Genotoxic Effect of Chronic Exposure to DDT on Lymphocytes, Oral Mucosa and Breast Cells of Female Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Laboratory Animals
2.2. Exposure Assays
2.3. Obtaining Samples
2.4. Micronuclei in Oral Mucosa Cells
2.5. Single Cell Electrophoresis (Comet Assay)
2.6. Determination of Free Radicals Through a Lipid Peroxidation Assay
2.7. Statistical Analysis
3. Results
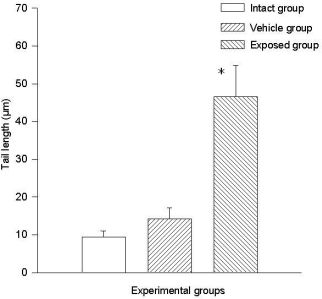
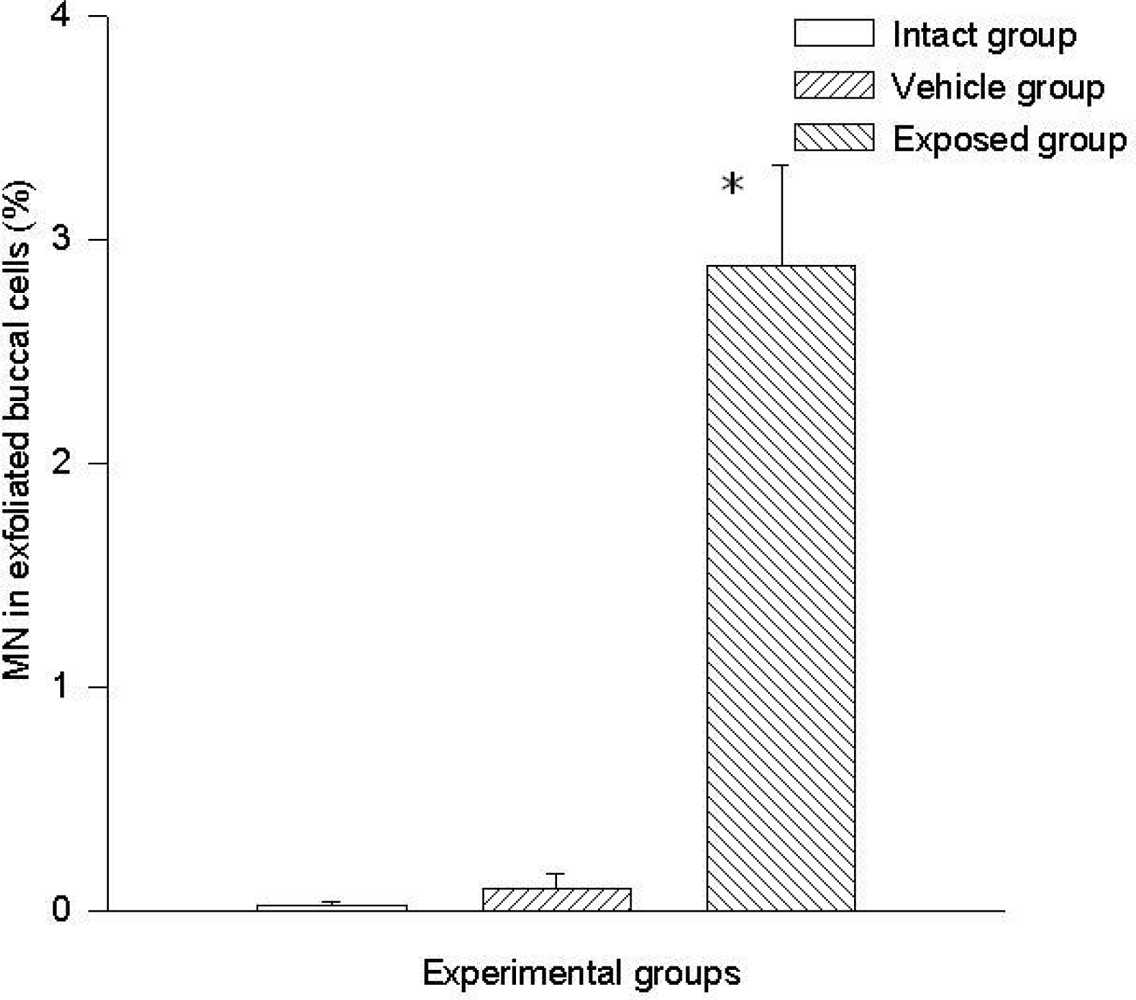
3.1 Micronuclei in Cells of Oral Mucosa Smears
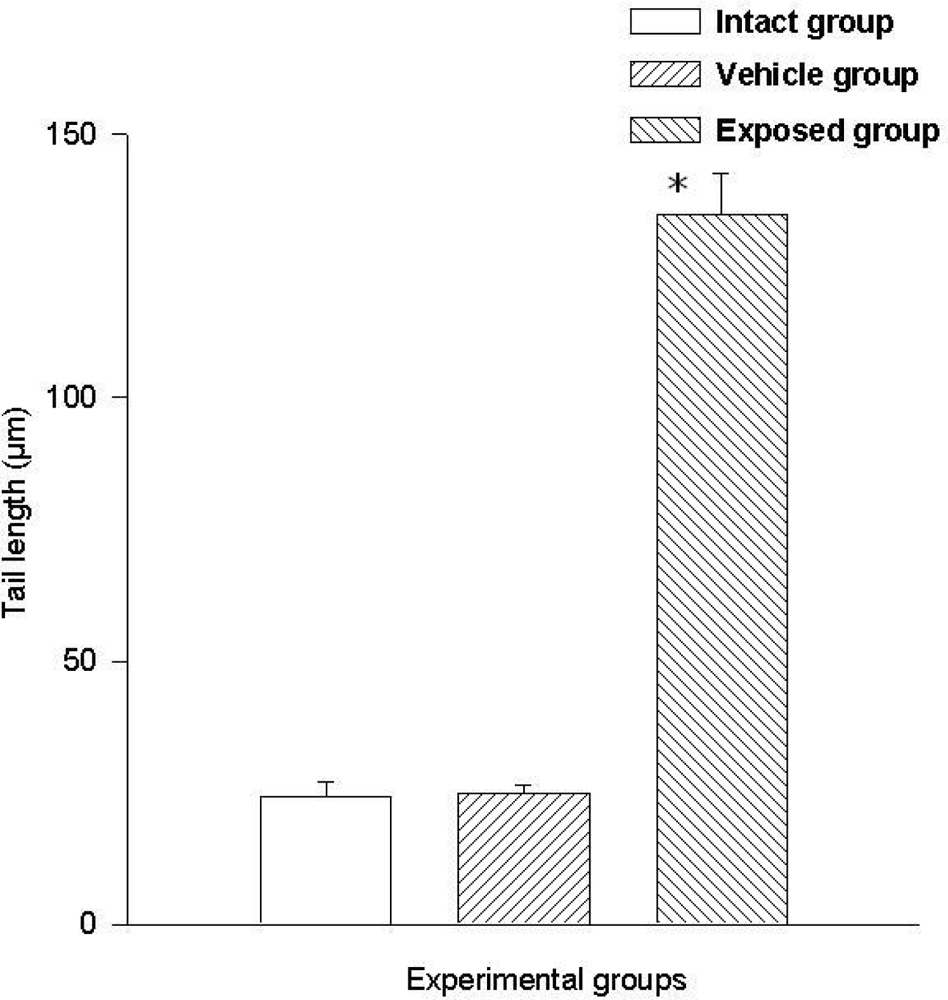
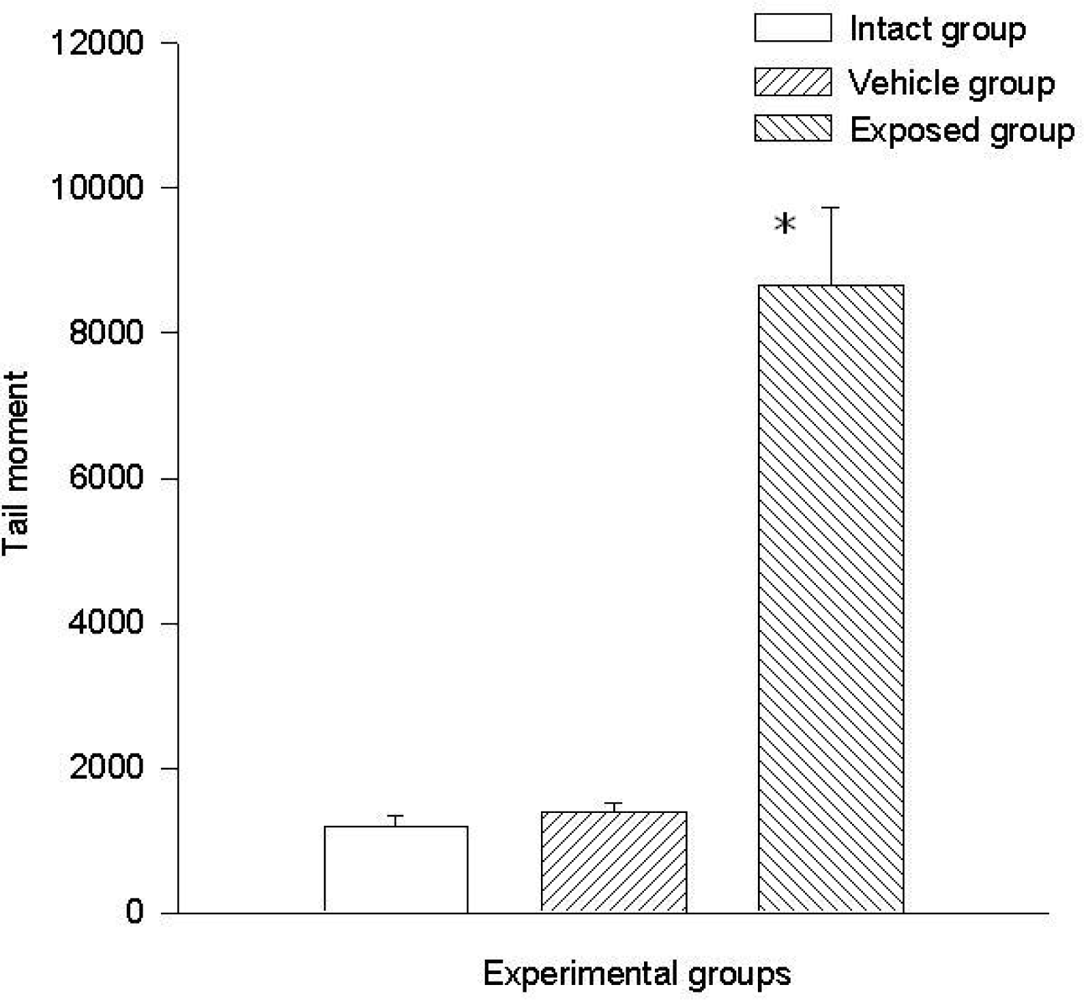
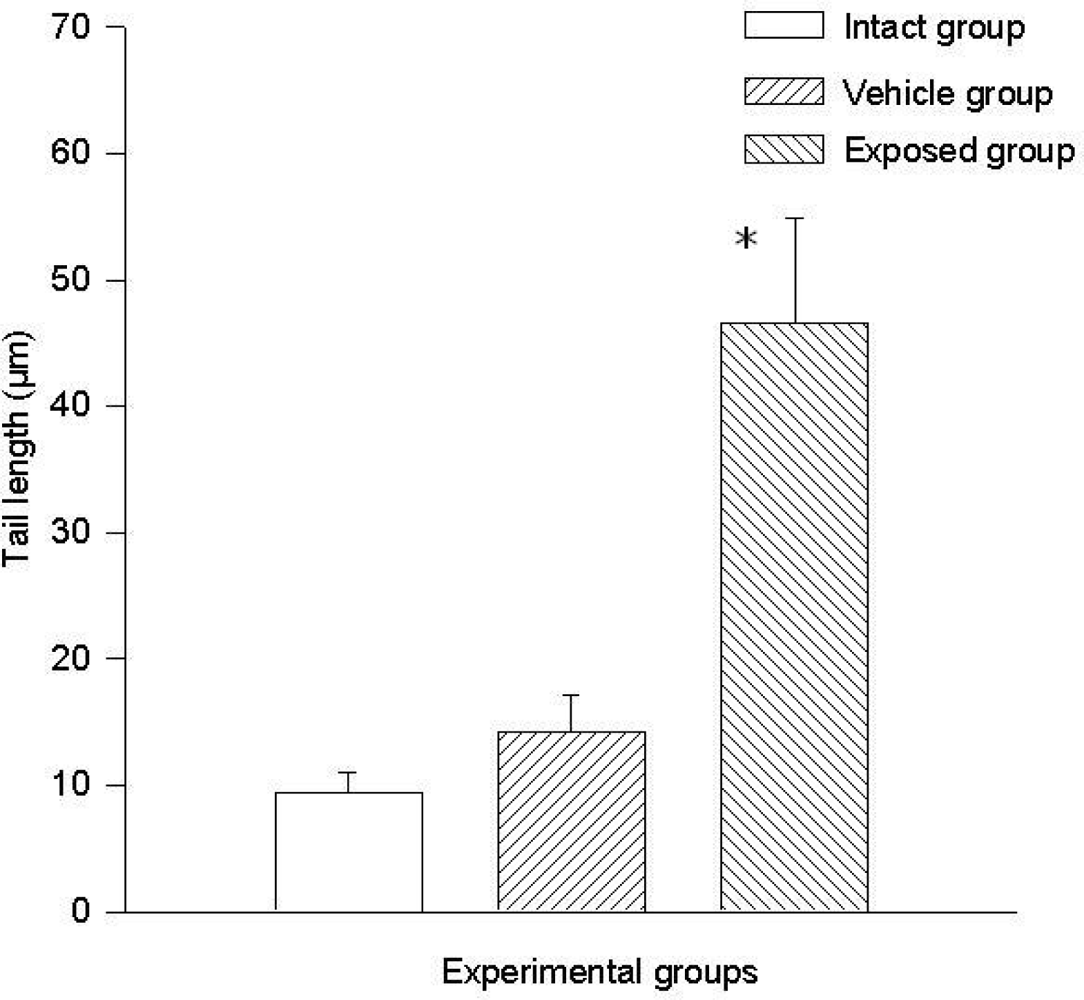
3.2. Lymphocyte Single Cell Electrophoresis Test
3.3. Single Cell Electrophoresis in Mammary Epithelial Cells
3.5. Free Radical Measurement
4. Discussion
5. Conclusions
Acknowledgments
Abbreviations
| DDT: | 1,1,1-trichloro-2,2-bis-(chlorophenyl)-ethane; |
| MN: | micronuclei; |
| MDA: | malondialdehyde; |
| DDE: | dichlorodiphenyldichloroethylene |


References and Notes
- Kristensen, VN; Kure, EH; Erikstein, B; Harada, N; Borresen-Dale, A. Genetic susceptibility and environmental estrogen-like compounds. Mutat. Res 2001, 482, 77–82. [Google Scholar]
