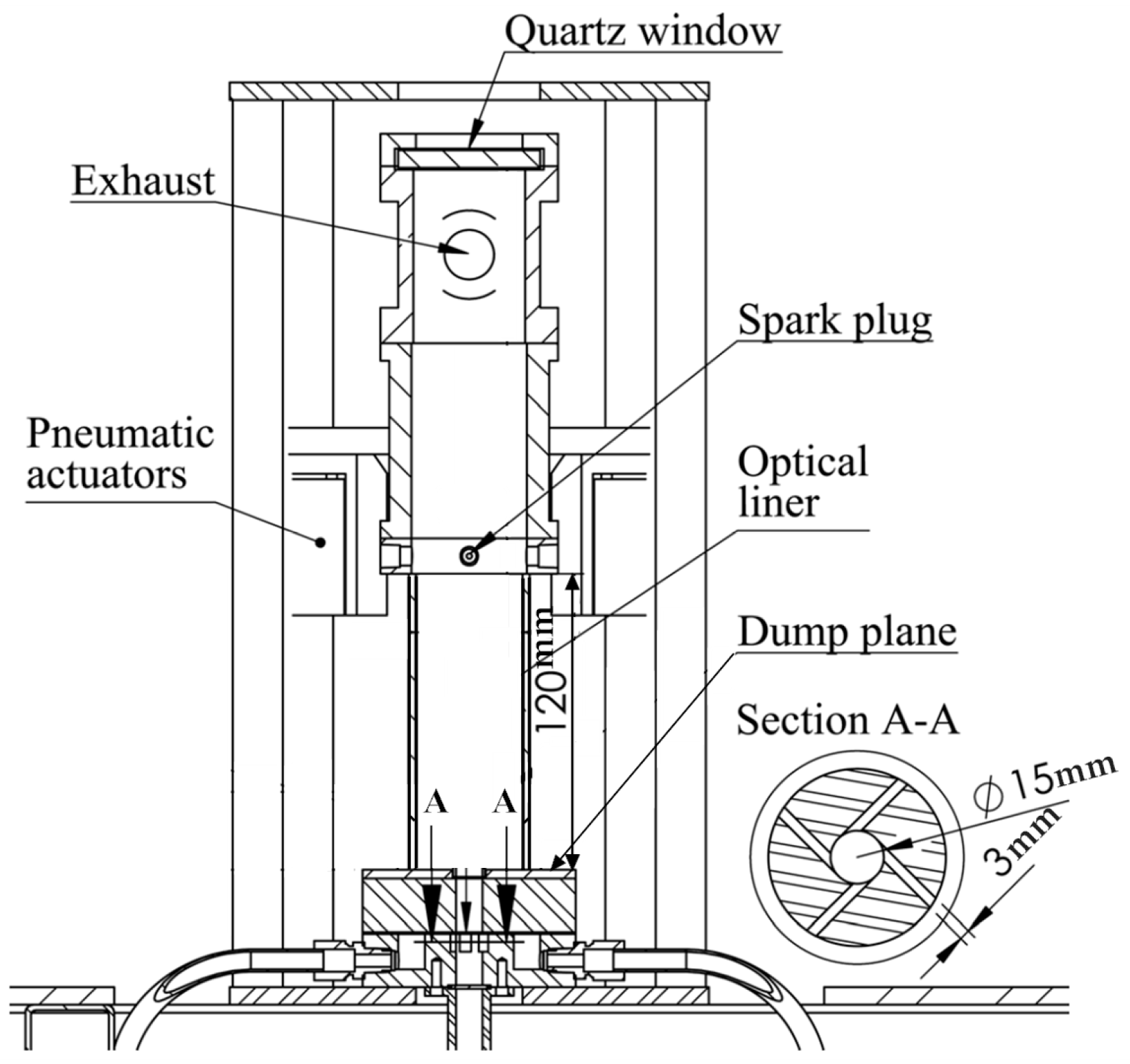
1. Introduction
Due to environmental problems, the low emission combustion technologies have attracted considerable attention. In the combustion concept, with the consideration of reducing pollutant emissions, the operating condition where combustion happens in the air with reduced oxygen concentration, so-called vitiated air, is commonly encountered. Regarding the combustion research field, the vitiated air refers to the air containing additional gas which is neither fuel nor oxygen. The air vitiation is commonly achieved by the recirculation of exhaust gas into the fresh air stream (referred to as exhaust gas recirculation (EGR), the burning of landfill gas (approximately forty to sixty percent is methane, with the remainder being mostly CO2), and the use of a staged combustion system, e.g., with additional fuel injection after the primary reaction zone. Vitiated air generally includes the combustion products such as CO2, H2O, CO, NOx and unburned hydrocarbons, along with N2 and O2.
The vitiated air has been used in many practical combustion systems, e.g., gas turbine combustors, furnaces, and piston engines, in order to reduce pollutant emissions and improve thermal efficiency [
1]. Compared to normal air, vitiated air has a lower oxygen concentration and contains a different composition which impacts the chemical and physical properties of the reactant mixtures. Hence, the process of fuel oxidation and flame propagation could be affected, which could make the burning of the fuel in vitiated air differ from what happens in pure air. For most combustion devices, the primary vitiation species are N
2, CO
2, and H
2O (steam). Therefore, investigation of the vitiation effects of N
2, CO
2, and H
2O are the main objectives of the current work.
