13C-NMR Assessment of the Pattern of Organic Matter Transformation during Domestic Wastewater Treatment by Autothermal Aerobic Digestion (ATAD)
Abstract
:1. Introduction
2. Material and Methods
2.1. Description of the Site and Sample Collection
2.2. Sample Preparation for NMR Analysis
2.3. Solid state Nuclear Magnetic Resonance Spectroscopy
2.3.1. NMR acquisition conditions and processing parameters
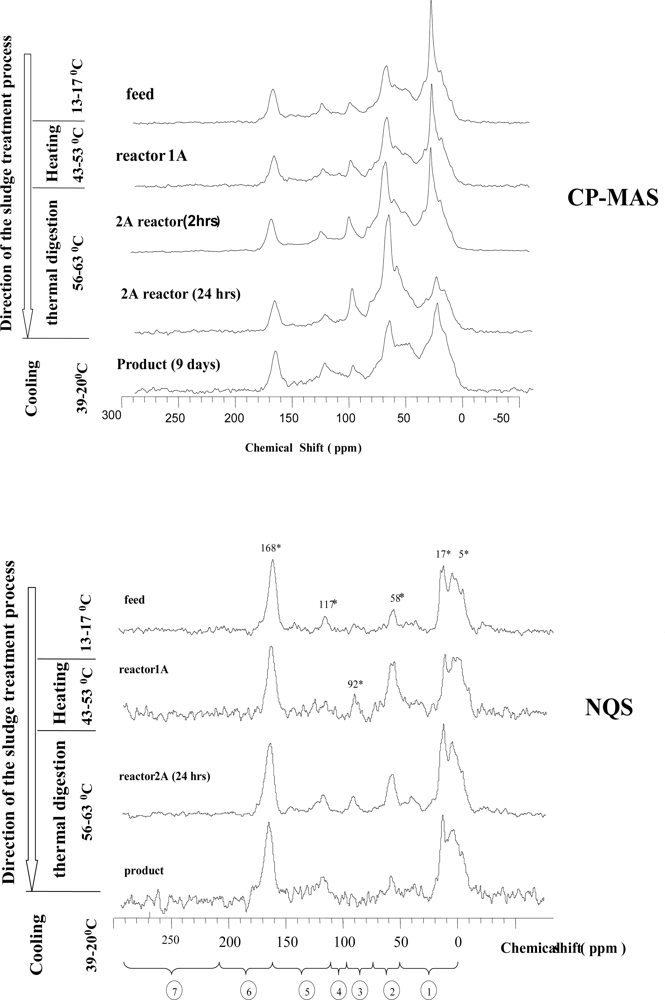
2.3.2. Spectral analysis and peaks assignment
2.4. Evaluation of the Specific Transformation of Different Types Changes in Different C-Domains during Different stages of the ATAD Process
2.5. Extent of the Decomposition Rate
3. Results and Discussion
3.1. Physico-Chemical Characteristics Obtained at Different Stage of the ATAD Process
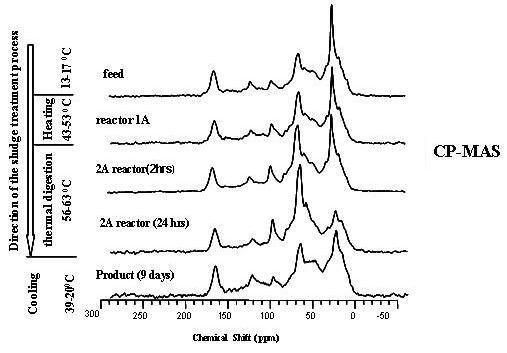
3.2. Spectra Characterisation and Peak Assignments
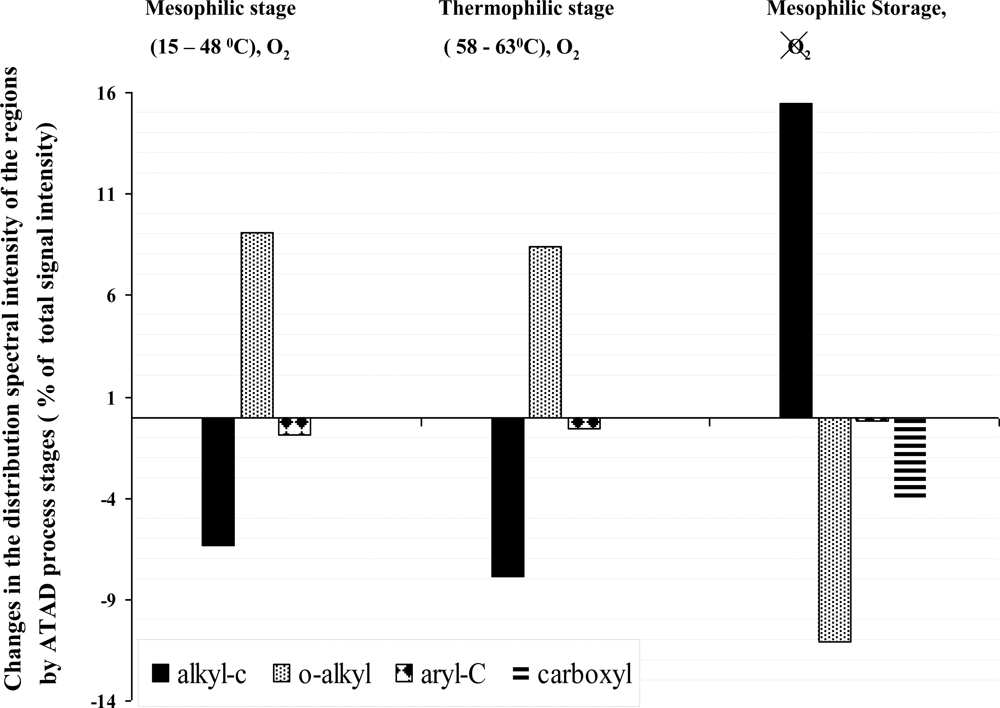
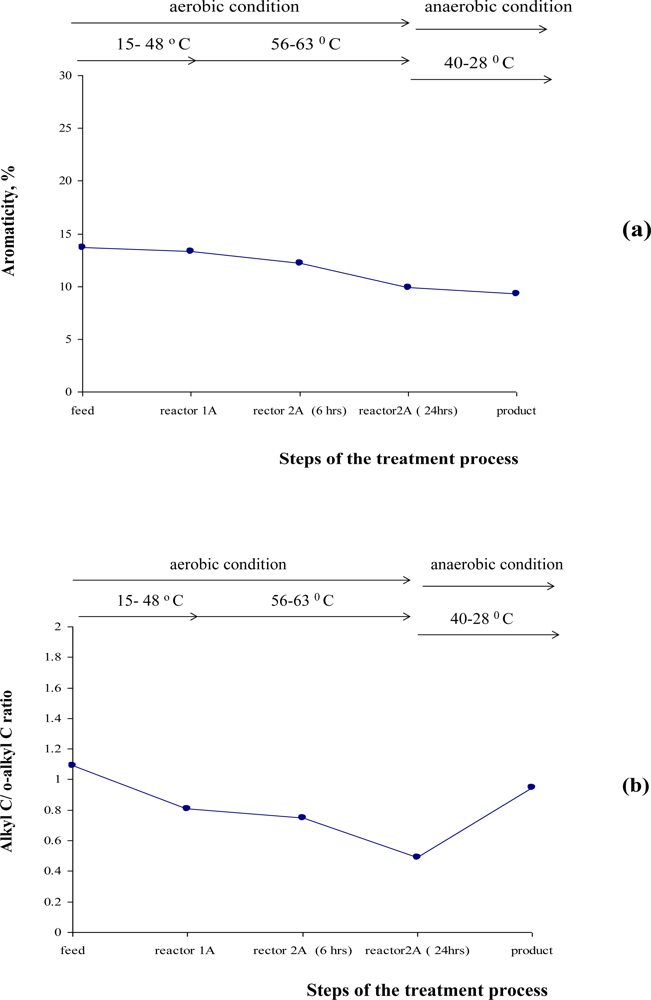
3.3. Evaluation of the Specific Transformation in Different Carbon Domains during the overall ATAD Process
3.4. Extent of the Decomposition Rate
4. Conclusions
Acknowledgments
References
- La Para, TM; Alleman, JE. Thermophilic aerobic biological wastewater treatment. Water Res 1999, 33, 895–908. [Google Scholar]
- Layden, N; Mavinic, D; Kelly, H; Moles, R; Bartlett, J. Autothermal thermophilic aerobic digestion (ATAD)–Part I: Review of origins, design, and process operation. J. Environ. Eng. Sci 2007, 6, 665–678. [Google Scholar]
- Tripathi, CS; Grant, AD. Comparison of mesophilic and thermophilic aerobic biological treatment in sequencing batch reactors treating bleached kraft pulp mill effluent. Water Res 1999, 33, 836–846. [Google Scholar]
- Heinonen-Tanski, H; Kiuru, T; Ruuskanen, J; Korhonen, K; Koivunen, J; Ruokojarvi, A. Thermophilic aeration of cattle slurry with whey and/or jam wastes. Bioresour. Technol 2005, 96, 247–252. [Google Scholar]
- Kelly, HG; Melcer, H; Mavinic, DS. Autothermal thermophilic aerobic digestion of municipal sludges: a one year full scale demonstration project. Water Res 1993, 27, 849–861. [Google Scholar]
- Kelly, HG. Emerging processes in biosolids treatment. J. Environ. Eng. Sci 2006, 5, 175–186. [Google Scholar]