- Pacakova, V; Loukotkova, L; Bosakova, Z; Stulik, K. Analysis for estrogens as environmental pollutants—A review. J. Sep. Sci 2009, 32, 867–882. [Google Scholar]
- Canales-Aguirre, A; De Celis, R; Salado Ponce, H; Feria-Velasco, A. Xenoestrógenos: Función y efectos. e-Gnosis 2003, 1, 1–11. [Google Scholar]
- Dees, C; Askari, M; Garrett, S; Gehrs, K; Henley, D; Ardies, CM. Estrogenic and DNA-damaging activity of Red No. 3 in human breast cancer cells. Environ. Health Perspect 1997, 105, 625–632. [Google Scholar]
- Rowe, LA; Degtyareva, N; Doetsch, PW. DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radic. Biol. Med 2008, 45, 1167–1177. [Google Scholar]
- De Celis, R; Pedron-Nuevo, N; Feria-Velasco, A. Toxicology of male reproduction in animals and humans. Arch. Androl 1996, 37, 201–218. [Google Scholar]
- Tucker, JD; Preston, RJ. Chromosome aberrations, micronuclei, aneuploidy, sister chromatid exchanges, and cancer risk assessment. Mutat. Res 1996, 365, 147–159. [Google Scholar]
- Clavel, J. Progress in the epidemiological understanding of gene-environment interactions in major diseases: Cancer. C. R. Biol 2007, 330, 306–317. [Google Scholar]