In combustion applications like EGR, N
2, CO
2, and H
2O constitute the majority of the vitiated air. CO
2 and H
2O were found to have a strong effect on chemical kinetics, while N
2 was found to predominantly have the thermal effect [
2,
3]. The previous study results indicated that CO
2 had an inhibiting effect on the fuel oxidation process [
2,
4]. More analysis on the chemical effect of CO
2 vitiation showed that the reduction of the fuel oxidation rate was primarily determined by the reaction CO + OH
CO
2 + H [
5,
6]. Hence a negative effect on the combustor operation range with low CO concentration can be expected due to the CO
2 vitiation. H
2O vitiation was found to have a positive chemical kinetic effect due to its contribution to the increase of H and OH radicals which come from the reaction step O + H
2O
OH + OH [
7,
8,
9] and H
2O
H + OH (the dissociation of the steam under high temperature) [
10]. The thermal effect of the vitiation results in a reduction of the flame temperature, which makes the vitiated combustion a feasible method for achieving NO
x reduction. CO emission has been studied in oxyfuel combustion where fuel is burned in O
2/CO
2 circumstance. The results showed that CO
2 had a significant effect on the increase of CO concentration [
11]. The H
2O vitiation effect on CO was also examined and the results showed that the CO did not seem to be affected significantly [
12]. The vitiation effect of N
2 and CO
2 on the LBO limits of the methane flame was investigated, and the results showed that CO
2 had a stronger effect on the LBO increase [
13]. A similar effect on LBO limits was observed in methane combustion with steam vitiation [
14].
According to the previous work, few experimental investigation results were exhibited to compare the effects of the primary vitiation species, e.g., N
2, CO
2, and H
2O. Hence, in present work, the vitiation effects of N
2, CO
2, and H
2O were investigated with a swirl-stabilized gas turbine model combustor under atmospheric pressure. Measurements were conducted at the conditions where 10%, 20%, and 30% mole fraction of the air was substituted by N
2, CO
2, and H
2O (steam), respectively. Chemical kinetics calculations were performed to understand the vitiation effects on the flame reaction zone by using the Chemkin software [
15] and the GRI-mech 3.0 mechanism [
16]. Time-averaged CH chemiluminescence images were captured to evaluate the flame shapes, anchored location, and intensity of the heat-release. The LBO limits of flame with and without vitiation were examined and compared to two combustor inlet temperatures (384 K/484 K). The flame instability prior to LBO was recorded by using the high-speed broadband chemiluminescence. CO emissions were measured at the combustor exit with a water-cooled probe. The current paper contributes to a better understanding of the vitiated flame on the stable and low CO operation, and the results will bolster the development of environmentally friendly combustion technologies.
3. Results and Discussion
The premixed turbulent combustion is a complex process which is determined by many factors, e.g., chemical reactions, turbulent flow, and heat and mass transfer. Therefore, the flame stability and emission performance strongly depend on these factors and the interactions among them. In the case of using reactant stream with different composition, the variation in physical properties and the chemical kinetics probably lead to new characteristics in combustion progress. In order to study the characteristics of vitiated methane flame, 10%, 20%, and 30% mole fraction of the air was substituted by N2, CO2, and steam, respectively. The mole fraction of vitiation gas was defined as the vitiation ratio which was used in the results analysis.
3.1. Reaction Zone Characteristics
In the premixed combustion, most of the chemical reactions occur in a narrow zone which is referred to as the reaction zone. The reaction zone separates the unburned reactant mixtures and the combustion products. Significant changes in temperature, combustion intermediate radicals, fuel, oxidizer, etc., are observed across the reaction zone.
Figure 3 shows the comparison of temperature profile across the flame reaction zone at equivalence ratio 0.8 and the vitiation effects on the adiabatic flame temperature at the equivalence ratio range of 0.3 to 1. The results were calculated, respectively, with a premixed laminar flame speed calculation reactor and equilibrium reactor in Chemkin software. Based on
Figure 3a, a reduction in temperature gradient was observed with 20% vitiation gas, which indicated a decrease in chemical reaction rate. In
Figure 3b, as a result of the changes in chemical and physical properties of the reactant mixtures, the final adiabatic flame temperature of vitiated flame had a significant decrease compared to the flame without vitiation. Among the three vitiation gas, combustion with 20% CO
2 had the minimum flame temperature. This was probably due to its specific heat capacity and the inhibiting effect on CO oxidization.
Figure 4 shows the concentration of H, O, OH, and CO across the flame reaction zone. A comparison of the concentration of these species has been made for the vitiated flame and flame without vitiation. The data were calculated with the premixed laminar flame speed calculation reactor in Chemkin software. Based on the data, there was a great concentration gradient between the unburned reactant mixtures and the reaction zone. Compared to the flame with no vitiation, the concentration of H, O, OH, and CO of the vitiated flame decreased significantly. With 20% vitiation ratio, the reduction in species concentration was expected because the amount of the active radicals was less due to the fact that less oxygen was available for the chemical reactions. If only the dilution effect of the vitiation plays a role in the chemical reactions, the concentration of the same species shown in
Figure 4 should stay the same regardless of the type of vitiation. However, the data in
Figure 4 clearly shows that the concentration of H, O, OH, and CO strongly depends on the type of the vitiation. This means, besides the vitiation dilution effect, the vitiation species can influence the combustion reaction process in a more complicated way due to the different chemical properties, physical properties, and the interaction between them.
