Cadmium Accumulation in Tissues of Sarotherodon melanotheron (Rüppel, 1852) from the Aby Lagoon System in Côte d’Ivoire
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area
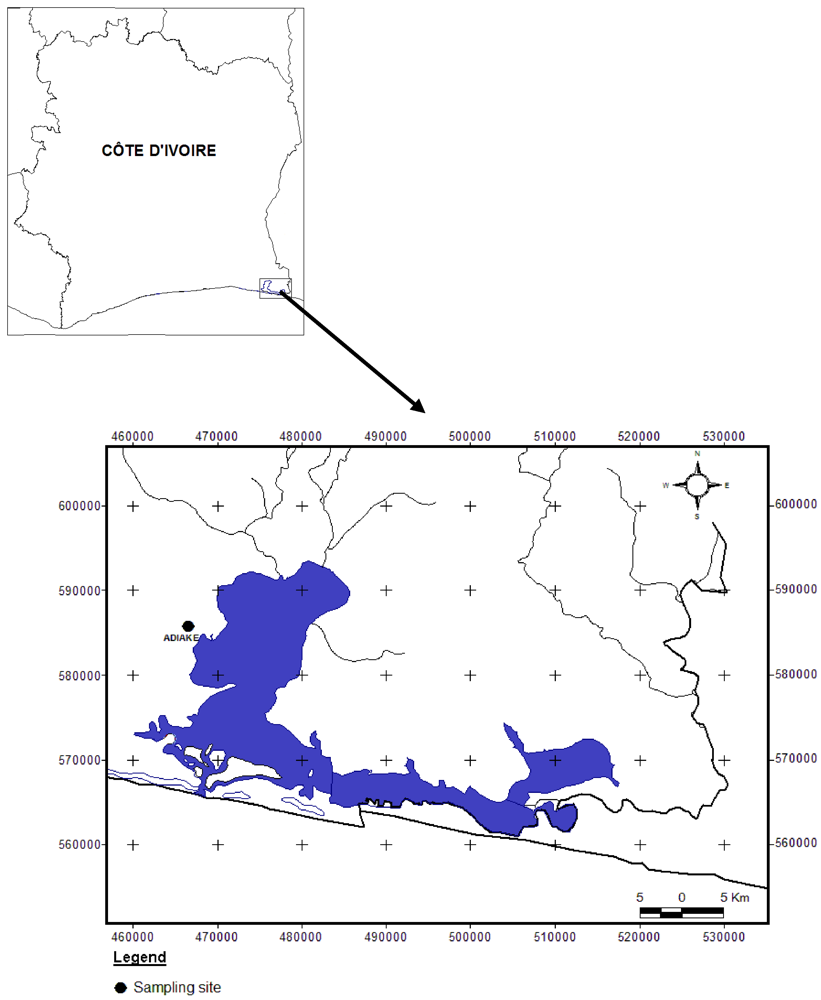
2.2. Sampling Method
2.3. Samples Preparation
| Dry Season (Jan.–Apr.) | Rainy Season (May–Aug.) | Flood Season (Sep.–Dec.) | ||||
|---|---|---|---|---|---|---|
| Juvenile | Adult | Juvenile | Adult | Juvenile | Adult | |
| M ± sd | 15.5 ± 0.28 | 22.8 ± 2.02 | 15.25 ± 0.1 | 21.1 ± 0.7 | 15.38 ± 0.3 | 21.7 ± 0.8 |
| Min-Max | [15.2–15.8] | [19.5–25.0] | [15.1–15.4] | [20.1–21.8] | [15.1–15.8] | [20.5–22.4] |
| %CV | 2 | 9 | 1 | 3 | 2 | 4 |
2.4. Preparation of Standard Solutions
2.5. Preparation of Samples for Assaying Through Atomic Absorption Spectrophotometer (AAS)
2.6. Calculations
2.7. Statistical Analyses
3. Results and Discussion
3.1. Results
| Dry Season | Rainy Season | Flood Season | |
|---|---|---|---|
| pH | 9.02 ± 0.02 | 8.5 ± 0.1 | 9.2 ± 0.1 |
| T°C | 30.6 ± 0.3 | 30.9 ± 0.4 | 31.9 ± 0.1 |
| Salinity | 6.2 ± 0.4 | 1.9 ± 0.3 | 1.5 ±0.1 |
| *DO | 10.81 ± 0.02 | 3.8 ± 0.1 | 3.04 ± 0.01 |
3.1.1. Cadmium Contamination
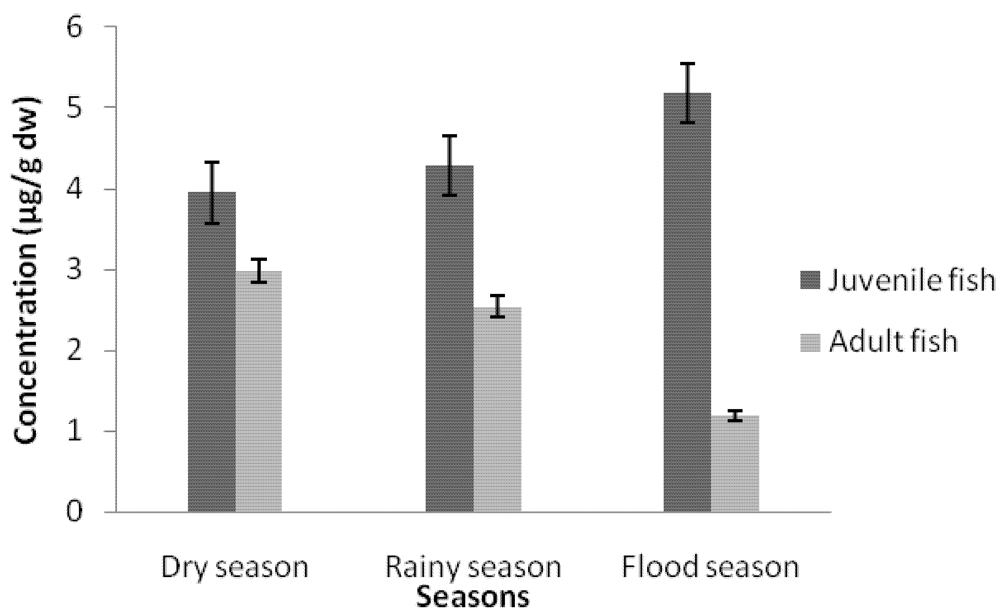
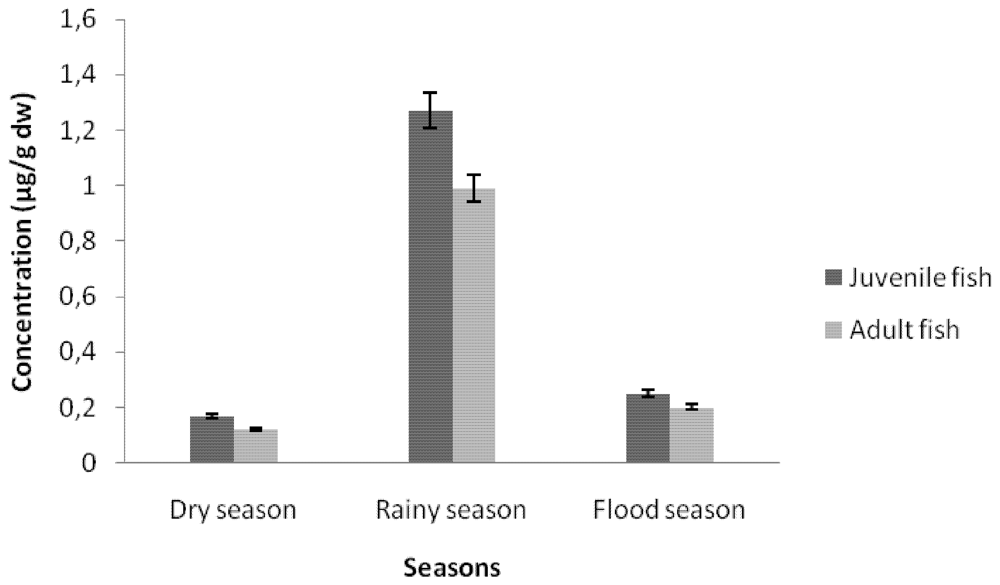

3.2. Discussion
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Güven, K.; Özbay, C.; Ünlü, E.; Satar, A. Acute lethal toxicity and accumulation of copper in Gammarus pulex (L.) (Amphipoda). Turk. J. Biol. 1999, 23, 513–521. [Google Scholar]
- Freedman, B. Environmental Ecology. The Impacts of Pollution and Other Stresses on Ecosystem Structure and Function; Academic Press: San Diego, CA, USA, 1989. [Google Scholar]
- Alibabić, V.; Vahčić, N.; Bajramovi, M. Bioaccumulation of metals in fish of Salmonidae family and the impact on fish meat quality. Environ. Monit. Assess. 2007, 131, 349–364. [Google Scholar]
- Allen, P. Chronic accumulation of cadmium in the edible tissues of Oreochromis aureus (Steindachner): Modification by mercury and lead. Arch. Environ. Contam. Toxicol. 1995, 29, 8–14. [Google Scholar]
