Analysis of Airborne Betula Pollen in Finland; a 31-Year Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Pollen Sampling Methods
2.3. Meteorological Data
2.4. Statistical Analyses
| N | total number of months; N = 372 (31 years × 12 months/year = 372) |
| i | month; i = 1, 2, 3, …, 372 |
| αi | monthly data for temperature or total precipitation or pollen count for the month i. |
| n | number of months being averaged; n = 12 |
2.5. Bonferroni Correction
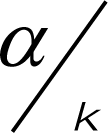 where α is the standard level of significance and k is the number of tests. In this study, we present the Bonferroni-adjusted significance levels where it is appropriate; however, all interpretations of results were presented using the standard significance level of 0.05.
where α is the standard level of significance and k is the number of tests. In this study, we present the Bonferroni-adjusted significance levels where it is appropriate; however, all interpretations of results were presented using the standard significance level of 0.05.3. Results
3.1. Meteorological Data
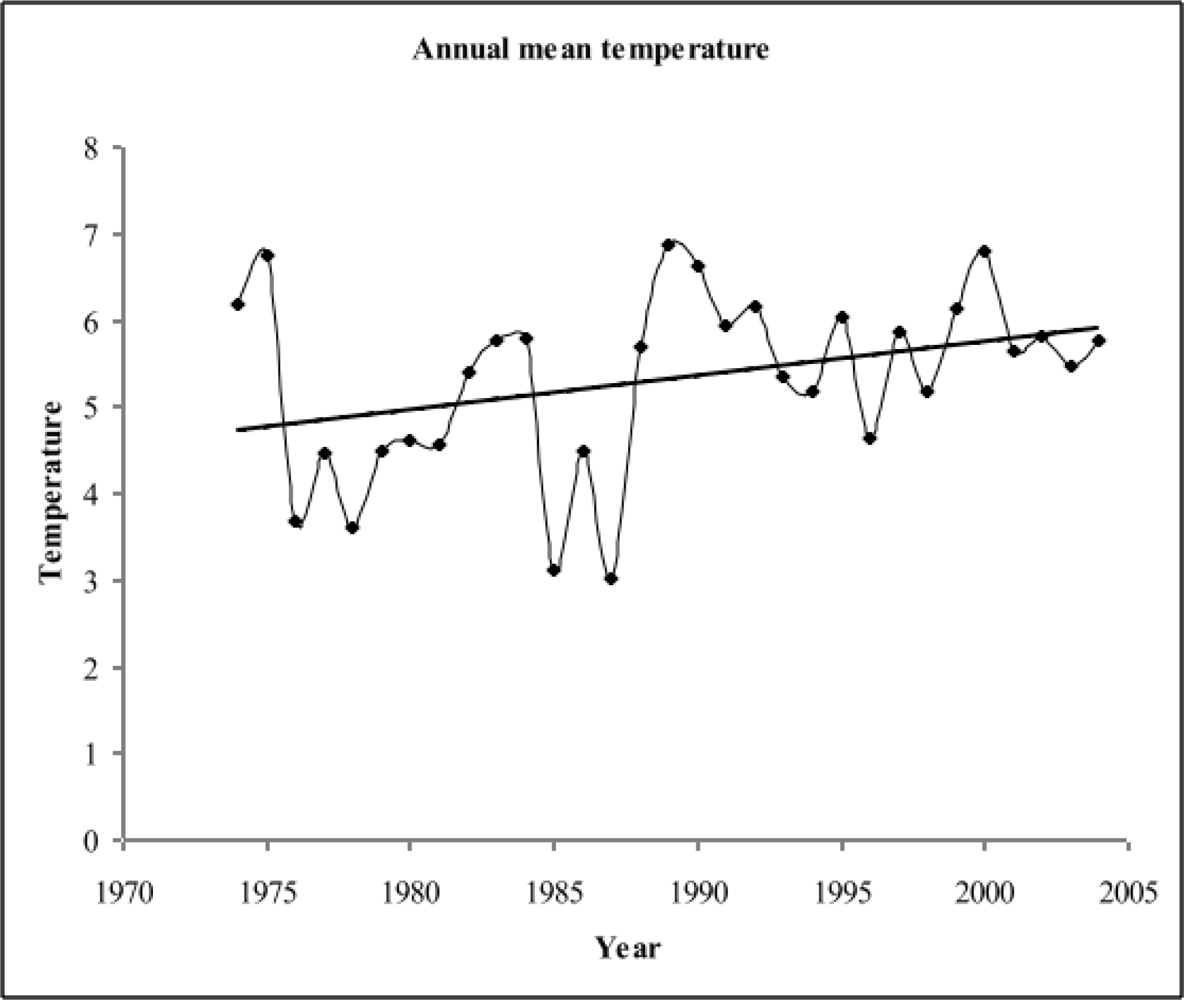
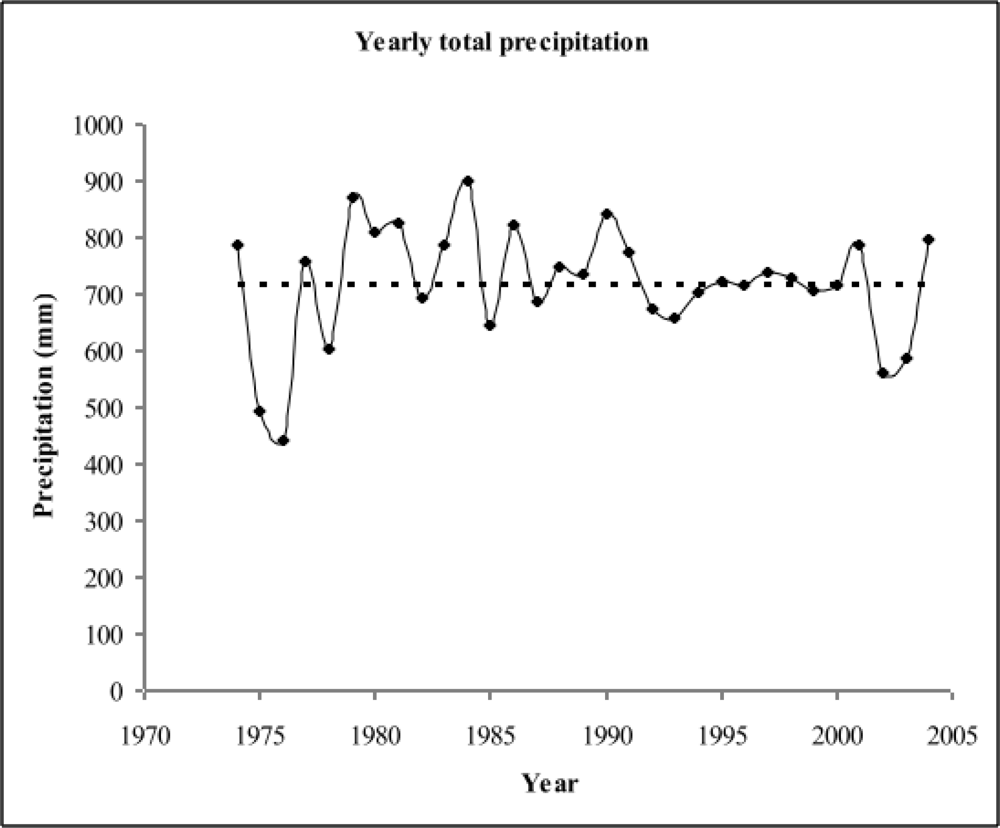
3.1.1. Long term trend in annual temperature and precipitation
3.1.2. Long term trend in monthly temperature and precipitation
3.2. Birch Pollen Data
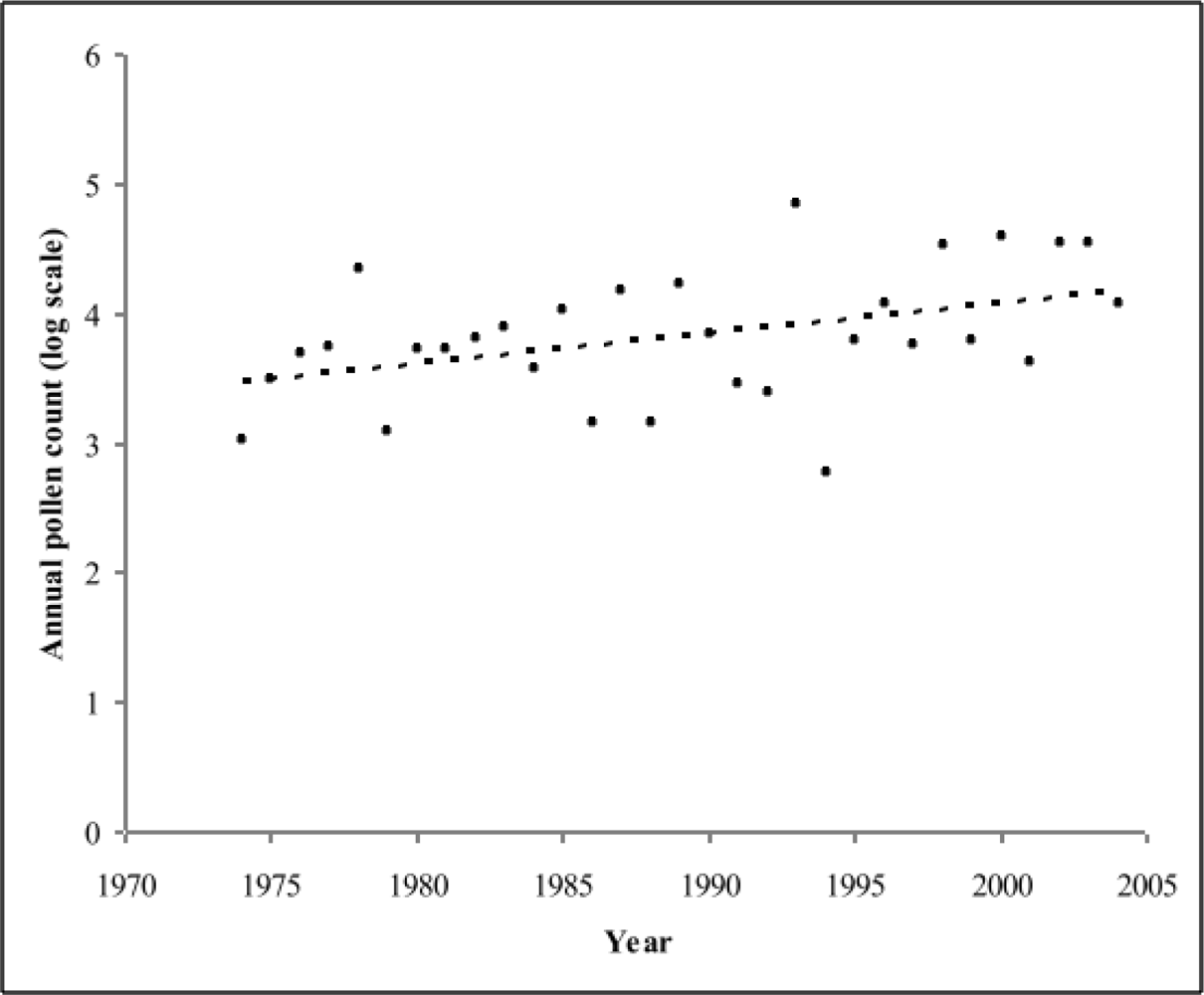
3.2.1. Trend in annual and seasonal pollen count
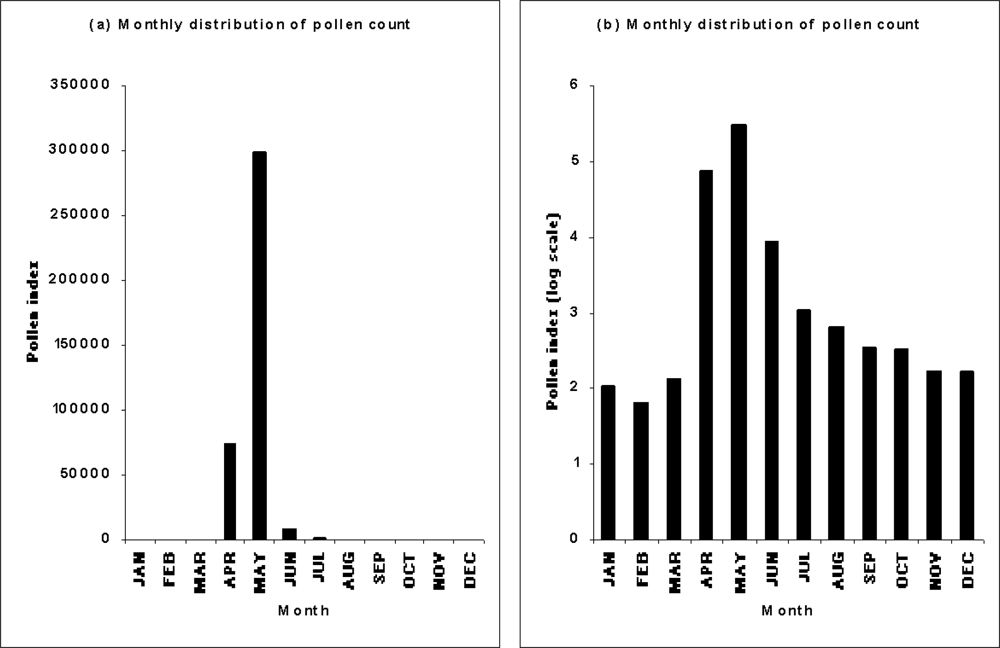
3.2.2. Behavior of pollen release and pollen transport
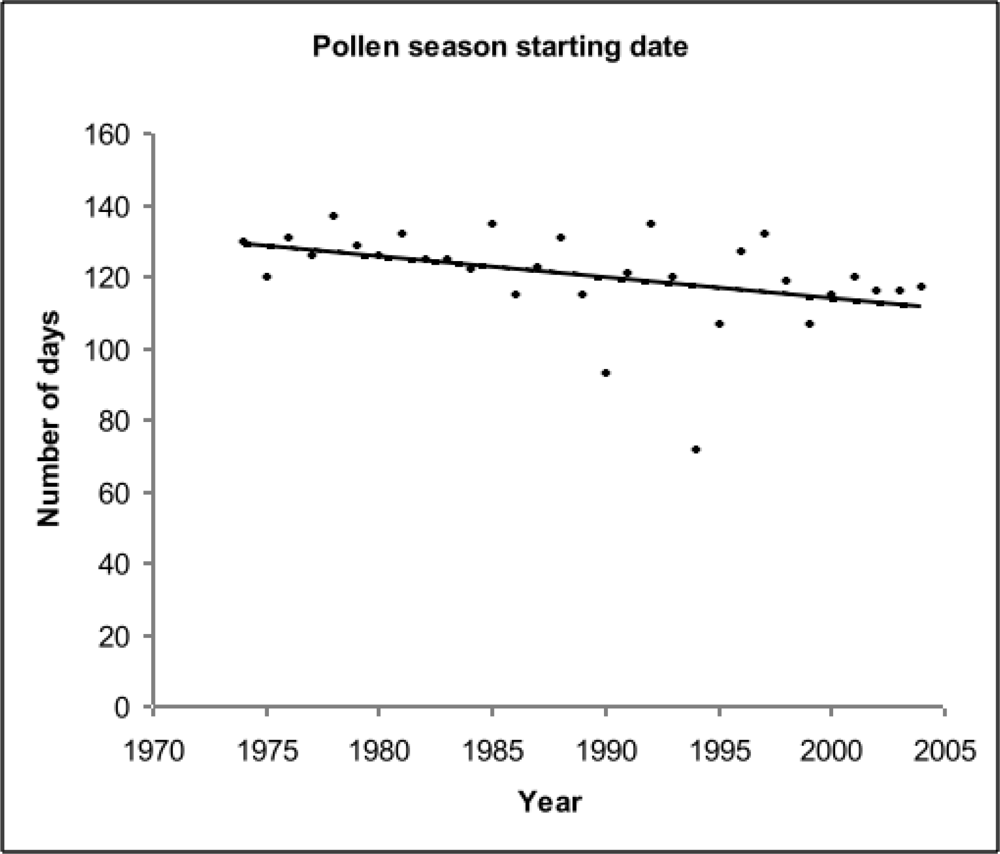
3.2.3. Birch pollen season
3.2.4. Associations between birch pollen season and meteorological parameters
3.2.5. Correlation of birch pollen concentration (annual, daily maximum, and date of daily maximum) with temperature and precipitation
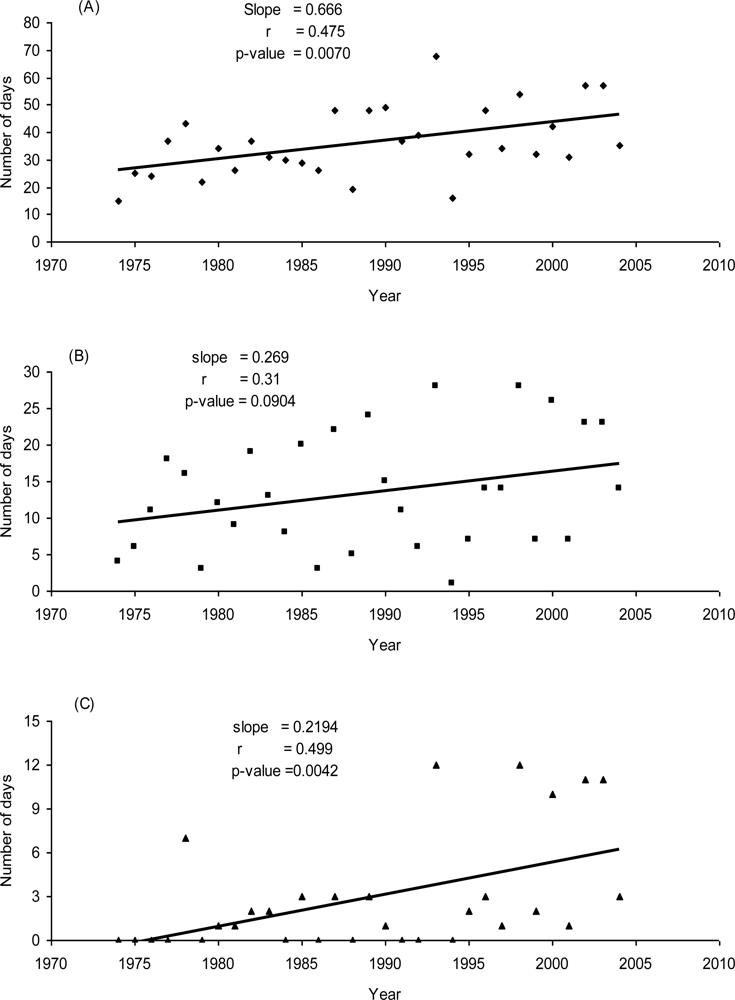
3.2.6. The number of days with daily birch pollen concentration exceeding 10, 100, and 1000 grains/m3 of air during seasons 1974–2004