- Piterina, AV; MacCausland, C; Bartlett, J; Pembroke, JT. Microbial ecology of autothermal aerobic digestion (ATAD): diversity, dynamics and activity of bacterial communities involved in treatment of municipal wastewater. In Modern Multidisciplinary Applied Microbiology, Exploiting Microbes and Their Interactions; Mendez-Vilas, A, Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 526–535. [Google Scholar]
- Li, JZ; Mavinic, DS; Kelly, HG. Pilot-scale operation of thermophilic aerobic digestion for volatile fatty acid production and distribution. J. Environ. Eng. Sci 2003, 2, 187–197. [Google Scholar]
- Mohaibes, M; Heinonen-Tanski, H. Aerobic thermophilic treatment of farm slurry and food wastes. Bioresour. Technol 2004, 95, 245–254. [Google Scholar]
- Zhou, J; Donald, SM; Harlan, GK; William, DR. Effects of temperatures and extracellular proteins on dewaterability of thermophilically digested biosolids. J. Environ. Eng. Sci 2002, 1, 409–414. [Google Scholar]
- Mikkelsen, LH; Gotfredsen, AK; Agerbk, ML; Nielsen, PH; Keiding, K. Effect of colloidal stability on clarification and dewatering of activated sludge. Water Sci. Technol 1996, 34, 449–457. [Google Scholar]
- Layden, N; Mavinic, D; Kelly, H; Moles, R; Bartlett, J. Autothermal thermophilic aerobic digestion (ATAD)–Part II: Review of research and full-scale operating experiences. J. Environ. Eng. Sci 2007, 6, 679–690. [Google Scholar]
- Bitton, A. Introduction to Wastewater Treatment. In Wastewater Microbiology; Bitton, G, Ed.; John Wiley & Sons: New York, NY, USA, 2005; pp. 5–50. [Google Scholar]
- Garcia, C; Hernandez, T; Costa, F. Changes in carbon fractions during composting and maturation of organic wastes. Environ. Mgmt 1991, 15, 433–439. [Google Scholar]
- Jackson, MJ; Line, MA. Organic composition of a pulp and paper mill sludge determined by FTIR, 13C-CPMAS NMR, and chemical extraction techniques. J. Agric. Food Chem 1997, 45, 2354–2358. [Google Scholar]
- Baldock, JA; Oades, JM; Nelson, PN; Skene, TM; Golchin, A; Clarke, P. Assessing the extent of decomposition of natural organic materials using solid-state 13C-NMR spectroscopy. Aust. J. Soil Res 1997, 35, 1061–1083. [Google Scholar]
- Hsu, J-H; Lo, S-L. Chemical and spectroscopic analysis of organic matter transformation during composting of pig manure. Environ. Pollut 1999, 104, 189–196. [Google Scholar]