- Karthikeyan, S.; Palaniappan, P.L.R.M.; Sabhanayakan, S. Influence of pH and water hardness upon Nickel accumulation in edible fish Cirrhinus mrigala. J. Environ. Biol. 2007, 28, 484–492. [Google Scholar]
- Metongo, B.S.; Gbocho, G.B. Biological accumulation of some trace elements (Cd, Cu, Mn, Zn) in a bivalve Arcasenilis. J. Ivoir. Oceanol. Limnol. 2007, 4, 11–21. [Google Scholar]
- Akpetou, K.L.; Kouassi, A.M.; Goula, B.T.A.; Assemian, S.; Aka, K. Nutrients induction on lead, cadmium, manganese, zinc and cobalt speciation in the sediments of Aby lagoon (Côte d’Ivoire). Int. J. Eng. Sci. Technol. 2010, 2, 3894–3900. [Google Scholar]
- Claon, S. Exposition de l’écosystème et des Populations Riveraines de la Lagune Aby au Mercure, arsenic et Sélénium. Ph.D. Dissertation, l’Université de Cocody, Abidjan, 2004. [Google Scholar]
- Henry, F.; Amara, R.; Courcot, L.; Lacouture, D.; Bertho, M.-L. Heavy metals in four fish species from the French coast of the Eastern English Channel and Southern Bight of the North Sea. Environ. Int. 2004, 30, 675–683. [Google Scholar]
- Al-Weher, S.M. Levels of heavy metal Cd, Cu and Zn in three fish species collected from the Northern Jordan Valley. Jordan J. Biol. Sci. 2008, 1, 41–46. [Google Scholar]
- McLusky, D.S. The Estuarine Ecosystem, 2nd ed; Chapman and Hall: New York, NY, USA, 1989; p. 214. [Google Scholar]
- FAO/WHO, Principles of the Safety Assessment of Food Additives and Contaminants in Food Environmental Health Criteria; FAO/WHO: Geneva, Switzerland, 1987; No. 70.
- Javed, M. Relationships among water, sediments and plankton for the uptake and accumulation of metals in the river Ravi. Ind. J. Plant Sci. 2003, 2, 326–331. [Google Scholar]
- Obodai, E.A.; Boamponsem, L.K.; Adokoh, C.K.; Essumang, D.K.; Villawoe, B.O.; Aheto, D.W.; Debrah, J.S. Concentrations of heavy metals in two Ghanaian Lagoons. Arch. Appl. Sci. Res. 2011, 3, 177–187. [Google Scholar]