4. Discussion
Acknowledgments
References
- Haahtela, T; von Hertzen, L; Mäkelä, M; Hannuksela, M. The Allergy Programme Working Group. Finnish Allergy Programme 2008–2018 - time to act and change the course. Allergy 2008, 63, 634–645. [Google Scholar]
- Viander, M; Koivikko, A. The seasonal symptoms of hyposensitized and untreated hay fever patients in relation to birch pollen counts: correlation with nasal sensitivity, prick test and RAST. Clin. Allergy 1978, 8, 387–396. [Google Scholar]
- World Health Organisation, Regional Office for Europe. Phenology and human health: allergic disorders: report on WHO meeting Rome, Italy 16–17 January 2003; WHO Regional Office for Europe: Copenhagen, Denmark, 2003. [Google Scholar]
- Aberg, N. Asthma and allergic rhinitis in Swedish conscripts. Clin. Exp. Allergy 1989, 19, 59–63. [Google Scholar]
- Eriksson, NE; Wihl, JA; Arrendal, H; Strandhede, SO. Tree pollen allergy. II. Sensitisation to various tree pollen allergens in Sweden. A multi-centre study. Allergy 1984, 36, 610–617. [Google Scholar]
- Haahtela, T; Lindholm, H; Bjorksten, F; Koskenvuo, K; Laitinen, LA. Prevalence of asthma in Finnish young men. Br. Med. J 1990, 301, 266–268. [Google Scholar]
- Moverare, R; Kosunen, TU; Haahtela, T. Change in the pattern of IgE reactivity to timothy grass and birch pollen allergens over a 20-year period. J. Inv. Allergol. Clin. Immunol 2006, 16, 274–278. [Google Scholar]
- Emberlin, J; Savage, M; Woodman, R. Annual variations in the concentrations of Betula pollen in the London area, 1961–1990. Grana 1993, 32, 359–363. [Google Scholar]
- Emberlin, J; Mullins, J; Corden, J; Millington, W; Brooke, M; Savage, M; Jones, S. The trend to earlier birch pollen seasons in the UK: Abiotic response to changes in weather conditions? Grana 1997, 36, 29–33. [Google Scholar]