- Pichler, M; Knicker, H; Kögel-Knabner, I. Changes in the chemical structure of municipal solid waste during composting as studied by solid-state dipolar dephasing and PSRE 13C-NMR and solid-state 15N NMR spectroscopy. Environ. Sci. Technol 2000, 34, 4034–4038. [Google Scholar]
- Almendros, G; Dorado, J; González-Vila, FJ; Blanco, MJ; Lankes, U. 13C-NMR assessment of decomposition patterns during composting of forest and shrub biomass. Soil Biol. Biochem 2000, 32, 793–804. [Google Scholar]
- Smernik, RJ; Oliver, IW; Merrington, W. Characterization of sewage sludge organic matter using solid-state carbon-13 nuclear magnetic resonance spectroscopy. J. Environ. Qual 2003, 32, 1516–1522. [Google Scholar]
- Smernik, J; Oliver, IW; Merrington, G. Advanced solid-state Carbon-13 nuclear magnetic resonance spectroscopic studies of sewage sludge organic matter: detection of organic domains. Environ. Qual 2003, 32, 1523–1533. [Google Scholar]
- Smernik, RJ; Oliver, IW; McLaughlin, MJ. Changes in the nature of sewage sludge organic matter during a twenty-one-month incubation. J. Environ. Qual 2004, 33, 1924–1929. [Google Scholar]
- Conte, P; Spaccini, R; Piccolo, A. State of the art of CPMAS 13C-NMR spectroscopy applied to natural organic matter. Prog. Nucl. Magn. Reson. Spectrosc 2004, 44, 215–223. [Google Scholar]
- de Alcântara, FA; Buurmanb, P; Curi, N; Furtini Netoa, AE; van Lagenb, B; Meijerb, EL. Changes in soil organic matter composition after introduction of riparian vegetation on shores of hydroelectric reservoirs (Southeast of Brazil). Soil Biol. Biochem 2004, 36, 1497–1508. [Google Scholar]
- Zbytniewski, R; Buszewski, B. Characterization of natural organic matter (NOM) derived from sewage sludge compost. Part 1: chemical and spectroscopic properties. Bioresour. Technol 2005, 96, 471–478. [Google Scholar]
- Tanga, L; Maieb, N; Tadab, Y; Katayamaa, A. Characterization of the maturing process of cattle manure compost. Process Biochem 2006, 41, 380–389. [Google Scholar]
- Bartoszeka, M; Polaka, J; Sułkowski, WW. NMR study of the humification process during sewage sludge treatment. Chemosphere 2008, 73, 1465–1470. [Google Scholar]
- de Groot, F; Kotani, F; Akio, S. Core Level Spectroscopy of Solids (Advances in Condensed Matter Science); CRS Press, Taylor and Francis Group: New York, NY, USA, 2008; pp. 1–100. [Google Scholar]
