3.2. Optimization of Starch Hydrolysis Process Operating Parameters
Once the optimal hydrolysis process is determined, the Box-Behnken design is used to test four different independent variables in the hydrolysis process. ANOVA was used to determine the effects of the independent variables (i.e., substrate loading, α-amylase concentration, amyloglucosidase concentration and stroke speed) on the dependent variable (i.e., reducing sugar concentration).
Table 4 showed that the regression coefficients of the linear terms
X1,
X2, and
X3 were significant at the 1% level as well as quadratic coefficients of
. The regression coefficients of the linear term
X4,
X2X4 and
X3X4 were significant at the 5% level as well as quadratic coefficients of
. The polynomial model for reducing sugar concentration was regressed by considering the significant terms and is shown in Equation (3) below:
In Equation (3), YRS represents the reducing sugar concentration (g/L), X1 represents the substrate loading, X2 represents the α-amylase concentration, X3 represents the amyloglucosidase concentration and X4 represents the stroke speed.
The ANOVA results for the starch hydrolysis process are presented in
Table 4. The reducing sugar concentrations were predicted using Equation (3) for each sample and the results are shown in
Table 5.
ANOVA was carried out to determine the statistical significance of the quadratic response surface model, and it can be seen from
Table 4 that the
p-value of the model was less than 0.0001, which indicates the model was statistically significant. The coefficient of determination (
R2) was found to be 0.9914, which indicates that 99.14% of the experimental data were relevant and only 0.86% of the total variations was not explained by the model. In general, a high value of
R2 indicates that there is good fit between the predicted data and experimental data.
The adjusted coefficient of determination (Ra2) was found to be very high with a value of 0.9828, which indicates that that the model accounted for 98.28% of the variability in the data. The coefficient of variation (C.V.) was found to be 3.05%, indicating that the deviations between the predicted data and experimental data were small. The lack of fit was found to be small with a value of 5.39, and this indicates that the model adequately described the relationship between the dependent variable (i.e., reducing sugar concentration) and the independent variables (i.e., substrate loading, α-amylase concentration, amyloglucosidase concentration and stroke speed). The corresponding p-value for the lack of fit parameter was 0.0593 (p-value > α), which indicates that there was no evidence that the model did not fit the experimental data.
From the experimental result inputs, the optimum conditions for enzymatic hydrolysis were generated by DOE. The optimum substrate loading, α-amylase concentration, amyloglucosidase concentration and stroke speed were 23.88% (w/v), 109.43 U/g, 65.44 U/mL and 74.87 spm, respectively. The optimum reducing sugar was found to be 196.23 g/L. Verification tests in triplicate were performed under the set of optimized parameters. Average reducing sugar of 194.98 g/L was achieved, at predicted optimum conditions of the variables.