- Hicks, S; Helander, M; Heino, S. Birch pollen production, transport and deposition for the period 1984–1993 at Kevo, northernmost Finland. Aerobiologia 1994, 10, 183–191. [Google Scholar]
- Ranta, H; Kubin, E; Siljamo, P; Sofiev, M; Linkosalo, T; Oksanen, A; Bondestam, K. Long-distance pollen transport cause problems for determining the timing of birch pollen season in Fennoscandia by using phenological observations. Grana 2006, 45, 297–304. [Google Scholar]
- Koenig, WD; Knops, JMH. Patterns of annual seed production by northern hemisphere trees: A global perspective. Amer. Naturalist 2000, 155, 59–69. [Google Scholar]
- Ranta, H; Oksanen, A; Hokkanen, T; Bondestam, K; Heino, S. Masting by Betula species; applying the resource budget model to North European data sets. Int. J. Biometeorol 2005, 49, 146–151. [Google Scholar]
- Linkosalo, T. Regularities and patterns in the spring phenology of some boreal trees. Silva Fenn 1999, 33, 237–245. [Google Scholar]
- Emberlin, J; Detandt, M; Gehrig, R; Jäger, S; Nolard, N; Rantio-Lehtimäki, A. Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperatures across Europe. Int. J. Biometeorol 2002, 46, 159–170. [Google Scholar]
- Emberlin, J; Laaidi, M; Detandt, M; Gehrig, R; Jäger, S; Myszkowska, D; Nolard, N; Rantio-Lehtimäki, A; Stach, A. Climate change and evolution of the pollen content of the air in seven European countries: The example of Birch. Rev. Fr. Allergol. Immunol. Clin 2007, 47, 57–63. [Google Scholar]
- British Aerobiology Federation. Airborne pollens and spores, a guide to trapping and counting; British Aerobiology Federation: Harpenden, UK, 1996. [Google Scholar]
- Mäkinen, Y. Random sampling in the study of microscopic slides. Rep. Aerobiol. Lab Univ. Turku 1981, 5, 27–43. [Google Scholar]
- Shirely, D; Stanley, W; Daniel, C. Statistics for research; John Wiley & Sons Inc: New York, USA, 2003. [Google Scholar]