- Ramamoorthy, HU. NMR Spectroscopy of Biological Solids; Taylor & Francis: New York, NY, USA, 2006; pp. 5–13. [Google Scholar]
- Oetjen, GW; Haseley, P. Freeze-Drying, 2nd ed; Wiley-VCH: Weinheim, Germany, 2003; pp. 30–333. [Google Scholar]
- Preston, CM; Newman, RH. Demonstration of spatial heterogeneity in the organic matter of de-ashed humin samples by solid-state 13C CPMAS NMR. Can. J. Soil Sci 1992, 6, 13–19. [Google Scholar]
- Preston, CM. Carbon-13 solid-state NMR of soil organic matter—using the technique effectively. Can. J. Soil Sci 2001, 81, 255–270. [Google Scholar]
- Keelera, C; Kellyb, EF; Maciela, GE. Chemical-structural information from solid-state 13C NMR studies of a suite of humic materials from a lower montane forest soil, Colorado, USA. Geoderma 2006, 130, 124–140. [Google Scholar]
- Jerzykiewicz, M; Drozd, J; Jezierski, A. Organic radicals and paramagnetic metal complexes in municipal solid waste compost. An EPR and chemical study. Chemosphere 1999, 39, 253–268. [Google Scholar]
- Coward-Kelly, G; Agbogbo, FK; Holtzapple, MT. Lime treatment of keratinous material for the generation of highly digestible animal feed: 2. Animal hair. Bioresour Technol 2006, 97, 1344–1352. [Google Scholar]
- Grady, PL; Daigger, GT; Lim, HC. Biological wastewater treatment, 2nd ed; CRS Press Taylor & Francis: New York, NY, USA, 1998; pp. 572–573. [Google Scholar]
- Maillard, LC. Reaction of amino acids on sugars: melanoidin formation through a methodologic way. Comptes Rendus de l’Académie des Sci 1912, 156, 148–149. [Google Scholar]
- Veeken, AHM; Adanid, F; Nieropb, KGJ; de Jagerc, PA; Hamelersa, HV. Degradation of Biomacromolecules during High-Rate Composting of Wheat Straw–Amended Feces. J. Environ. Qual 2001, 30, 1675–1684. [Google Scholar]
- Leinweber, P; Blumenstein, O; Schulten, HR. Organic matter composition in sewage farm soils: Investigations by 13C-NMR and pyrolysis-field ionization mass spectrometry. Eur. J. Soil Sci 1996, 47, 71–80. [Google Scholar]
- Liu, Y; Fang, HHP. Influences of extracellular polymeric substances (EPS) on flocculation, settling, and dewatering of activated sludge. Crit. Rev. Env. Sci. Technol 2003, 33, 237–273. [Google Scholar]