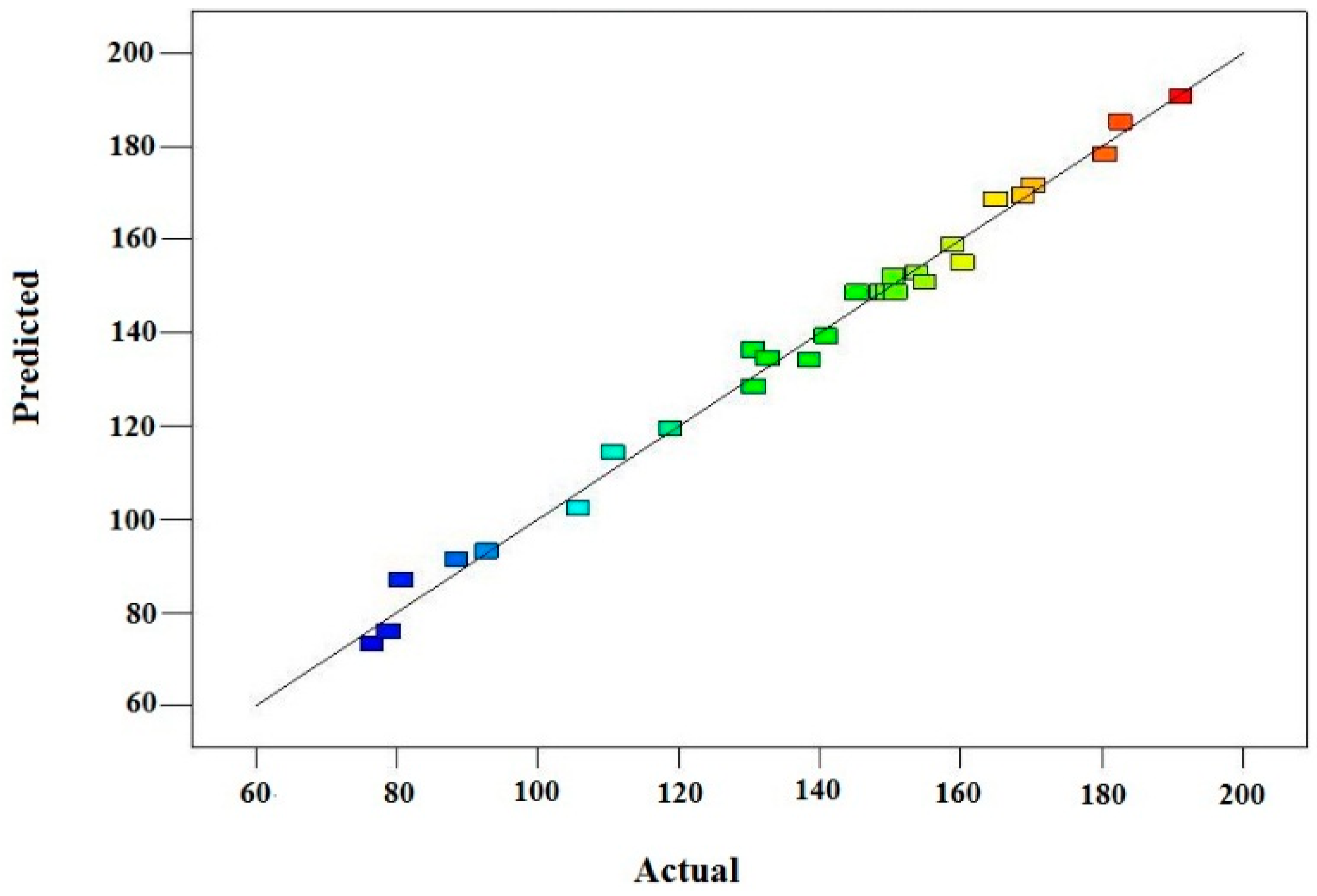
The predicted reducing sugar concentrations are plotted against the experimental values, as shown in
Figure 1. It can be observed that all of the data points lie within the proximity of the regression line, which indicates that there is good agreement between the predicted and experimental data.
