Health Risk and Biological Effects of Cardiac Ionising Imaging: From Epidemiology to Genes
Abstract
:1. Introduction
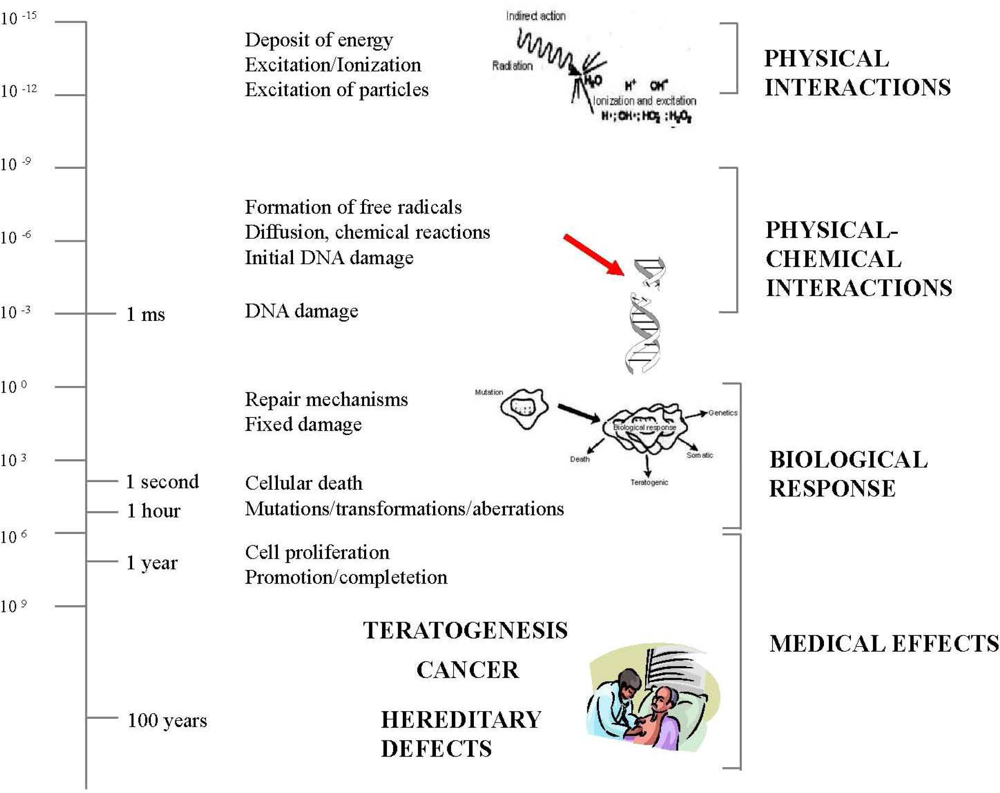
2. Biological Effects of Ionizing Radiation
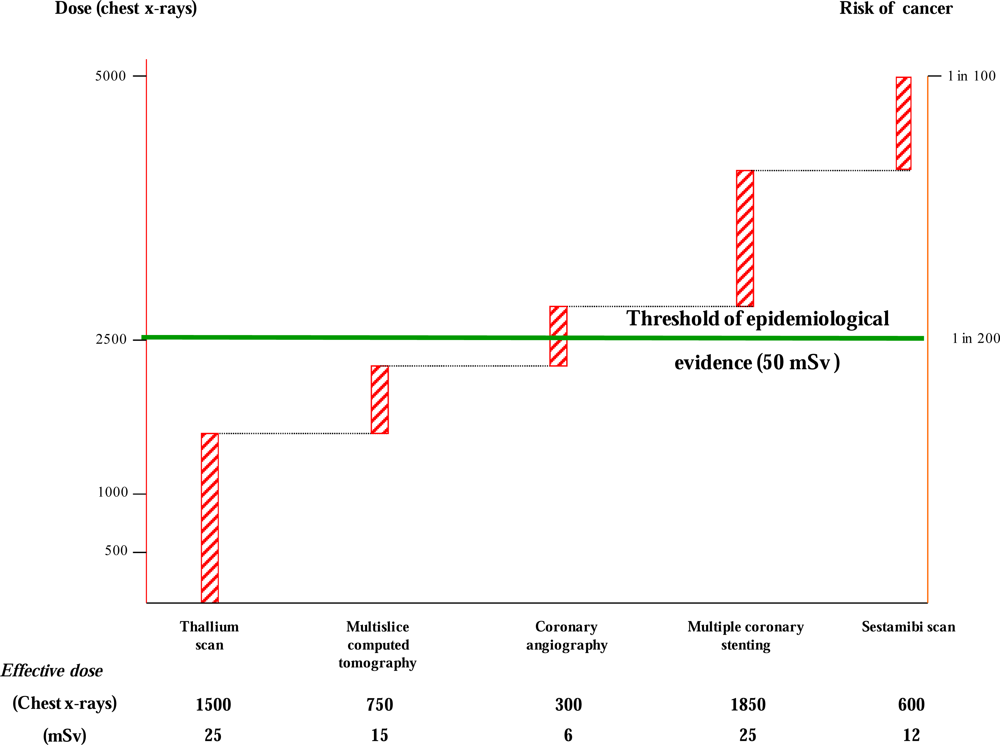
3. Clinical Risk of Medical Ionizing Radiation Exposure
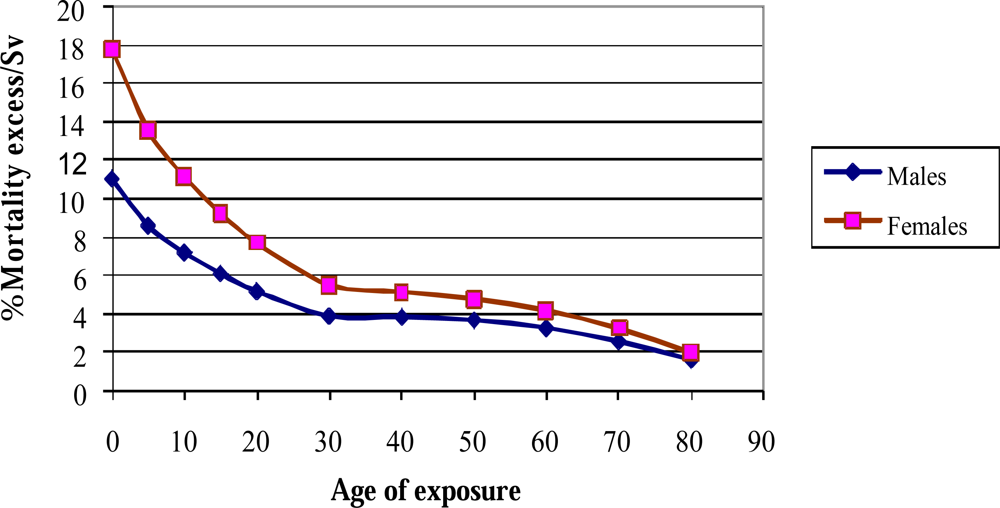
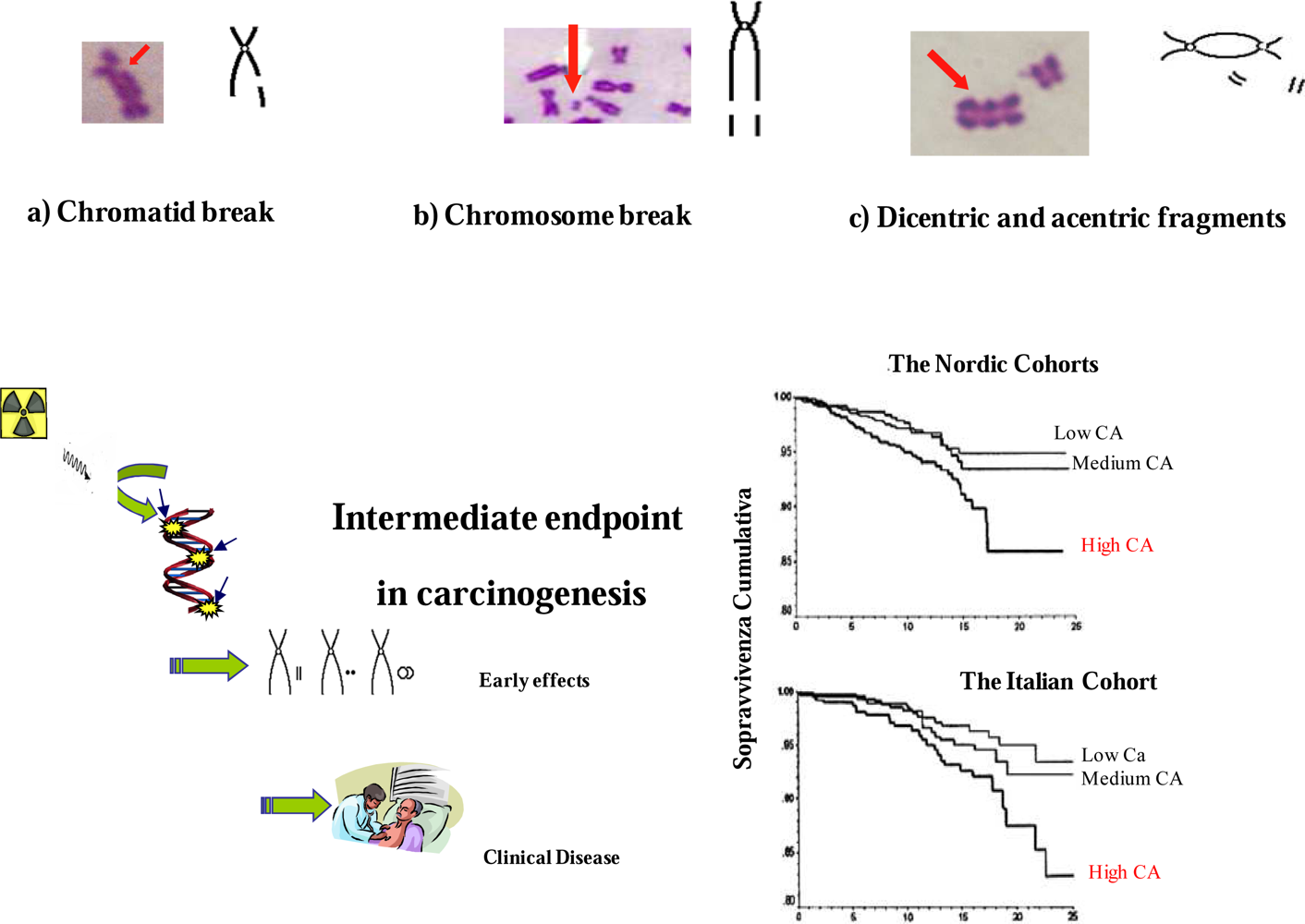
4. The Limitation of Risk Assessment from Epidemiological Studies
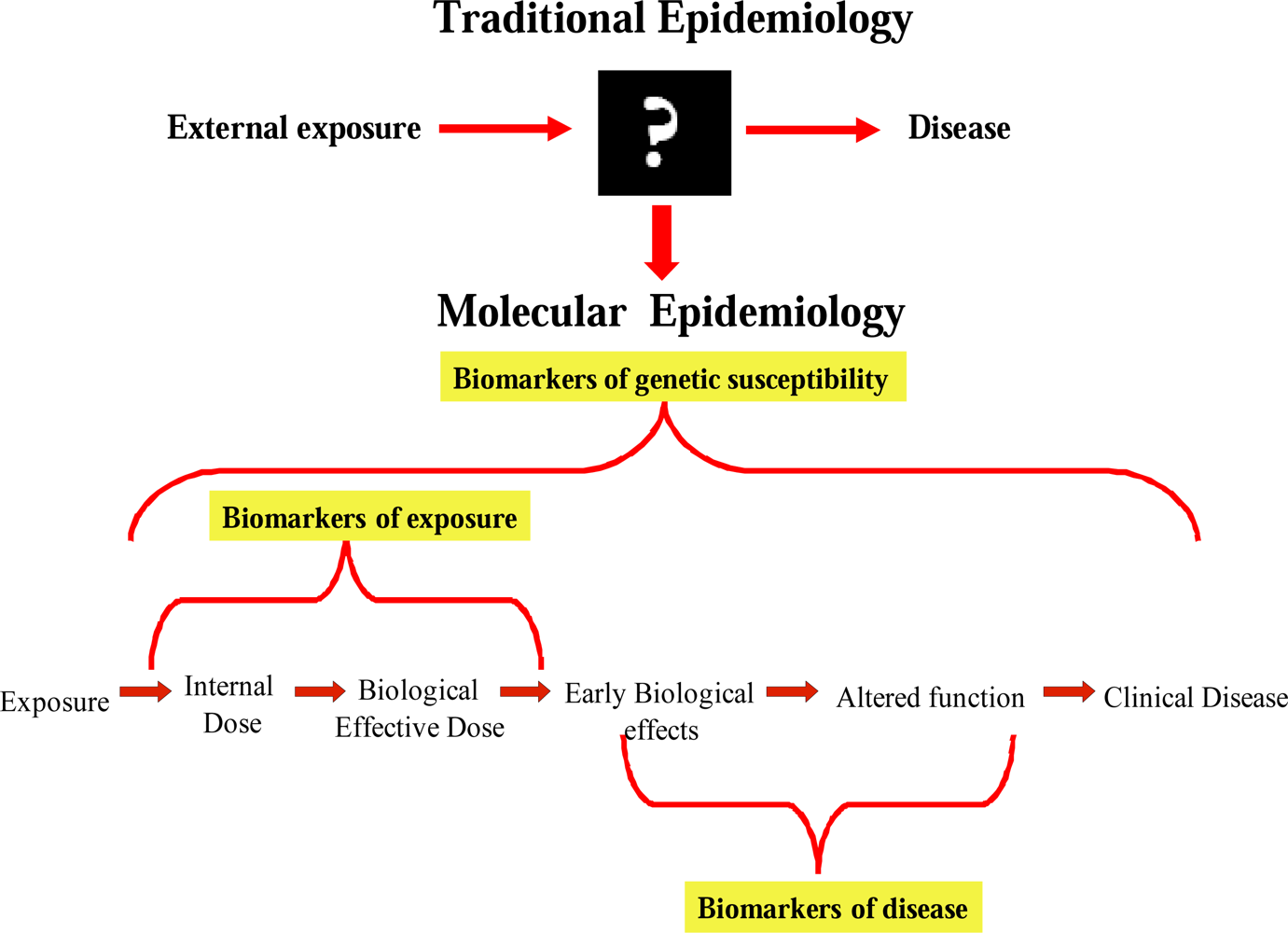
5. Biomarkers in the Assessment of Radiation Exposure Support BEIR VII Estimates
References
- Picano, E. Sustainability of medical imaging. Education and debate. BMJ 2004, 328, 578–580. [Google Scholar]