- Jorand, F; Bouge-Bigne, F; Block, JC; Urbain, V. Hydrophobic/hydrophilic properties of activated sludge exopolymeric substances. Water Sci. Technol 1998, 37, 307–316. [Google Scholar]
- Kögel-Knabner, I. Analytical approaches for characterizing soil organic matter. Org. Geochem 2000, 31, 609–625. [Google Scholar]
- Golchin, A; Clarke, P; Oades, JM. The heterogeneous nature of microbial products as shown by solid-state 13C CP/MAS NMR spectroscopy. Biogeochemistry 1996, 34, 71–97. [Google Scholar]
- Bohm, N; Kulicke, WM. Optimization of the use of polyelectrolytes for dewatering industrial sludge of various origins. Colloid. Polym. Sci 1997, 275, 73–81. [Google Scholar]
| Organic domain | Chemical shift region (ppm) | |
|---|---|---|
| alkyl | Aliphatic carbons (–CH3, –CH2) (Biopolymers, lipid, proteins) | 0–55 |
| O-alkyl | Methoxyl (–OCH3) | 45–72 |
| C–O of carbohydrate-type compounds; C-2, C-3, C-4, C-5 and C-6 non-crystalline component of the cellulose | 72–97 | |
| Anomeric carbon of polysaccharides and C-1 of the cellulose or xylan or aromatic lignins) | 97–118 | |
| aryl | Unsubstituted and alkyl-substituted aromatic-C; Aromatic lignins | 110–140 |
| Lignins, phenols Aromatic ethers or amines moieties | 140–165 | |
| carboxyl | Carboxyl-C in aliphatics and aromatics groups; C - in amidic groups | 165–205 |
| Obtained samples | T,°C | pH | TS, % | VS, % | Color | Viable bacterial number (Live/Dead Viability assay) |
|---|---|---|---|---|---|---|
| Feed (primary +secondary sludge) | 11 | 6.3 | 6.3 | 82,3 | grey | (7.3 +0.4) × 106 |
| Reactor 1A | 43 | 7.0 | 5.8 | 56.2 | grey -brown | (8.2 +0.1) ×1011 |
| Reactor 2A (2hrs) | 53 | 8.1 | 5.1 | 59.1 | Brown | (4 + 0.2) × 10 8 |
| Reactor 2A (24 hrs)-Fresh biosolids | 63 | 9.1 | 4.2 | 43.1 | dark brown | (4.9+ 0.1) ×10 11 |
| Product-(9 day storage in holding tank) | 14 | 7.8 | 4.6 | 39.5 | grey-Brown | (6.2+ 0.3) ×106 |
| CP-MAS | |||||||
|---|---|---|---|---|---|---|---|
| Chemical shift regions (ppm) | |||||||
| 205-165 | 165-140 | 140-118 | 118-97 | 97-72 | 72-55 | 55-0 | |
| Carbon Distribution (%) | |||||||
| Feed | 8.9 | 4.4 | 6.6 | 6.8 | 17.4 | 14.0 | 41.9 |
| Reactor 1A | 8.1 | 4.1 | 6.7 | 7.9 | 20.4 | 14.3 | 38.5 |
| Reactor 2A (2hrs) | 7.0 | 3.7 | 6.4 | 8.5 | 23.4 | 15.4 | 35.6 |
| Reactor 2A (24hrs) | 7.0 | 3.8 | 5.7 | 9.7 | 29.2 | 16.8 | 27.7 |