3.3. Relationship between the Independent Variables of the Starch Hydrolysis Process
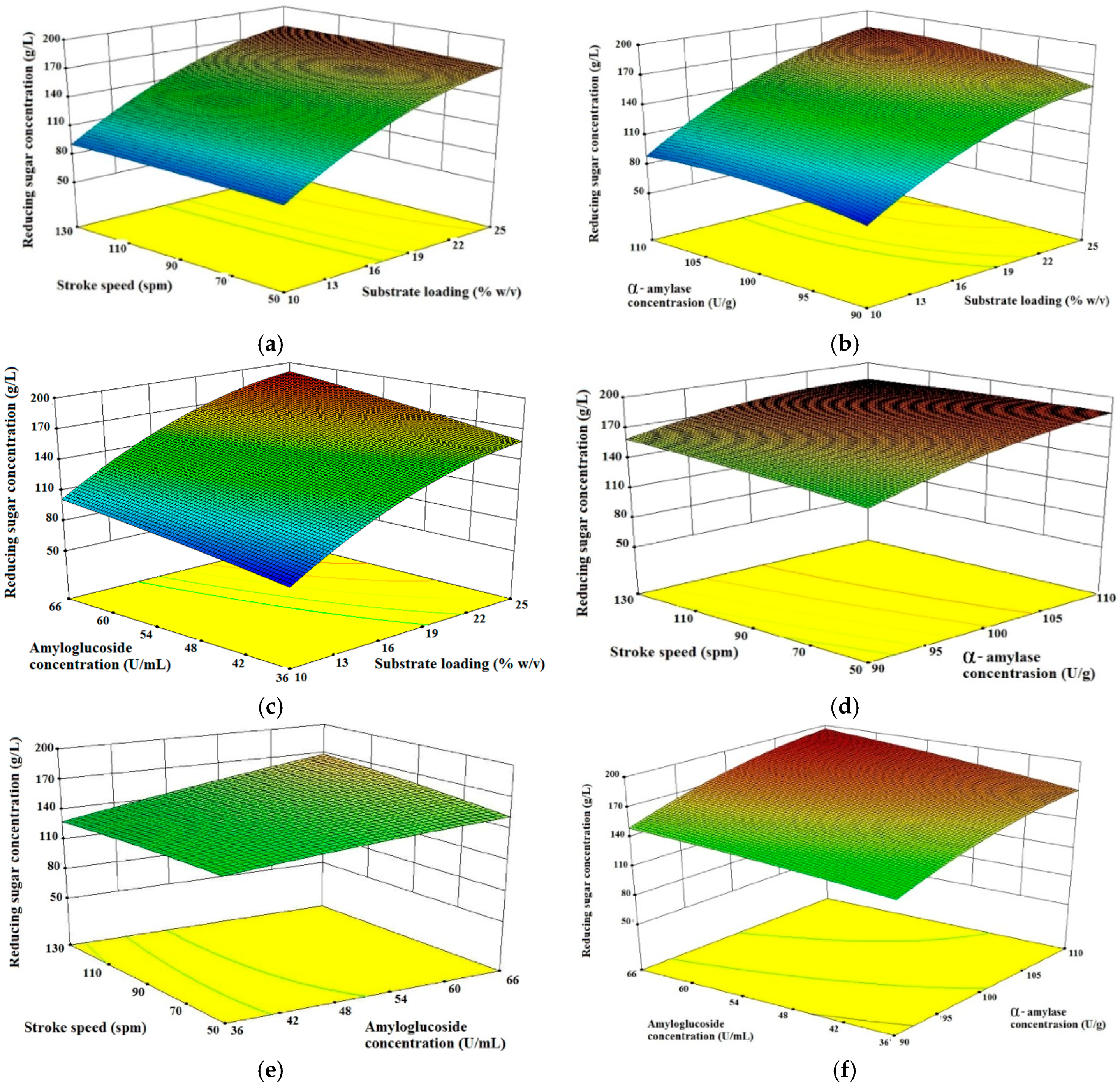
In this study, the operating parameters of the starch hydrolysis process (i.e., substrate loading, α-amylase concentration, amyloglucosidase concentration and stroke speed) were varied in order to determine the interaction effects of these parameters on the produced reducing sugar concentration. The results are presented in the form of three-dimensional surface plots.
Figure 2a–c illustrates that there were significant interactions between substrate loading and other process parameters affecting the results of reducing sugar.
Figure 2a–c showed that the substrate loading had a more significant influence on the results of reducing sugar compared with stroke speed, α-amylase concentration and amyloglucosidase concentration. In general, an increase in the substrate loading hastened the improvement of reducing sugar concentration. However, an increase in stroke speed had an insignificant effect on the reducing sugar concentration, and it was evident that for a fixed substrate loading, increasing the stroke speed would only increase reducing sugar production by a slight amount.