By comparing the species concentration with the vitiation of N
2, CO
2, and H
2O, N
2 was found to have minimum changes in active radical concentration. In other words, N
2 had a minimum influence on combustion process among the three vitiation gas. The primary reason could be that the adiabatic flame temperature with N
2 is higher than that with CO
2 and H
2O. As a result, the reaction rate is enhanced and the radical concentration is higher. In the flame reaction zone, the CO
2 and H
2O had a close peak concentration for H radical. A similar performance for O radical was observed with the vitiation of CO
2 and H
2O. With N
2 vitiation gas, the peak concentration of H and O radicals was about double that with CO
2 and H
2O. According to the OH concentration profile across the reaction zone, it was observed that the H
2O and N
2 vitiation gas had almost the same peak concentration, although the adiabatic flame temperature with H
2O vitiation gas was lower. This can be explained by the results of the previous research work, which indicated that more OH radicals were produced based on the chemical reaction step O + H
2O
OH + OH [
8,
9]. At high flame temperature, the steam dissociation could also contribute to OH production [
10]. The flame vitiated with CO
2 had the lowest adiabatic flame temperature according to the data in
Figure 3. However, it showed a CO peak concentration which was comparable to flame with N
2 vitiation. Studies have shown that the CO oxidation is primarily determined by the reaction step CO + OH
CO
2 + H [
18]. Hence, the 20% CO
2 vitiation and lower concentration of OH could lead to a higher CO concentration in the flame reaction zone.
3.2. Flame Visualization
In the present measurement, the characteristics of vitiated flames were investigated with a swirl number of 0.58, which can produce a center recirculation zone in the combustor, according to the previous particle image velocimetry (PIV) measurement. Time-averaged CH chemiluminescence was used to provide the information about the flame shapes, stabilized location, flame length, and so on.
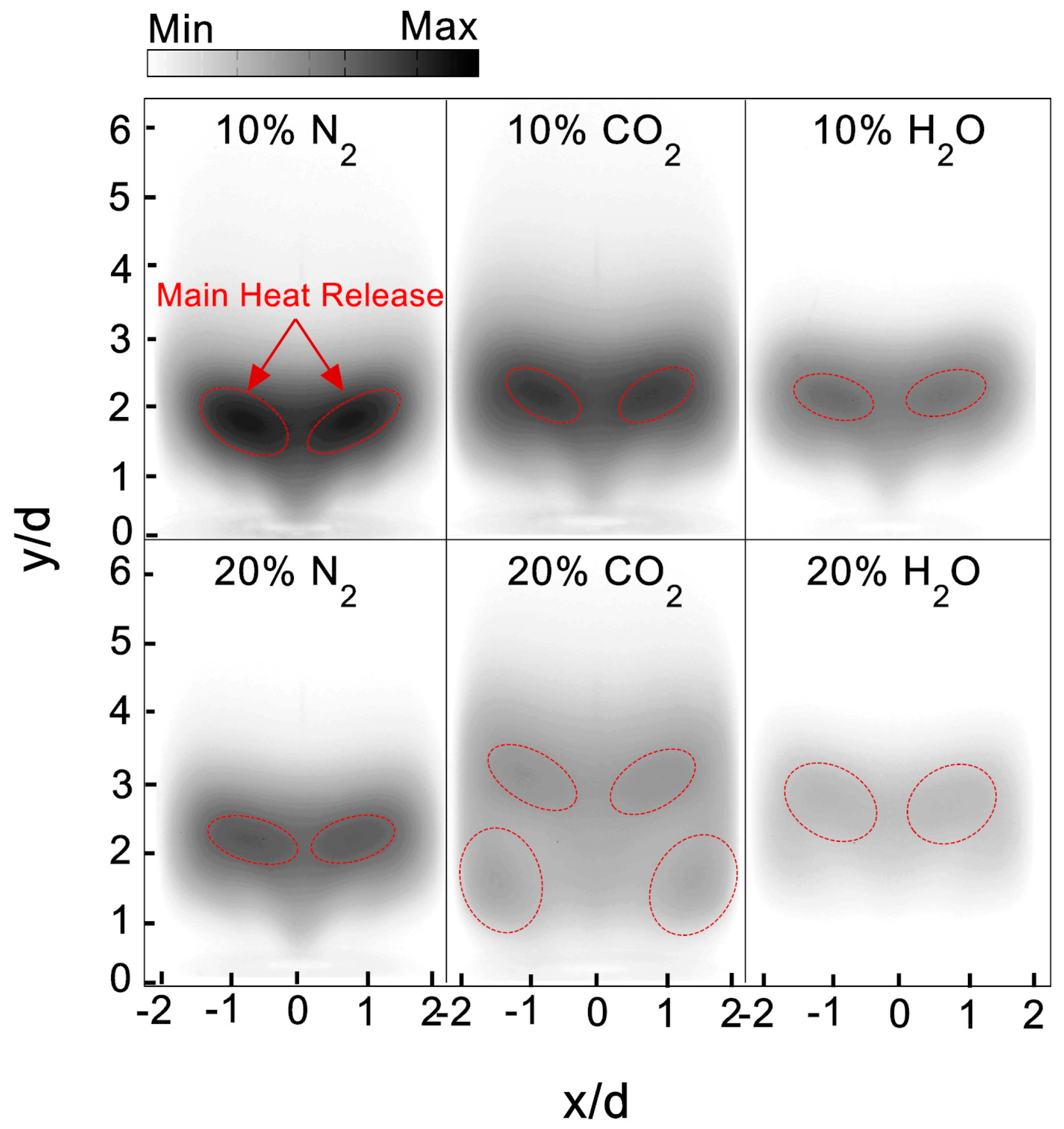
Figure 5 depicts the flame shape’s response to the vitiation of N
2, CO
2, and H
2O with 10% vitiation ratio. The high CH intensity area circled by the red dashed line could provide the indication for the main reaction zone. For the flame without vitiation, the CH intensity was stronger compared to the flame with vitiation. The 10% N
2 did not make significant changes on flame shape, however—the CH intensity in the main reaction zone was lower. It can be seen from
Figure 5 that CO
2 and H
2O had an observable influence on the flame height and the location of the main reaction zone. With 10% CO
2, the flame height was increased and the flame root had a tendency to detach from the edge of the combustor inlet. The lower CH intensity in the main reaction zone showed CO
2 had a stronger inhibiting effect on the chemical reaction than N
2. This is probably due to the relatively larger heat capacity of CO
2 and its negative effect on CO oxidization. The H
2O seemed to have the maximal reduction in CH intensity; at the same time, there was a small lift-off height between the flame root and the combustor inlet. This indicated that the chemical reaction rate was decreased greatly by H
2O. This could be due to the high emissivity of steam, which leads to a remarkable heat loss.