- Nilsson, S; Persson, S. Tree pollen spectra in the Stockholm region (Sweden), 1973–1980. Grana 1981, 20, 179–182. [Google Scholar]
- Jäger, S; Spieksma, FTM; Nolard, N. Fluctuations and trends in airborne concentrations of some abundant pollen types, monitored at Vienna, Leiden and Brussels. Grana 1991, 30, 309–312. [Google Scholar]
- Spieksma, FTM; Emberlin, JC; Hjelmroos, M; Jäger, S; Leuschner, RM. Atmospheric birch (Betula) pollen in Europe: Trends and fluctuations in annual quantities and the starting dates of the seasons. Grana 1995, 34, 51–57. [Google Scholar]
- Frei, T. The effects of climate change in Switzerland 1969–1996 on airborne pollen quantities from hazel, birch and grass. Grana 1998, 37, 172–179. [Google Scholar]
- Rasmussen, AA. The effects of climate change on the birch pollen season in Denmark. Aerobiologia 2002, 18, 253–265. [Google Scholar]
- Mendez, J; Comtois, P; Iglesias, I. Betula pollen: One of the most important aeroallergens in Ourense, Spain. Aerobiological studies from 1993 to 2000. Aerobiologia 2005, 21, 115–123. [Google Scholar]
- Dahl, A; Strandhede, SO. Predicting the intensity of the birch pollen season. Aerobiologia 1996, 12, 97–106. [Google Scholar]
- Stach, A; Emberlin, J; Smith, M; Adams-Groom, B; Myszkowska, D. Factors that determine the severity of Betula spp. pollen seasons in Poland (Poznan and Krakow) and the United Kingdom (Worcester and London). Int. J. Biometeorol 2008, 52, 311–321. [Google Scholar]
- Sparks, T; Jeffree, EP; Jeffree, CE. An examination of the relationship between flowering times and temperature at the national scale using long-term phonological records from the UK. Int. J. Biometeorol 2000, 44, 82–87. [Google Scholar]
- Menzel, A. Phenological anomalies in Germany and their relation to air temperature and NAO. Clim. Change 2003, 57, 243–263. [Google Scholar]
- Root, TL; Price, JT; Hall, KR; Schneider, SH; Rosenzweig, C; Pounds, JA. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar]