- Laar, C.; Fianko, J.R.; Akiti, T.T.; Osae, S.; Brimah, A.K. Determination of heavy metals in the black-chin tilapia from the Sakumo Lagoon, Ghana. Res. J. Environ. Earth Sci. 2011, 3, 8–13. [Google Scholar]
- Idodo-Umeh, G. Water quality assessment of water bodies in Olomoro, Isoko South, Delta State, Nigeria using Physical, Chemical and Biological Indices. Ph.D. Dissertation, University of Benin, Benin City, Nigeria, 2002. [Google Scholar]
- Okoronkwo, I.P. Heavy metals content in Water and Fish of River Niger at Yelwa-Yauri. M.Sc. Thesis, University of Benin, Benin City, Nigeria, 1992. [Google Scholar]
- Dural, M.; Goksu, M.Z.L.; Ozak, A.A. Investigation of heavy metal levels in economically important fish species captured from the Tuzla Lagoon. Food Chem. 2007, 102, 415–421. [Google Scholar]
- Ploetz, D.M.; Fitts, B.E.; Rice, T.M. Differential accumulation of heavy metals in muscles and liver of a marine fish (King Mackerel, Scomberomorus cavalla, Cuvier) from the Northern Gulf of Mexico, USA. Bull. Environ. Contam. Toxicol. 2007, 78, 134–137. [Google Scholar]
- Yilmaz, F.; Ozdemir, N.; Demirak, A.; Tuna, A.L. Heavy metal levels in two fish species Leuciscus cephalus and Lepomis gibbosus. Food Chem. 2007, 100, 830–835. [Google Scholar]
- Pratap, H.B.; Bonga, W.S.E. Calcium homeostasis in low and high calcium water acclimatized Oreochromis mossambicus exposed to ambient and dietary cadmium. J. Environ. Biol. 2007, 28, 385–393. [Google Scholar]
- Othumpangat, S.; Kashon, M.; Joseph, P. Eukaryotic translation initiation factor 4E is a cellular target for toxicity and death due to exposure to cadmium chloride. J. Biol. Chem. 2005, 280, 162–169. [Google Scholar]
- Satoh, M.; Kaji, T.; Tohyama, C. Lowdose exposure to cadmium and its health effects. (3) Toxicity in laboratory animals and cultured cells. Nihon Eiseigaku Zasshi 2003, 57, 615–623. [Google Scholar] [CrossRef]