Figure 6 illustrates the comparison of flame shapes for the three vitiation species at the vitiation ratio of 10% and 20%. At the equivalence ratio of 0.98, with the increase of vitiation ratio from 10% to 20%, a substantial reduction of CH intensity was observed for N
2, CO
2, and H
2O. For N
2, the CH images showed that the flame stabilized position was slightly shifted downstream. With 20% CO
2, four stabilized positions of flame were achieved. Two of the stabilized positions were shifted downstream, compared to 10% CO
2 case. The other two were located upstream at the combustor corner. A minimal CH intensity was observed in flame with 20% steam vitiation. Even at the main reaction zone, a much stronger chemical reaction rate was still not observed. Therefore, it could be expected that the flame with 20% steam has a weak flame stabilization ability compared with the other vitiated flames.
3.3. The LBO of Vitiated Flame
The flame LBO should be avoided in practice combustor operation. Hence, it is essential to know the LBO limits in order to have an economical and safe operation. The LBO limits, defined as the LBO equivalence ratio, were determined by gradually reducing the fuel until the flame was physically away from the combustor. LBO limits were measured for the premixed methane flame with vitiation of N
2, CO
2, and H
2O. 10%, 20%, and 30% vitiation ratio were examined at combustor inlet temperature of 484 K.
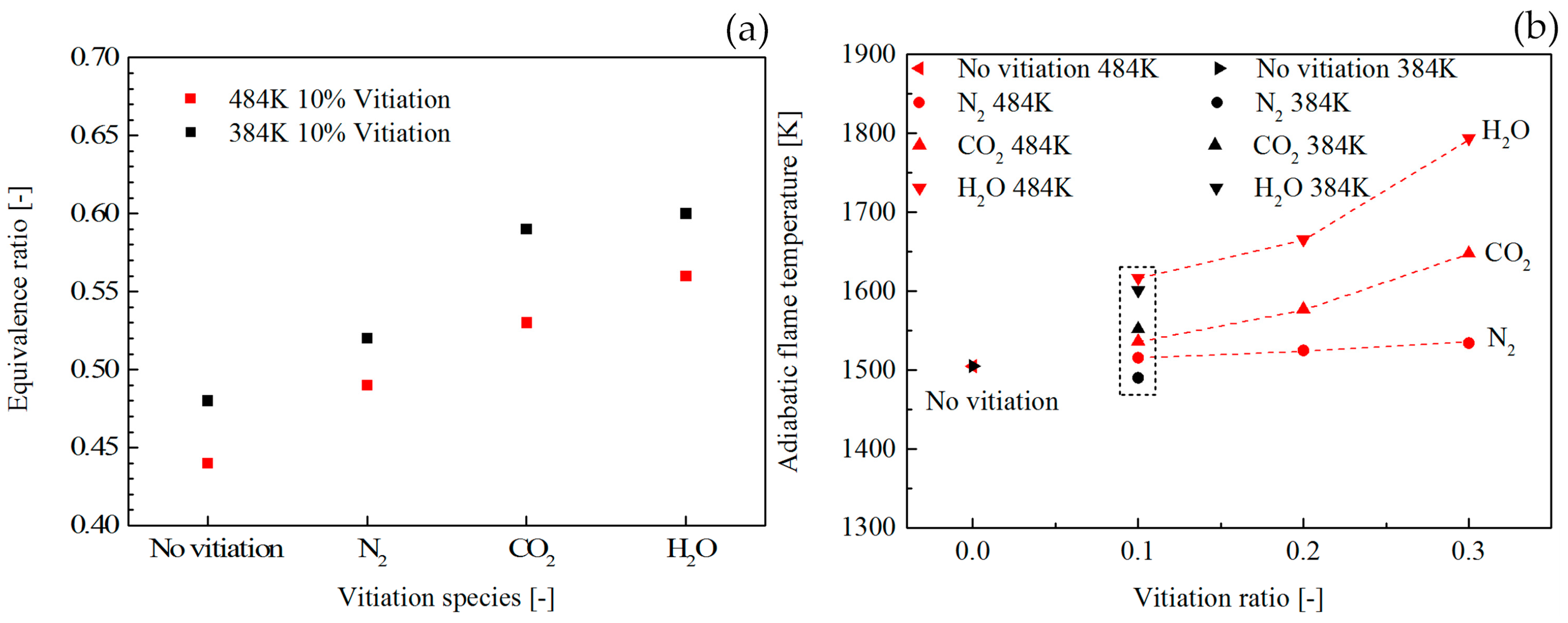
Figure 7a shows the vitiation effect on the LBO limits. For all the vitiation species, the LBO limits went up with the increase of vitiation ratio. It was found that the LBO limits with the vitiation of CO
2 and H
2O had a similar trend which was highlighted by the shadow region. Compared to the flame vitiated by CO
2 and H
2O, the vitiation ratio had a relatively small influence on flame vitiated by N
2. The reason for the LBO limits trend in
Figure 7a could be understood based on the discussion in
Section 3.1, where the vitiation effects on the flame reaction zone were analyzed. In
Figure 7b, the LBO limits were analyzed based on the Damköhler number at the LBO condition. The Damköhler number was defined as:
which is a time scale ratio between the residence time and the chemical reaction time. Regarding the calculation method for the Damköhler number, the readers are suggested to refer to the previous work [
14].