- Linet, MS. Evolution of cancer epidemiology. Epidemiol. Rev 2000, 22, 35–56. [Google Scholar]
- Hartmann, A; Fender, H; Speit, G. Comparative biomonitoring study of workers at a waste disposal site using cytogenetic tests and the comet (single-cell gel) assay. Environ. Mol. Mutagen 1998, 32, 17–24. [Google Scholar]
- Kim, BS; Park, JJ; Edler, L; Von Fournier, D; Haase, W; Sautter-Bihl, ML; Gotzes, F; Thielmann, HW. New measure of DNA repair in the single-cell gel electrophoresis (comet) assay. Environ. Mol. Mutagen 2002, 40, 50–56. [Google Scholar]
- Padilla-Camberos, E; Feria-Velasco, A. Prueba de Ames, usos e importancia en detección de agentes genotóxicos. In Genética Ambiente y Salud; Alvarez-Moya, C, Ed.; Universidad de Guadalajara: Guadalajara, Mexico, 2001; pp. 117–126. [Google Scholar]
- Fenech, M. The micronucleus assay determination of chromosomal level DNA damage. Methods Mol. Biol 2008, 410, 185–216. [Google Scholar]
- Hallare, AV; Gervasio, MK; Gervasio, PL; Acacio-Claro, PJ. Monitoring genotoxicity among gasoline station attendants and traffic enforcers in the city of Manila using the micronucleus assay with exfoliated epithelial cells. Environ. Monit. Assess 2009, 156, 331–341. [Google Scholar]