- Crepinsek, Z; Kajfez-Bogataj, L; Bergant, K. Modelling of weather variability effect on phytophenology. Ecol. Model 2006, 194, 256–265. [Google Scholar]
- Hjelmroos, M. Evidence of long-distance transport of Betula pollen. Grana 1991, 30, 215–228. [Google Scholar]
- Estrella, N; Menzel, A; Krämer, U; Behrendt, H. Integration of flowering dates in phenology and pollen counts in aerobiology: analysis of their spatial and temporal coherence in Germany (1992–1999). Int. J. Biometeorol 2006, 51, 49–59. [Google Scholar]
- Corden, J; Millington, W; Bailey, J; Brookes, M; Caulton, E; Emberlin, J; Mullins, J; Simpson, C; Wood, AA. UK regional variations in Betula pollen. Aerobiologia 2000, 16, 227–232. [Google Scholar]
- Emberlin, J. Interaction between air pollutants and aeroallergens. Clin. Exp. Allergy 1995, 25, 33–39. [Google Scholar]
- Skjøth, CA; Geels, C; Hvidberg, M; Hertel, O; Brandt, J; Frohn, LM; Hansen, KM; Hedegård, GB; Christensen, J; Moseholm, L. An inventory of tree species in Europe - an essential data input for air pollution modelling. Ecol Model 2008. [Google Scholar]
- Björksten, B; Dumitrascu, D; Foucard, T; Khetsuriani, N; Khaitov, R; Leja, M; Lis, G; Pekkanen, J; Priftanji, A; Riikjarv, MA. Prevalence of childhood asthma, rhinitis and eczema in Scandinavia and Eastern Europe. Eur. Respir. J 1998, 12, 432–437. [Google Scholar]
- Latvala, J; von Hertzen, L; Lindholm, H; Haahtela, T. Trends in prevalence of asthma and allergy in Finnish young men: a nationwide study from 1966 to 2003. Br. Med. J 2005, 330, 1186–1187. [Google Scholar]
- Von Mutius, E; Martinez, FD; Fritzsch, C; Nicolai, T; Roell, G; Thiemann, HH. Prevalence of asthma and atopy in two areas of west and East Germany. Am. J. Respir. Crit. Care Med 1994, 149, 358–364. [Google Scholar]