The dashed line in
Figure 7b shows a two-dimensional flammability range, which is controlled by the equivalence ratio flammability limit and the Damköhler number flammability limit. Above the equivalence ratio flammability limit, a sufficiently high temperature is guaranteed to keep the combustion reaction rate (in other words, to keep the flame propagation). However, although the flame can propagate towards the unburned premixed reactant mixtures, the propagation speed may be lower than the flow speed, which will lead to the combustion LBO. Based on a time concept, it is to say an adequate time must be ensured to complete the chemical reaction in order to avoid LBO. Therefore, it is important to know the critical Damköhler number limit above which the flame can survive in the combustor. According to
Figure 7b, the changes of vitiation species and vitiation ratio did not have a significant influence on the Damköhler number flammability limit.
Figure 8a shows the comparison of LBO limits between combustor inlet temperature of 384 K and 484 K with 10% vitiation ratio. The results indicated that the higher combustor inlet temperature could extend the LBO limits to the lower level.
Figure 8b shows the adiabatic flame temperature at the LBO limits. The vitiation ratio influence on the adiabatic flame temperature was examined with 484 K combustor inlet temperature. The results indicated that higher vitiation ratio could lead to an increase of the adiabatic flame temperature at LBO. The data marked with the dashed line shows the comparison between 484 K and 384 K. According to the results, the inlet temperature influence on the adiabatic flame temperature at LBO is very little.
In the vicinity of LBO limits, the flame reaction zone can be very sensitive to the heat loss from the flame, the fluctuation of the high strain rate, and the local equivalence ratio. Therefore, the local flame extinction and flame re-ignition process can happen [
19]. The local flame extinction can detach the flame from its stabilized location and the flame re-ignition process can make the flame propagate back to the previously stabilized place again. This flame instability behavior sometimes can stop the flame re-ignition and attachment process, which finally leads to the LBO.
Figure 9 shows the flame re-ignition and detachment phenomena which were recorded by the high-speed chemiluminescence imaging at a frequency of 1 kHz. Due to the usage of swirling flow, the flame had a rotating movement along the combustor center line besides the flame movements in the vertical direction. At 0 ms, it was observed that the flame stayed away from the dump plane of the combustor. Later, the flame re-ignition and attachment process were observed, which made the flame travel upstream (from 2 ms to 6 ms). The flame was found in the outer recirculation zone for a short time interval (from the time 7 ms to 12 ms). Then, the flame detachment was found, which made the flame travel downstream along the combustor liner. In the end, a complete flame detachment from the dump plane and the outer recirculation zone was achieved. The described flame unstable phenomena can be observed in all the examined flame cases when approaching the LBO limits.
According to the previous literature, the flame chemiluminescence is correlated with time-dependent variation of heat-release rate [