| Product (9days) | 2.9 | 0.7 | 7.6 | 5.9 | 21.2 | 18.5 | 43.2 |
| NQS | |||||||
| Chemical shift regions (ppm) | |||||||
| 205-165 | 165-140 | 140-118 | 118-97 | 97-72 | 72-55 | 55-0 | |
| Carbon Distribution (%) | |||||||
| Feed | 26.6 | 3.5 | 5.0 | 2.7 | 7.5 | 5.4 | 49.4 |
| Reactor 1A | 18.6 | 3.0 | 6.8 | 3.9 | 10.9 | 6.3 | 50.4 |
| Reactor 2A (2hrs) | 19.7 | 3.9 | 5.8 | 4.7 | 11.9 | 6.4 | 47.6 |
| Reactor 2A (24hrs) | 21.6 | 3.7 | 3.2 | 2.6 | 17.8 | 7.9 | 43.2 |
| Product (9days) | 24.5 | 6.3 | 7.5 | 0.7 | 6.4 | 5.1 | 49.4 |
| Source of the material | Decomposition Indexes | Refs | ||
|---|---|---|---|---|
| Arom,% | A/O-Alkyl (b) | Hydr | ||
| Sludges and manures | ||||
| Pig faeces | 10 | 0,57 | 0,64 | [17] |
| Primary settling tank | 20.5 | – | – | [17] |
| Secondary treatment | 28.7 | – | – | [17] |
| Pulp industry | – | 0.44 | – | [15] |
| Raw Cattle manure | – | 0.27 | – | [38] |
| Composted Cattle manure | – | 0.56 | – | [38] |
| Composted mixture (faeces + straw) | 15 | 0.23 | 0.39 | [38] |
| Straw | 06 | 0.15 | 0.29 | [38] |
| ATAD | ||||
| Feed | 14 | 1.1 | 1.12 | This study |
| Fresh Biosolids | 10.4 | 0.48 | 0.62 | This study |
| Biosolids (9 days storage) | 9.7 | 0.9 | 0.48 | This study |
| Soil | ||||
| Farmed sludge soil | 13 | 0.29 | 0.40 | [19] |
| Planted forest litter | 11 | 0.35 | 0.31 | [39] |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Piterina, A.V.; Barlett, J.; Pembroke, J.T. 13C-NMR Assessment of the Pattern of Organic Matter Transformation during Domestic Wastewater Treatment by Autothermal Aerobic Digestion (ATAD). Int. J. Environ. Res. Public Health 2009, 6, 2288-2306. https://doi.org/10.3390/ijerph6082288
Piterina AV, Barlett J, Pembroke JT. 13C-NMR Assessment of the Pattern of Organic Matter Transformation during Domestic Wastewater Treatment by Autothermal Aerobic Digestion (ATAD). International Journal of Environmental Research and Public Health. 2009; 6(8):2288-2306. https://doi.org/10.3390/ijerph6082288
Chicago/Turabian StylePiterina, Anna V., John Barlett, and J.Tony Pembroke. 2009. "13C-NMR Assessment of the Pattern of Organic Matter Transformation during Domestic Wastewater Treatment by Autothermal Aerobic Digestion (ATAD)" International Journal of Environmental Research and Public Health 6, no. 8: 2288-2306. https://doi.org/10.3390/ijerph6082288