- Mettler, FA; Thomadsen, BR; Bhargavan, M; Gilley, DB; Gray, JE; Lipoti, JA; McCrohan, J; Yoshizumi, TT; Mahesh, M. Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys 2008, 95, 502–507. [Google Scholar]
- Amis, ES; Butler, PF; Applegate, KE; Birnbaum, SB; Brateman, LF; Hevezi, JM; Mettler, FA; Morin, RL; Pentecost, MJ; Smith, GG; Strauss, KJ; Zeman, RK. American College of Radiology white paper on radiation dose in medicine. J. Am. Coll. Radiol 2007, 4, 272–284. [Google Scholar]
- Picano, E; Vano, E; Semelka, R; Regulla, D. The American College of Radiology white paper on radiation dose in medicine: deep impact on the practice of cardiovascular imaging. Cardiovasc. Ultrasound 2007, 5, 37. [Google Scholar]
- Karatzis, EN; Danias, PG. Exposure to ionizing radiation from cardiovascular imaging and therapeutic procedures may be a considerable unrecognized risk for subsequent cancer. J. Am. Coll. Radiol 2008, 5, 694–695. [Google Scholar]
- Gerber, TC; Carr, JJ; Arai, AE; Dixon, RL; Ferrari, VA; Gomes, AS; Heller, GV; McCollough, CH; McNitt-Gray, MF; Mettler, FA; Mieres, JH; Morin, RL; Yester, MV. Ionizing radiation in cardiac imaging. A science advisory from the American Heart Association Committee on cardiac imaging of the council on clinical cardiology and committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention. Circulation 2009, 119, 1056–1065. [Google Scholar]
- Bedetti, G; Botto, N; Andreassi, MG; Traino, C; Vano, E; Picano, E. Cumulative patient effective dose in cardiology. Br. J. Radiol 2008, 81, 699–705. [Google Scholar]
- Hampton, T. Researchers examine long-term risks of exposure to medical radiation. JAMA 2006, 296, 913–920. [Google Scholar]
- Hall, EJ. Radiobiology for the Radiologist; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Report of the United States Nations Scientific Committee on the Effects of Atomic Radiation to the General Assembly Annex G: Biological effects at low radiation doses; UNSCEAR United Nations: New York, USA, 2001.