- Majer, BJ; Laky, B; Knasmuller, S; Kassie, F. Use of the micronucleus assay with exfoliated epithelial cells as a biomarker for monitoring individuals at elevated risk of genetic damage and in chemoprevention trials. Mutat. Res 2001, 489, 147–172. [Google Scholar]
- Dhawan, A; Bajpayee, M; Parmar, D. Comet assay: A reliable tool for the assessment of DNA damage in different models. Cell Biol. Toxicol 2009, 25, 5–32. [Google Scholar]
- Binelli, A; Riva, C; Cogni, D; Provini, A. Genotoxic effects of p,p’-DDT (1,1,1-trichloro-2,2-bis-(chlorophenyl) ethane) and its metabolites in Zebra mussel (D. polymorpha) by SCGE assay and micronucleus test. Environ. Mol. Mutagen 2008, 49, 406–415. [Google Scholar]
- Wojewodzka, M; Buraczewska, I; Kruszewski, M. A modified neutral comet assay: Elimination of lysis at high temperature and validation of the assay with anti-single-stranded DNA antibody. Mutat. Res 2002, 518, 9–20. [Google Scholar]
- Mandelker, L. Oxidative stress: The role of mitochondria, free radicals, and antioxidants. Vet Clin North Am Small Anim Pract 2008, 38, ix–xi. [Google Scholar]