- Cai, Y.; Aoshima, K.; Katoh, T.; Teranishi, H.; Kasuya, M. Renal tubular dysfunction in male inhabitants of a cadmium-polluted area in Toyama, Japan—An, eleven-year follow-up study. J. Epidemiol. 2001, 11, 180–189. [Google Scholar]
- Satoh, M.; Koyama, H.; Kaji, T.; Kito, H.; Tohyama, C. Perspectives on cadmium toxicity research. Tohoku J. Exp. Med. 2002, 196, 23–32. [Google Scholar]
- Banerjee, S.; Flores-Rozas, H. Cadmium inhibits mismatch repair by blocking the ATPase activity of the MSH2-MSH6 complex. Nucleic Acids Res. 2005, 33, 1410–1419. [Google Scholar]
- Hovland, D.N.; Cantor, R.M.; Lee, G.S.; Machado, A.F.; Collins, M.D. Identification of a murine locus conveying susceptibility to cadmium induced forelimb malformations. Genomics 2000, 63, 193–201. [Google Scholar]
- Kim, S.D.; Moon, C.K.; Eun, S.Y.; Ryu, P.D.; Jo, S.A. Identification of ASK1, MKK4, JNK, c-Jun, and caspase-3 as a signalling cascade involved in cadmium-induced neuronal cell apoptosis. Biochem. Biophys. Res. Commun. 2005, 328, 326–334. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cyrille, Y.D.A.; Victor, K.; Sanogo, T.A.; Boukary, S.; Joseph, W. Cadmium Accumulation in Tissues of Sarotherodon melanotheron (Rüppel, 1852) from the Aby Lagoon System in Côte d’Ivoire. Int. J. Environ. Res. Public Health 2012, 9, 821-830. https://doi.org/10.3390/ijerph9030821
Cyrille YDA, Victor K, Sanogo TA, Boukary S, Joseph W. Cadmium Accumulation in Tissues of Sarotherodon melanotheron (Rüppel, 1852) from the Aby Lagoon System in Côte d’Ivoire. International Journal of Environmental Research and Public Health. 2012; 9(3):821-830. https://doi.org/10.3390/ijerph9030821
Chicago/Turabian StyleCyrille, Yapi Dope Armel, Kouame Victor, Tidou Abiba Sanogo, Sawadogo Boukary, and Wethe Joseph. 2012. "Cadmium Accumulation in Tissues of Sarotherodon melanotheron (Rüppel, 1852) from the Aby Lagoon System in Côte d’Ivoire" International Journal of Environmental Research and Public Health 9, no. 3: 821-830. https://doi.org/10.3390/ijerph9030821