- National Radiological Protection Board. Documents of the National Radiological Protection Board 7; HMSO: London, UK, 1996. [Google Scholar]
- Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation; Nuclear and Radiation Studies Board, Division on Earth and Life Studies, National Research Council of the National Academies. In Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2; The National Academies Press: Washington, DC, USA, 2006.
- International Commission on Radiological Protection. Recommendations on the protection of man and the environment against ionising radiation.
- Vano, E; Gonzalez, L; Guibelalde, E; Aviles, P; Fernandez, JM; Prieto, C; Galvan, C. Evaluation of risk of deterministic effects in fluoroscopically guided procedures. Radiat. Prot. Dosim 2005, 117, 190–194. [Google Scholar]
- Vano, E; Gonzalez, L; Fernández, JM; Haskal, ZJ. Eye lens exposure to radiation in interventional suites: caution is warranted. Radiology 2008, 248, 945–953. [Google Scholar]
- Tubiana, M; Aurengo, A; Averbeck, D; Masse, R. The debate on the use of linear no threshold for assessing the effects of low doses. J. Radiol. Prot 2006, 26, 317–324. [Google Scholar]
- Feinendegen, LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br. J. Radiol 2005, 78, 3–7. [Google Scholar]
- Azzam, EI; Little, JB. The radiation-induced bystander effect: Evidence and significance. Hum. Exp. Toxicol 2004, 23, 61–65. [Google Scholar]
- Royal, HD. Effects of low level radiation—what’s new? Semin. Nucl. Med 2008, 38, 392–402. [Google Scholar]
- Brenner, DJ; Hall, EJ. Current concepts - Computed tomography - An increasing source of radiation exposure. N. Engl. J. Med 2007, 357, 2277–2284. [Google Scholar]
- Einstein, AJ; Henzlova, MJ; Rajagopalan, S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007, 298, 317–323. [Google Scholar]
- Brenner, DJ; Doll, R; Goodhead, DT; Hall, EJ; Land, CE; Little, JB; Lubin, JH; Preston, DL; Preston, RJ; Puskin, JS; Ron, E; Sachs, RK; Samet, JM; Setlow, RB; Zaider, M. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc. Natl. Acad. Sci. USA 2003, 25, 13761–13766. [Google Scholar]
- Andreassi, MG. The biological effects of diagnostic cardiac imaging on chronically exposed physicians: the importance of being non-ionizing. Cardiovasc. Ultrasound 2004, 2, 25. [Google Scholar]
- Neta, R. The promise of molecular epidemiology in defining the association between radiation and cancer. Health Phys 2000, 79, 77–84. [Google Scholar]
- Vineis, P; Perera, F. Molecular epidemiology and biomarkers in etiologic cancer research: the new in light of the old. Cancer Epidem. Biomarker. Prev 2007, 16, 1954–1965. [Google Scholar]
- Hagmar, L; Bonassi, S; Stromberg, U; Brogger, A; Knudsen, LS; Norppa, H; Reuterwall, C. Chromosomal aberrations in lymphocytes predict human cancer: a report from the European Study Group on Cytogenetic Biomarkers and Health (ESCH). Cancer Res 1998, 58, 4117–4121. [Google Scholar]
- Bonassi, S; Znaor, A; Ceppi, M; Lando, C; Chang, WP; Holland, N; Kirsch-Volders, M; Zeiger, E; Ban, S; Barale, R; Bigatti, MP; Bolognesi, C; Cebulska-Wasilewska, A; Fabianova, E; Fucic, A; Hagmar, L; Joksic, G; Martelli, A; Migliore, L; Mirkova, E; Scarfi, MR; Zijno, A; Norppa, H; Fenech, M. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis 2007, 28, 625–631. [Google Scholar]
- Andreassi, MG; Ait-Ali, L; Botto, N; Manfredi, S; Mottola, G; Picano, E. Cardiac catheterization and long-term chromosomal damage in children with congenital heart disease. Eur. Heart J 2006, 27, 2703–2708. [Google Scholar]