Figure 2a–c indicates that high reducing sugar results could be achieved on a substrate loading amounted to 23.88% (
w/
v).
Figure 2b,d,f showed there were significant interactions between α-amylase concentration and other process parameters that affected the results of reducing sugar.
Figure 2b,d,f showed that high reducing sugar results could be achieved at a concentration of α-amylase amounted to 109.43 U/g.
Figure 2b showed that at high substrate loading, the result of reducing sugar increased significantly with increased α-amylase concentration. It is also shown in
Figure 2f that increased α-amylase concentration which was followed by an increase in amyloglucosidase concentration also produced maximum reducing sugar.
Figure 2c,e,f showed there were significant interactions of amyloglucosidase concentration with other process parameters that affected the results of reducing sugar. This can be seen in
Figure 2c,e,f, which shows that high reducing sugar results could be achieved at 65.44 U/mL amyloglucosidase concentration. However, in
Figure 2e showed that the increase in stroke speed did not have a significant impact on increasing the reducing sugar.
The hydrolysis process (liquefaction and saccharification) for
M. glaziovii starch is a process for converting starch molecules into reducing sugar that is carried out by the enzyme α-amylse and amyloglucosidase.
Figure 2b,c shows reducing sugar levels rise with increasing concentration of the enzyme α-amylse and amyloglucosidase used in the hydrolysis process. This is due to the enzyme α-amylse and amyloglucosidase hydrolyzing α-1,4-glucoside and α-1,6-glucoside producing reducing sugar. From
Figure 2a–f, the maximum concentration reducing sugar produced was 196.23 g/L (82%) when the substrate loading, α-amylase, amyloglucosidase concentration and stroke speed were 23.88% (
w/
v), 109.43 U/g, 65.44 U/mL and 74.87 spm, respectively. The results of reducing in this study are similar to a study by Naguleswaran et al. [