- Huang, YL; Sheu, JY; Lin, TH. Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin. Biochem 1999, 32, 131–136. [Google Scholar]
- Burton, A. Pesticides: Toward DDT-free malaria control. Environ. Health Perspect 2009, 117, A344–A347. [Google Scholar]
- Bornman, R; De Jager, C; Worku, Z; Farias, P; Reif, S. DDT and urogenital malformations in newborn boys in a malarial area. BJU Int 2009, 106, 405–411. [Google Scholar]
- Wrobel, M; Mlynarczuk, J; Kotwica, J. The adverse effect of dichlorodiphenyltrichloroethane (DDT) and its metabolite (DDE) on the secretion of prostaglandins and oxytocin in bovine cultured ovarian and endometrial cells. Reprod. Toxicol 2009, 27, 72–78. [Google Scholar]
- Tarone, RE. DDT and breast cancer trends. Environ. Health Perspect 2008, 116, A374–A377. [Google Scholar]
- Snedeker, SM. Pesticides and breast cancer risk: A review of DDT, DDE, and dieldrin. Environ. Health Perspect 2001, 109, 35–47. [Google Scholar]
- Rehwagen, C. WHO recommends DDT to control malaria. BMJ 2006, 333, 622–646. [Google Scholar]
- WHO gives indoor use of DDT a clean bill of health for controlling malaria. Indian J Med Sci 2006, 60, 439–441.