- Rimpelä, A; Savonius, B; Rimpelä, M; Haahtela, T. Asthma and allergic rhinitis among Finnish adolescents in 1977–1991. Scand. J. Soc. Med 1995, 23, 60–65. [Google Scholar]
- Von Hertzen, LC; Mäkelä, MJ; Petäys, T; Jousilahti, P; Kosunen, TU; Laatikainen, T; Vartiainen, E; Haahtela, T. Growing disparities in atopy between the Finns and the Russians - a comparison of two generations. J. Allergy Clin. Immunol 2006, 117, 151–157. [Google Scholar]
- Björksten, F; Suoniemi, I; Koski, V. Neonatal birch-pollen contact and subsequent allergy to birch pollen. Clin. Allergy 1980, 10, 585–591. [Google Scholar]
- Kihlström, A; Lilja, G; Pershagen, G; Hedlin, G. Exposure to high doses birch pollen during pregnancy, and risk of sensitization and atopic disease in the child. Allergy 2003, 58, 871–877. [Google Scholar]
- Kihlström, A; Lilja, G; Pershagen, G. Exposure to birch pollen in infancy and development of atopic disease in childhood. J. Allergy Clin. Immunol 2002, 110, 78–84. [Google Scholar]
- Björksten, F; Suoniemi, I. Time and intensity of first pollen contacts and risk of subsequent pollen allergies. Acta Med. Scand 1981, 209, 299–303. [Google Scholar]
- Karachaliou, FH; Panagiotopoulou, K; Manousakis, M; Sinaniotis, K; Papageorgiou, F. Month of birth, atopic disease, and sensitisation to common aeroallergens in Greece. Ped. Allergy Immunol 1995, 6, 216–219. [Google Scholar]
| Temperature | Precipitation | |||||
|---|---|---|---|---|---|---|
| Month | Slope | Coefficient of determination (R2) | p-value | Slope | Coefficient of determination (R2) | p-value |
| Jan | 0.0673 | 0.023 | 0.4159 | 0.4188 | 0.020 | 0.4429 |
| Feb | 0.0741 | 0.028 | 0.3711 | 0.1385 | 0.001 | 0.8713 |
| Mar | 0.0652 | 0.080 | 0.1221 | 0.3700 | 0.032 | 0.3379 |
| Apr | 0.0804 | 0.203 | 0.0109* | −0.0545 | 0.001 | 0.8917 |
| May | −0.0094 | 0.003 | 0.7750 | −0.0799 | 0.001 | 0.8782 |
| Jun | 0.0074 | 0.001 | 0.8403 | 0.6473 | 0.043 | 0.2637 |
| Jul | 0.0873 | 0.230 | 0.0063** | −0.0716 | 0.0002 | 0.9346 |