25], with corn and triticale (80.8%–84.5%) as a feedstock, but slightly lower than that of wheat (90.8%–95.1%). Gumienna et al. [
22] also conduct the enzyme hydrolysis for corn starch and obtained reducing sugar of 11.7 g/L (69.40%). Moreover, Tasić et al. [
24] performed acid hydrolysis on potato and produced a 114 g/L (64%) concentration of reducing sugar. It was showed that optimization of hydrolysis improved the reducing sugar and increased ethanol yield.
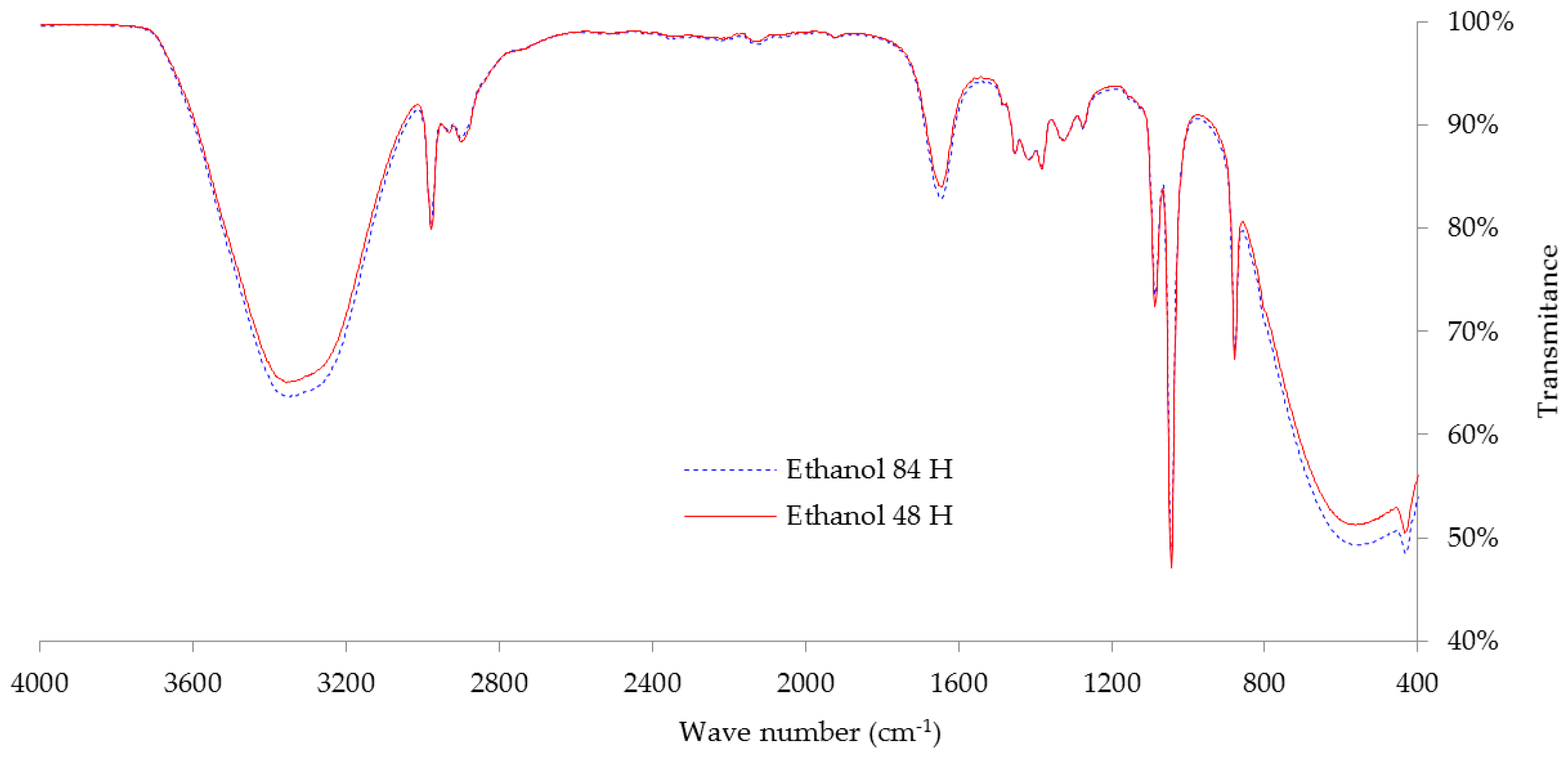
3.4. Ethanol Production from Manihot glaziovii
The highest initial sugar from the hydrolysis process (
Section 3.2) was fermented to produce bioethanol. The production of bioethanol in this study was conducted by batch fermentation.
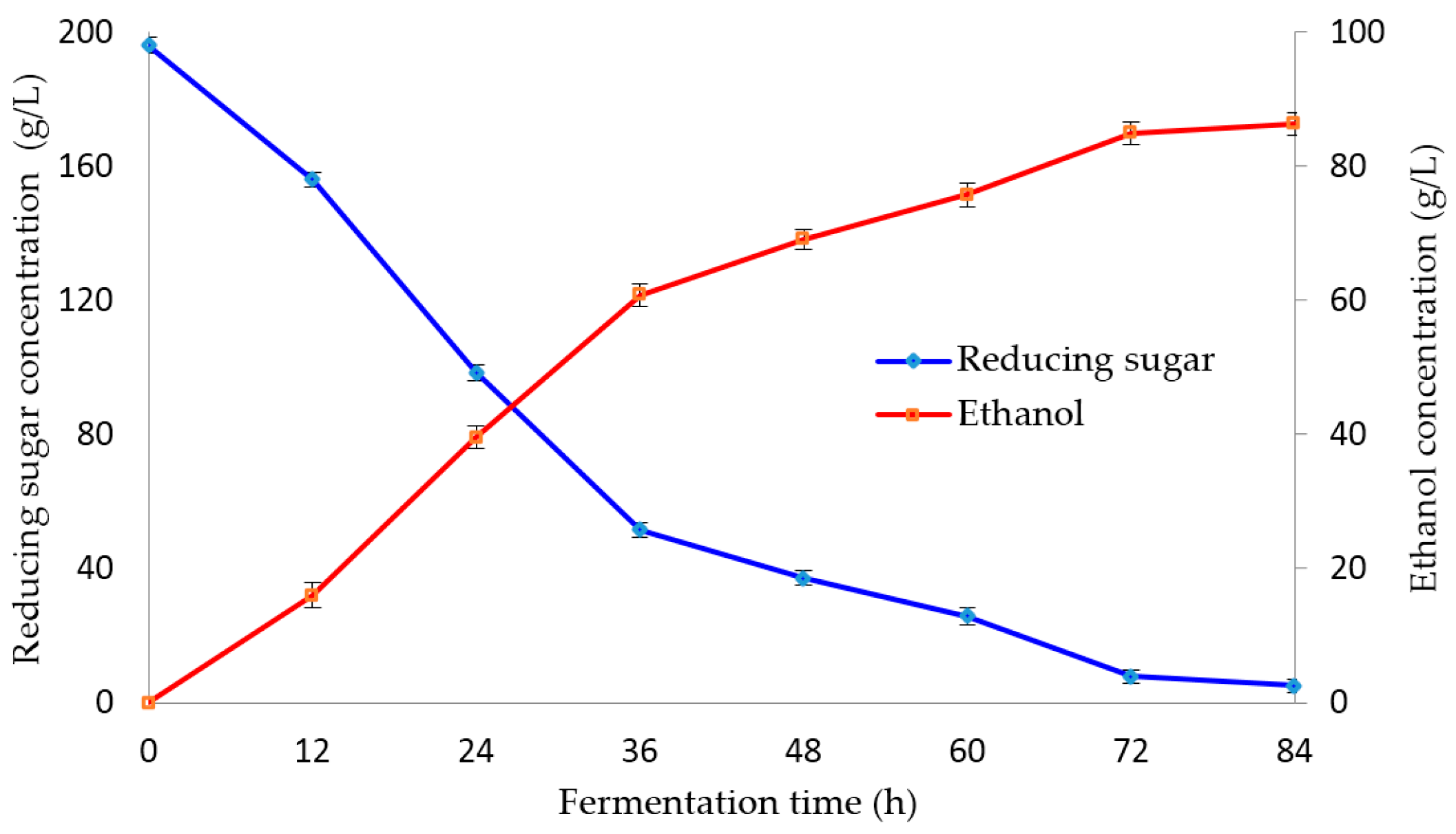
Figure 3 shows the production of ethanol from fermentation process by
S. cerevisiae cultivated in
M. glaziovii starch solution. During the first 12 h to 84 h of fermentation, reducing sugar consumed in