20]. Therefore, the dynamics of high-speed chemiluminescence are very helpful for providing the information of heat-release rate fluctuation, which is essentially produced by the coupling of fluctuation between the heat-release and the pressure. The high-speed chemiluminescence was recorded in time sequence. The spatial average of intensity was calculated for each image and the time-dependent fluctuation of the average intensity was analyzed by FFT (Fast Fourier Transform).
Figure 10 illustrates the FFT analysis result, which shows the frequency peaks for the chemiluminescence intensity oscillation observed in the proximity of LBO. Results showed that the dominant frequency of intensity fluctuation was not affected significantly by the 10% vitiation gas.
3.4. The Vitiation Effects on CO Emission
In this section, the CO emission performance of vitiated flames was examined with three vitiation ratios and two combustor inlet temperatures.
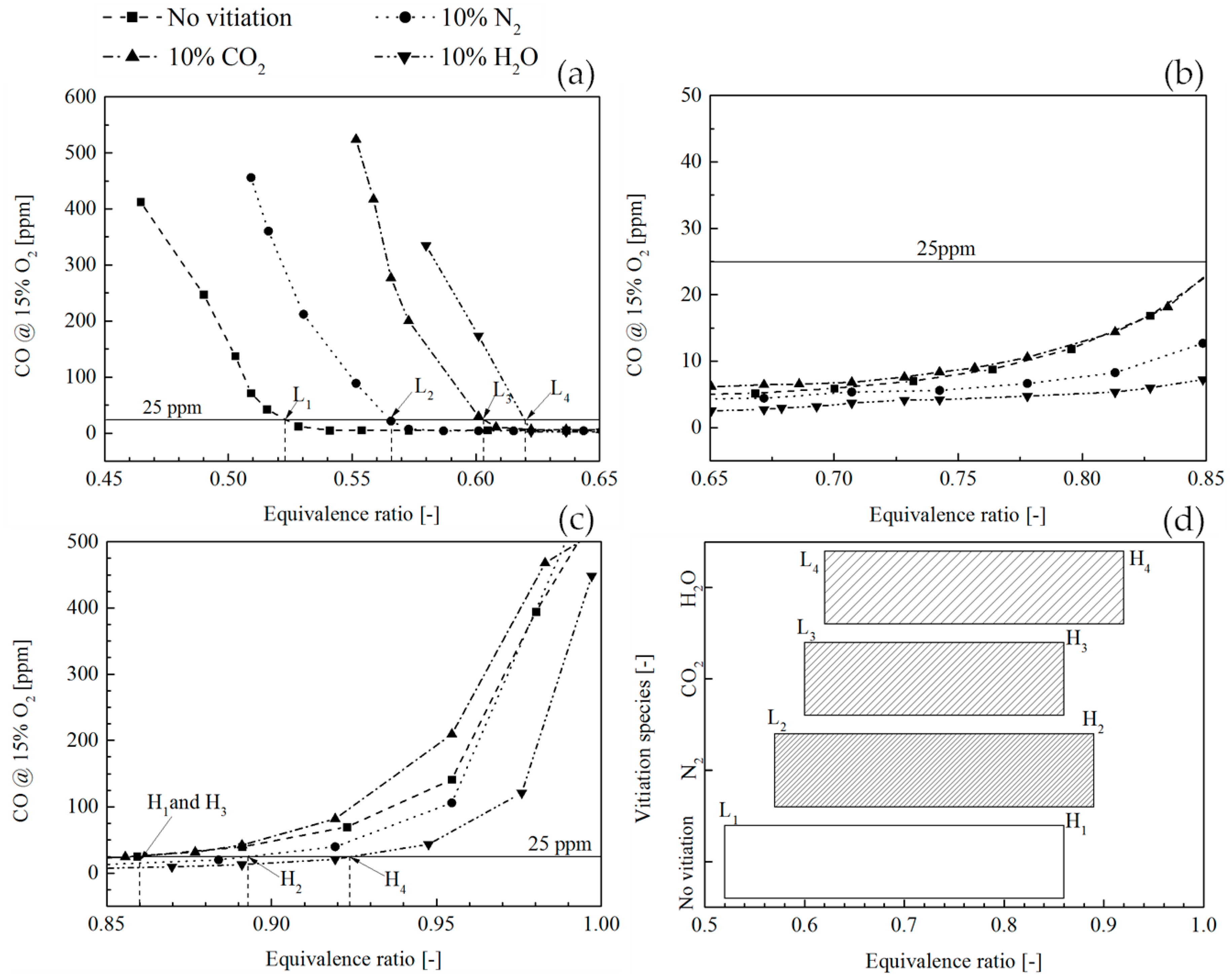
Figure 11 shows a comparison of CO concentration in the combustion products between the flame without vitiation and the flame with 10% vitiation ratio. In order to compare the vitiation effect on CO emission, a concentration limit was defined at 25 ppm below which the CO is considered acceptable.
Figure 11a shows that the combustion operated in a very lean condition leads to a high CO concentration in the combustion products. This is due to the low flame temperature, which can reduce the chemical reaction rate for CO oxidation. The equivalence ratios where CO equals to 25 ppm are denoted by L
1, L
2, L
3, and L
4, and the subscript stands for the corresponding flame case.
Figure 11b shows the equivalence ratio range from 0.65 to 0.85, where the CO of all flames is smaller than 25 ppm. In this range, the flame temperature was increased and the CO oxidation rate was improved; therefore, the CO concentration was reduced. It was observed that the flame with 10% CO
2 vitiation basically had the same CO performance compared to the flame without vitiation. The CO behavior with CO
2 vitiation could be understood from the two contrary effects on the CO emission. One is the inhibiting effect on CO formation which has been indicated by many studies. The other one is the flame temperature reduction effect which weakens the dissociation from CO
2 to CO. In the end, the CO concentration is determined by the joint effects of the two contrary effects. With 10% N
2 and 10% H
2O vitiation, a more and more obvious CO reduction effect was found as the increase of equivalence ratio. This was probably because the adiabatic flame temperature is low, hence the CO
2 dissociation is not strong.
Figure 11c shows the CO profile when approaching stoichiometric condition; the dissociation of CO
2 plays a more important role and yields an increase of CO concentration. The equivalence ratios where CO equals to 25 ppm are denoted by H
1, H
2, H
3, and H
4.
Figure 11a–c shows the CO concentration trend along the increase of equivalence ratio. The operation with CO lower than 25 ppm was only possible between the two equivalence ratio limits which are shown in
Figure 11d. It was found that the 10% vitiation gas led to a smaller equivalence ratio range for CO lower than 25 ppm. Among the three vitiation species, CO
2 had the most significant effects on low CO operation.