| Aug | 0.0706 | 0.163 | 0.0242* | −0.1536 | 0.001 | 0.8491 |
| Sep | 0.0401 | 0.053 | 0.2147 | −0.8842 | 0.040 | 0.2790 |
| Oct | 0.0101 | 0.002 | 0.8060 | 0.0119 | 0.00001 | 0.9862 |
| Nov | −0.0478 | 0.039 | 0.2872 | −0.7388 | 0.024 | 0.4104 |
| Dec | 0.0381 | 0.013 | 0.5445 | 0.4399 | 0.014 | 0.5242 |
| Annual1 | 0.0399 | 0.123 | 0.0533 | 0.0439 | 0.00001 | 0.9836 |
| Year | Annual cumulative pollen (grains) | Daily maximum(grain/m3) | Number of days to daily maximum | Month | Day |
|---|---|---|---|---|---|
| 1974 | 1065 | 231 | 137 | 5 | 17 |
| 1975 | 3119 | 646 | 133 | 5 | 13 |
| 1976 | 4931 | 798 | 136 | 5 | 15 |
| 1977 | 5574 | 679 | 140 | 5 | 20 |
| 1978 | 22194 | 5859 | 141 | 5 | 21 |
| 1979 | 1235 | 126 | 141 | 5 | 21 |
| 1980 | 5379 | 1004 | 129 | 5 | 8 |
| 1981 | 5413 | 1383 | 136 | 5 | 16 |
| 1982 | 6493 | 1683 | 128 | 5 | 8 |
| 1983 | 7956 | 1579 | 128 | 5 | 8 |
| 1984 | 3804 | 658 | 136 | 5 | 15 |
| 1985 | 10823 | 1933 | 139 | 5 | 19 |
| 1986 | 1454 | 194 | 118 | 4 | 28 |
| 1987 | 15291 | 3910 | 137 | 5 | 17 |
| 1988 | 1459 | 252 | 133 | 5 | 12 |
| 1989 | 17042 | 6321 | 117 | 4 | 27 |
| 1990 | 7078 | 1550 | 114 | 4 | 24 |
| 1991 | 2883 | 269 | 151 | 5 | 31 |
| 1992 | 2512 | 595 | 143 | 5 | 22 |
| 1993 | 70445 | 16291 | 122 | 5 | 2 |
| 1994 | 590 | 103 | 127 | 5 | 7 |
| 1995 | 6125 | 2208 | 111 | 4 | 21 |
| 1996 | 11924 | 3581 | 133 | 5 | 12 |
| 1997 | 5708 | 1207 | 135 | 5 | 15 |
| 1998 | 33933 | 5737 | 123 | 5 | 3 |
| 1999 | 6125 | 2208 | 111 | 4 | 21 |
| 2000 | 38713 | 8832 | 120 | 4 | 29 |
| 2001 | 4181 | 1659 | 121 | 5 | 1 |
| 2002 | 35215 | 5526 | 123 | 5 | 3 |
| 2003 | 35209 | 5526 | 123 | 5 | 3 |
| 2004 | 11897 | 1670 | 121 | 4 | 30 |
| Slope of regression line | 0.0533 | 0.0604 | −0.0046 | ||
| Correlation coefficient | 0.417 | 0.424 | −0.536 | ||
| p-value | 0.0197 | 0.0175 | 0.0019 |
| Monthly | Slope | Coefficient of determination (R2) | p-value |