- Torres-Bugarin, O; De Anda-Casillas, A; Ramirez-Munoz, MP; Sanchez-Corona, J; Cantu, JM; Zuniga, G. Determination of diesel genotoxicity in firebreathers by micronuclei and nuclear abnormalities in buccal mucosa. Mutat. Res 1998, 413, 277–281. [Google Scholar]
- Singh, NP; McCoy, MT; Tice, RR; Schneider, EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res 1988, 175, 184–191. [Google Scholar]
- Helma, C; Uhl, M. A public domain image-analysis program for the single-cell gel-electrophoresis (comet) assay. Mutat. Res 2000, 466, 9–15. [Google Scholar]
- Colorimetric Assay for Lipid Peroxidation; Product No FR 12; Oxford Biomedical Research: Oxford, MI, USA, 2003.
- Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 1976, 72, 248–254. [Google Scholar]
- Hemnani, T; Parihar, MS. Reactive oxygen species and oxidative DNA damage. Indian J. Physiol. Pharmacol 1998, 42, 440–452. [Google Scholar]
- Epe, B. Role of endogenous oxidative DNA damage in carcinogenesis: What can we learn from repair-deficient mice? Biol. Chem 2002, 383, 467–475. [Google Scholar]
- Bolognesi, C. Genotoxicity of pesticides: A review of human biomonitoring studies. Mutat. Res 2003, 543, 251–272. [Google Scholar]
- De Ferrari, M; Artuso, M; Bonassi, S; Bonatti, S; Cavalieri, Z; Pescatore, D; Marchini, E; Pisano, V; Abbondandolo, A. Cytogenetic biomonitoring of an Italian population exposed to pesticides: Chromosome aberration and sister-chromatid exchange analysis in peripheral blood lymphocytes. Mutat. Res 1991, 260, 105–113. [Google Scholar]
- Gauthier, JM; Dubeau, H; Rassart, E. Induction of micronuclei in vitro by organochlorine compounds in beluga whale skin fibroblasts. Mutat. Res 1999, 439, 87–95. [Google Scholar]
- Carrano, AV; Natarajan, AT. International commission for protection against environmental mutagens and carcinogens. ICPEMC publication No. 14. Considerations for population monitoring using cytogenetic techniques. Mutat. Res 1988, 204, 379–406. [Google Scholar]
- Anderson, D; Plewa, MJ. The international comet assay workshop. Mutagenesis 1998, 13, 67–73. [Google Scholar]
- Fairbairn, DW; Olive, PL; O’Neill, KL. The comet assay: A comprehensive review. Mutat. Res 1995, 339, 37–59. [Google Scholar]
- Gonzalez-Mille, DJ; Ilizaliturri-Hernández, C; Espinosa-Reyes, G; Costilla-Salazar, R; Díaz-Barriga, F; Ize-Lema, I; Mejía-Saavedra, J. Exposure to persistent organic pollutants (POPs) and DNA damage as an indicador of environmental stress in fish of different feeding habits of Coatzacoalcos, Veracruz, Mexico. Ecotoxicology 2010, 19, 1238–1248. [Google Scholar]
- Dhawan, A; Bajpayee, M; Parmar, D. Comet assay: A reliable tool for the assessment of DNA damage in different models. Cell Biol. Toxicol 2009, 25, 5–32. [Google Scholar]
- Sekihashi, K; Yamamoto, A; Matsumura, Y; Ueno, S; Watanabe-Akanuma, M; Kassie, F; Knasmüller, S; Tsuda, S; Sasaki, YF. Comparative investigation of multiple organs of mice and rats in the comet assay. Mutat. Res 2002, 517, 53–75. [Google Scholar]
- Vaghef, H; Nygren, P; Edling, C; Bergh, J; Hellman, B. Alkaline single-cell gel electrophoresis and human biomonitoring for genotoxicity: A pilot study on breast cancer patients undergoing chemotherapy including cyclophosphamide. Mutat. Res 1997, 395, 127–138. [Google Scholar]