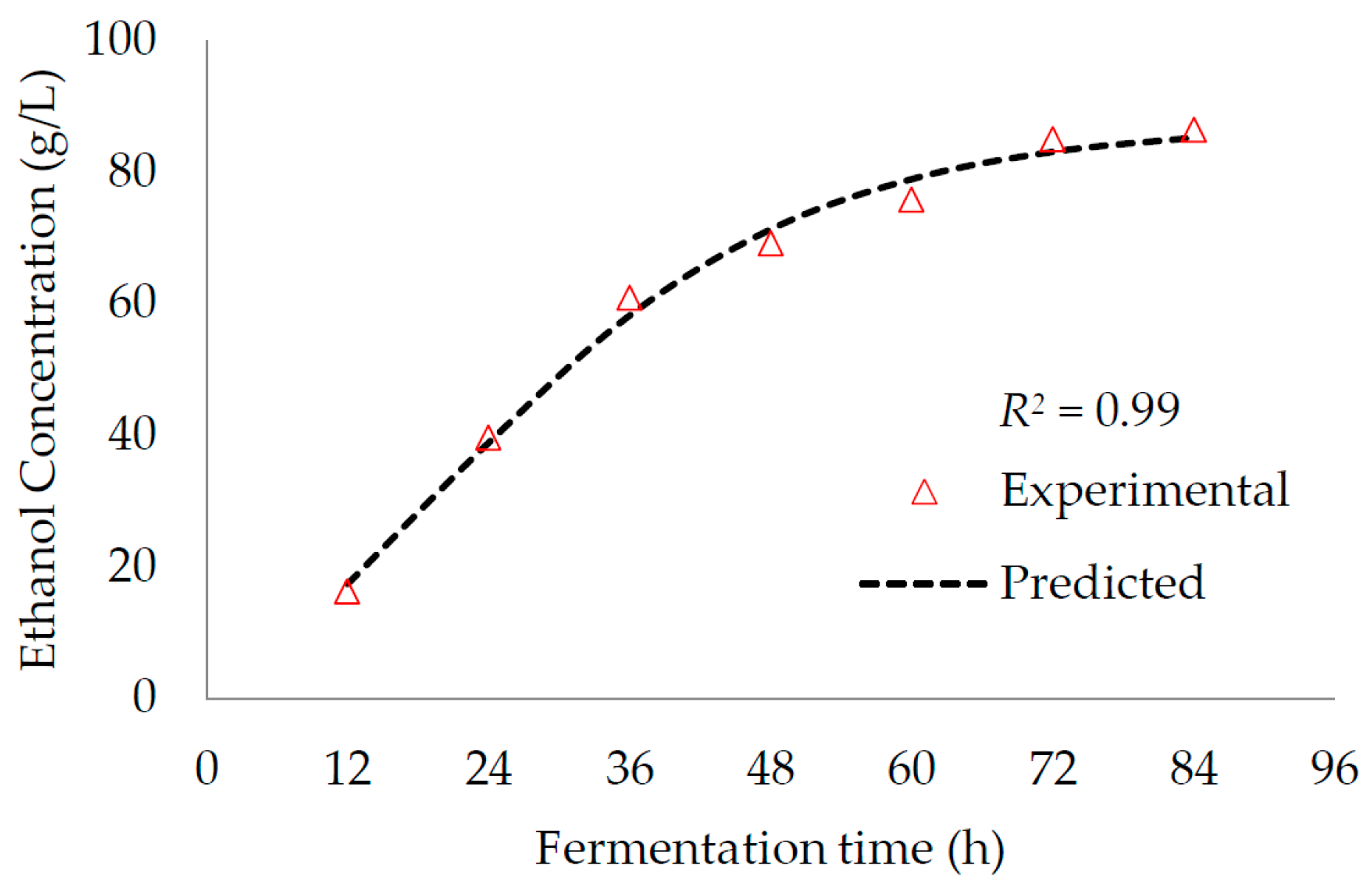
M. glaziovii starch solution changed rapidly. After completing 84 h of fermentation, an ethanol production conversion ratio of 0.41–0.45 g ethanol/g reducing sugar was observed, which is equivalent to 81.94%–88.61% of theoretical ethanol yield (0.51 g ethanol/g reducing sugar). This also produced an ethanol concentration of 86.37 g/L after 84 h fermentation. The result for ethanol yields in this study was similar to study of Moshi et al. [
14] and a little higher, where their ethanol yield was 82%–84% of theoretical ethanol yield. However, it was slightly lower than the results of the study Moshi et al. [
15,
29] which are equal to 95% and 94%. In addition, it was slightly higher when compared with the results of a study by Sivamani et al. [
30], where they used cassava peel and ethanol yield was 83% of the theoretical ethanol yield. The same is true when the result is compared with the study by Gumienna et al. [
22] where the outcome of each was 81.33%.