- Andreassi, MG; Cioppa, A; Manfredi, S; Palmieri, C; Botto, N; Picano, E. Acute chromosomal DNA damage in human lymphocytes after radiation exposure in invasive cardiovascular procedures. Eur. Heart J 2007, 28, 2195–2199. [Google Scholar]
- Andreassi, MG; Cioppa, A; Botto, N; Joksic, G; Manfredi, S; Federici, C; Ostojic, M; Rubino, P; Picano, E. Somatic DNA damage in interventional cardiologists: a case-control study. FASEB J 2005, 19, 998–999. [Google Scholar]
- Vano, E; Gonzalez, L; Fernandez, JM; Alfonso, F; Macaya, C. Occupational radiation doses in interventional cardiology: a 15-year follow-up. Br. J. Radiol 2006, 79, 383–388. [Google Scholar]
- Venneri, L; Rossi, F; Botto, N; Andreassi, MG; Salcone, N; Emah, A; Lazzeri, M; Gori, C; Vano, E; Picano, E. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am. Heart J 2009, 157, 118–124. [Google Scholar]
- Au, WW. Heritable susceptibility factors for the development of cancer. J. Radiat. Res 2006, 47, 13–17. [Google Scholar]
- Cornetta, T; Festa, F; Testa, A; Cozzi, R. DNA damage repair and genetic polymorphisms: assessment of individual sensitivity and repair capacity. Int. J. Radiat. Oncol. Biol. Phys 2006, 66, 537–545. [Google Scholar]
- Aka, P; Mateuca, R; Buchet, JP; Thierens, H; Kirsch-Volders, M. Are genetic polymorphisms in OGG1, XRCC1 and XRCC3 genes predictive for the DNA strand break repair phenotype and genotoxicity in workers exposed to low dose ionising radiations? Mutat. Res 2004, 556, 169–181. [Google Scholar]
- Andreassi, MG; Foffa, I; Manfredi, S; Botto, N; Coppa, A; Picano, E. Genetic polymorphisms in XRCC1, OGG1, APE1 and XRCC3 DNA repair genes, ionizing radiation exposure and chromosomal DNA damage in interventional cardiologists. Mutat. Res 2009, 666, 57–63. [Google Scholar]
- Andrieu, N; Easton, DF; Chang-Claude, J; Rookus, MA; Brohet, R; Cardis, E; Antoniou, AC; Wagner, T; Simard, J; Evans, G; Peock, S; Fricker, JP; Nogues, C; Van’t Veer, L; van Leeuwen, FE; Goldgar, DE. Effect of chestX-rays on the risk of breast cancer among BRCA1/2 mutation carriers in the international BRCA1/2 carrier cohort study: a report from the EMBRACE, GENEPSO, GEO-HEBON, and IBCCS collaborators’ group. J. Clin. Oncol 2006, 24, 3361–3366. [Google Scholar]
- Millikan, RC; Player, JS; Decotret, AR; Tse, CK; Keku, T. Polymorphisms in DNA repair genes, medical exposure to ionizing radiation, and breast cancer risk. Cancer Epidem. Biomarker. Prev 2005, 14, 2326–2334. [Google Scholar]
| Deterministic effects | Stochastic effects | |
|---|---|---|
| Dose | Medium-High | Low |
| Occurrence time | Short | Long |
| Threshold dose | Yes | No |
| Cell Biology | Cell Death | DNA damage |
| Clinical effects | Skin lesions, erythema, ulcers, epilation, cataracts, permanent sterility | Cancer, genetic effects |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Foffa, I.; Cresci, M.; Andreassi, M.G. Health Risk and Biological Effects of Cardiac Ionising Imaging: From Epidemiology to Genes. Int. J. Environ. Res. Public Health 2009, 6, 1882-1893. https://doi.org/10.3390/ijerph6061882
Foffa I, Cresci M, Andreassi MG. Health Risk and Biological Effects of Cardiac Ionising Imaging: From Epidemiology to Genes. International Journal of Environmental Research and Public Health. 2009; 6(6):1882-1893. https://doi.org/10.3390/ijerph6061882
Chicago/Turabian StyleFoffa, Ilenia, Monica Cresci, and Maria Grazia Andreassi. 2009. "Health Risk and Biological Effects of Cardiac Ionising Imaging: From Epidemiology to Genes" International Journal of Environmental Research and Public Health 6, no. 6: 1882-1893. https://doi.org/10.3390/ijerph6061882