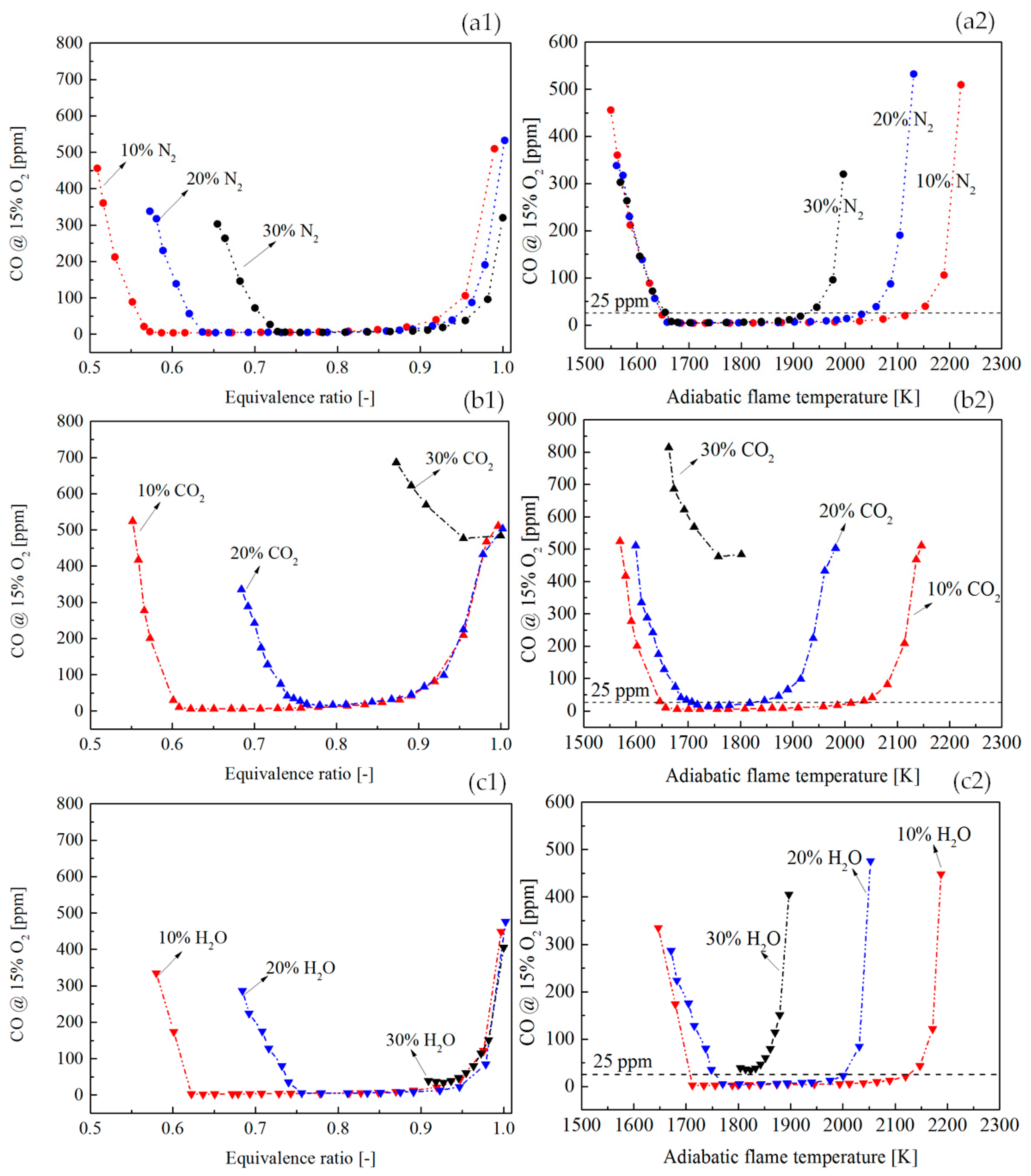
Figure 12(a1,b1,c1) shows a comparison of CO profile with the vitiation ratio of 10%, 20%, and 30%.
Figure 12(a2,b2,c2) shows the dependency of CO on the adiabatic flame temperature. As the increase of vitiation ratio, the flame temperature was decreased. Therefore, at the LBO side, the CO rise due to the low flame temperature shifted to higher equivalence ratios. This tendency has been presented in
Figure 12(a1,b1,c1). At the side where the equivalence ratio was close to 1, the CO rise was found in all the flames. A common phenomenon was that the higher vitiation ratio made the location where CO had a rapid rise shift to lower adiabatic flame temperatures. This was the result of the joint effects of the CO oxidation rate and the CO
2 dissociation rate. When the CO
2 dissociation rate was stronger than the CO oxidation rate due to the dilution effects of the vitiation, the CO concentration was increased even the flame temperature is not high.
Figure 12(a2,b2,c2) shows that there was a limit of vitiation ratio, above which the CO was never below 25 ppm (even the temperature was still high). This is because the CO oxidation rate is not only determined by flame temperature but also by the reactant concentration. A very high vitiation ratio could lead to a reaction rate which is not sufficiently fast to complete the CO reaction in a limited residence time in the combustor. The vitiation ratio limit was proven to be determined by the vitiation species by the current results in
Figure 12(a2,b2,c2). It could be observed that the vitiation limit of N
2 was larger than 30%. However, for CO
2 and H
2O, their vitiation limits were below 30%.
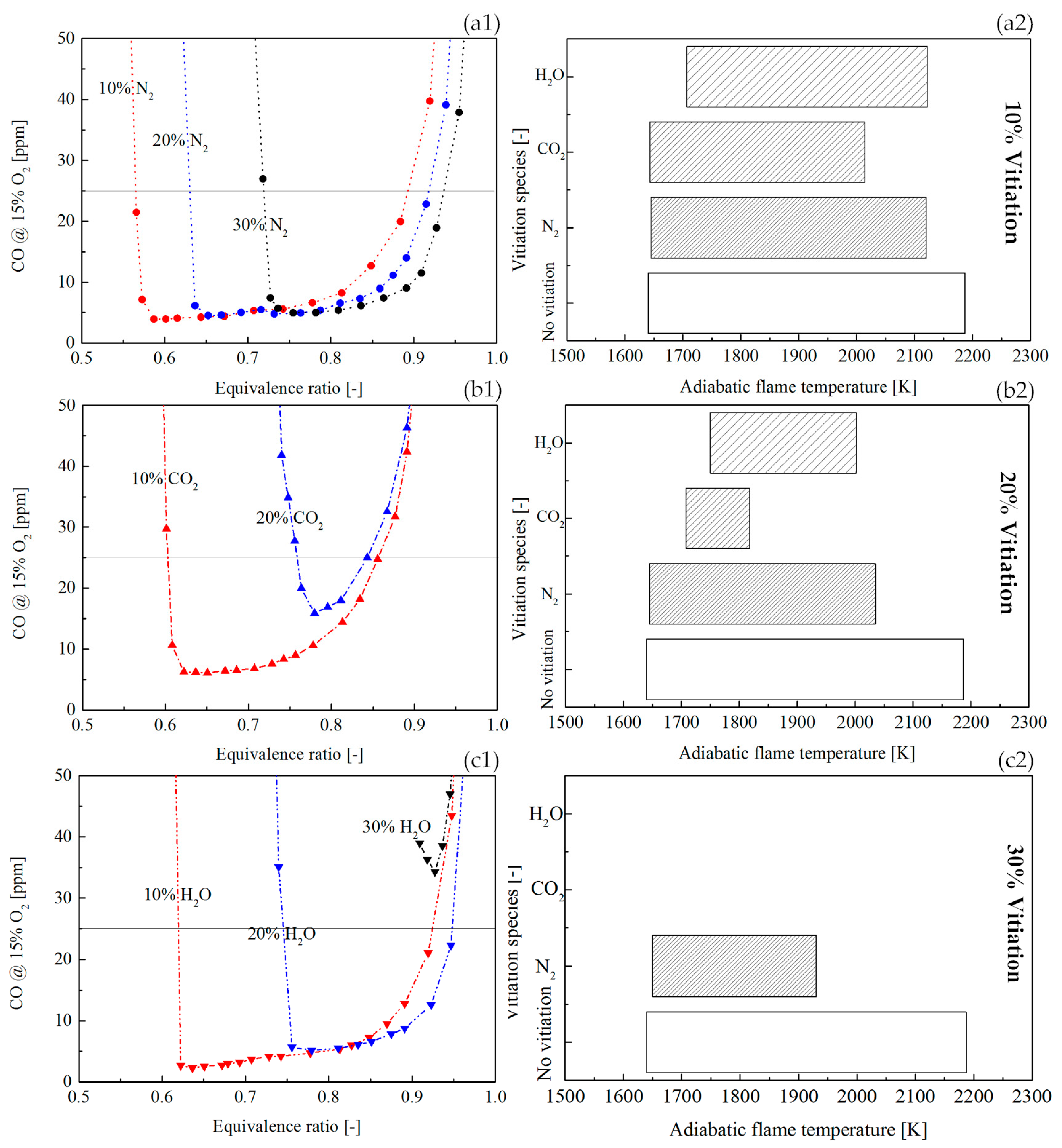
Figure 13(a1,b1,c1) shows the CO profile in a scale range from 0 to 50 ppm, so that the vitiation effects can be observed when CO was low. Comparing the vitiation of N
2, CO
2, and H
2O, a reduction of equivalence ratio range was found for CO emission below 25 ppm, as the increase of vitiation ratio. With 30% vitiation, CO was never below 25 ppm for the vitiation of CO
2 and H
2O. The adiabatic flame temperature range for the CO below 25 ppm is shown in
Figure 13(a2,b2,c2). The results indicated that the increase of vitiation ratio resulted in a smaller temperature region for low CO operation.
4. Conclusions
An experimental investigation was performed on a premixed and swirl-stabilized gas turbine model combustor at atmospheric pressure. The characteristics of the vitiated methane combustion have been demonstrated. 10%, 20%, and 30% mole fraction of the air were substituted by N2, CO2, and steam, respectively. The vitiation effects on the flame shapes, anchored location, LBO limits, and CO emission were studied experimentally. The measurement techniques, such as time-averaged CH chemiluminescence, high-speed broadband chemiluminescence, and CO emission monitoring were employed to characterize the features of the vitiated flame. Due to the variation of physical and chemical properties of the reactant mixtures, the chemical kinetics calculation was conducted to investigate vitiation effects on the flame reaction zone. Based on the analysis of the experimental and chemical kinetics calculation results, the below conclusions can be summarized as:
The chemical kinetics calculation showed a clear discrepancy of the flame reaction zone between the flame without vitiation and the vitiated flame. The calculation results revealed that across the flame reaction zone, the temperature gradient, the concentration of radicals (H, O, and OH), and CO emission were decreased by the vitiation. A reduction in adiabatic flame temperature was also found with the vitiation. Among the vitiation of N2, CO2, and H2O, N2 had the minimum influence on the flame reaction zone.
The time-averaged CH chemiluminescence showed that both the flame shapes and anchored location were affected by the vitiation. The increase in vitiation ratio (10% to 30%) could lead to the alteration of flame shapes and the reduction of CH intensity. Among the three types of vitiation, H2O was found to have the maximum influence, the next was CO2, and the N2 had the minimum influence.
LBO limits and flammability boundaries of the Damköhler number were identified. Measurements results showed that the LBO limits were increased by the vitiation species; however, the increase could be compensated for by improving the combustor inlet temperature. With the vitiation of CO2 and H2O, LBO limits had a similar increase tendency with the vitiation ratio rise between 10% and 30%. However, for the flame vitiated by N2, the LBO limits increased at a slower rate. A significant influence on the Damköhler number flammability limit was not found with the changes of vitiation species and the vitiation ratio.
High-speed broadband chemiluminescence showed a common flame re-ignition and detachment process prior to the LBO limits. For this process, the time-dependent fluctuation of the average broadband intensity was analyzed by FFT and the results showed that flames vitiated by N2, CO2, and H2O had approximately the same peak frequency.
A reduction of the equivalence ratio range for low CO operation (below 25 ppm) was found in vitiated flames. Among the three vitiation species, CO2 has the most significant effects. It should be noticed that the CO concentration in the combustion products was determined by the joint impacts of flame temperature, reactant concentration, and vitiation species. Low CO operation was not possible with a sufficiently high vitiation ratio.
Overall, the present work provided an insight into the vitiated combustion. In the vitiated flames, the limit of the vitiation ratio existed below which a stable flame operation with low CO emission was possible, with a constant combustor inlet temperature.