- Pfohl-Leszkowicz, A; Weber-Lotfi, F; Masfaraud, JF; Devaux, A; Laouedj, A; Guillemaut, P; Malaveille, C; Rether, B; Monod, G; Dirheimer, G. DNA adduct detection: Some applications in monitoring exposure to environmental genotoxic chemicals. IARC Sci. Publ 1993, 124, 373–378. [Google Scholar]
- Karuzina, II; Archakov, AI. The oxidative inactivation of cytochrome P450 in monooxygenase reactions. Free Radic. Biol. Med 1994, 16, 73–97. [Google Scholar]
- Freeman, BA. Oxygen: The air-borne nutrient that both sustains and threatens life. Nutrition 2000, 16, 478–480. [Google Scholar]
- Barreto, G; Madureira, D; Capani, F; Aon-Bertolino, L; Saraceno, E; Alvarez-Giraldez, LD. The role of catechols and free radicals in benzene toxicity: An oxidative DNA damage pathway. Environ. Mol. Mutagen 2009, 50, 771–780. [Google Scholar]
- Siems, WG; Pimenov, AM; Esterbauer, H; Grune, T. Metabolism of 4-hydroxynonenal, a cytotoxic lipid peroxidation product, in thymocytes as an effective secondary antioxidative defense mechanism. J. Biochem 1998, 123, 534–539. [Google Scholar]
- Esterbauer, H; Schaur, RJ; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med 1991, 11, 81–128. [Google Scholar]
- Bagchi, D; Bagchi, M; Hassoun, EA; Stohs, SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology 1995, 104, 129–140. [Google Scholar]
- Nath, RG; Ocando, JE; Chung, FL. Detection of 1, N2-propanodeoxyguanosine adducts as potential endogenous DNA lesions in rodent and human tissues. Cancer Res 1996, 56, 452–456. [Google Scholar]
- Wang, M; Dhingra, K; Hittelman, WN; Liehr, JG; de Andrade, M; Li, D. Lipid peroxidation-induced putative malondialdehyde-DNA adducts in human breast tissues. Cancer Epidemiol. Biomarkers Prev 1996, 5, 705–710. [Google Scholar]
- Perez-Maldonado, IN; Herrera, C; Batres, LE; Gonzalez-Amaro, R; Diaz-Barriga, F; Yanez, L. DDT-induced oxidative damage in human blood mononuclear cells. Environ. Res 2005, 98, 177–184. [Google Scholar]
- Olinski, R; Jurgowiak, M. The role of reactive oxygen species in mutagenesis and carcinogenesis processes. Postepy. Biochem 1999, 45, 50–58. [Google Scholar]
- Panayiotidis, M. Reactive oxygen species (ROS) in multistage carcinogenesis. Cancer Lett 2008, 266, 3–5. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Canales-Aguirre, A.; Padilla-Camberos, E.; Gómez-Pinedo, U.; Salado-Ponce, H.; Feria-Velasco, A.; De Celis, R. Genotoxic Effect of Chronic Exposure to DDT on Lymphocytes, Oral Mucosa and Breast Cells of Female Rats. Int. J. Environ. Res. Public Health 2011, 8, 540-553. https://doi.org/10.3390/ijerph8020540
Canales-Aguirre A, Padilla-Camberos E, Gómez-Pinedo U, Salado-Ponce H, Feria-Velasco A, De Celis R. Genotoxic Effect of Chronic Exposure to DDT on Lymphocytes, Oral Mucosa and Breast Cells of Female Rats. International Journal of Environmental Research and Public Health. 2011; 8(2):540-553. https://doi.org/10.3390/ijerph8020540
Chicago/Turabian StyleCanales-Aguirre, Alejandro, Eduardo Padilla-Camberos, Ulises Gómez-Pinedo, Hugo Salado-Ponce, Alfredo Feria-Velasco, and Ruth De Celis. 2011. "Genotoxic Effect of Chronic Exposure to DDT on Lymphocytes, Oral Mucosa and Breast Cells of Female Rats" International Journal of Environmental Research and Public Health 8, no. 2: 540-553. https://doi.org/10.3390/ijerph8020540