|---|---|---|---|
| Apr | 0.1021 | 0.429 | < 0.0001*** |
| May | 0.0121 | 0.035 | 0.3123 |
| Jun | 0.0010 | 0.0004 | 0.9196 |
| Jul | 0.0134 | 0.017 | 0.2297 |
| Temperature | Precipitation | ||||||
|---|---|---|---|---|---|---|---|
| Month | Starting date | Ending date | Duration | Starting date | Ending date | Duration | |
| Prior Year | Jun | 0.10 | −0.45* | −0.40* | −0.14 | 0.23 | 0.25 |
| Jul | −0.26 | −0.29 | −0.06 | 0.12 | 0.02 | −0.11 | |
| Aug | 0.16 | −0.15 | −0.15 | −0.21 | 0.17 | 0.22 | |
| Sep | 0.08 | −0.63*** | −0.47** | 0.31 | 0.00 | 0.08 | |
| Oct | 0.16 | 0.08 | 0.05 | 0.06 | −0.19 | −0.29 | |
| Nov | 0.40* | −0.04 | −0.19 | 0.42* | −0.03 | −0.24 | |
| Dec | −0.04 | −0.01 | −0.05 | −0.37* | −0.02 | 0.15 | |
| Current Year | Jan | −0.17 | 0.01 | 0.11 | −0.42* | −0.14 | 0.17 |
| Feb | −0.01 | −0.36* | −0.08 | 0.02 | −0.19 | −0.04 | |
| Mar | −0.24 | −0.12 | 0.18 | −0.53** | 0.35 | 0.39* | |
| Apr | −0.59*** | −0.29 | 0.09 | −0.21 | 0.24 | 0.36* | |
| May | 0.14 | −0.18 | −0.26 | 0.04 | −0.10 | −0.02 | |
| FMA1 | −0.25 | −0.36* | 0.04 | −0.25 | 0.06 | 0.24 | |
| FMAM2 | −0.19 | −0.37* | −0.03 | −0.22 | 0.02 | 0.22 | |
| Annual3 | −0.31 | −0.19 | 0.15 | −0.08 | 0.02 | 0.15 | |
| Temperature | Precipitation | ||||||
|---|---|---|---|---|---|---|---|
| Month | Annual | Daily maximum | Number of days to daily maximum | Annual | Daily maximum | Number of days to daily maximum | |
| Prior year | Jun | 0.60** | 0.58*** | −0.11 | −0.25 | −0.17 | −0.05 |
| Jul | 0.40* | 0.38* | −0.49** | 0.15 | 0.10 | 0.21 | |
| Aug | 0.36 | 0.27 | −0.05 | −0.22 | −0.16 | −0.08 | |
| Sep | 0.45* | 0.47** | −0.45* | 0.02 | −0.03 | 0.29 | |
| Oct | −0.26 | −0.15 | 0.09 | 0.20 | 0.31 | −0.03 | |
| Nov | −0.05 | −0.05 | 0.29 | 0.05 | 0.09 | 0.23 | |
| Dec | 0.01 | 0.09 | −0.07 | −0.18 | −0.12 | −0.25 | |
| Current year | Jan | −0.07 | −0.01 | −0.21 | −0.02 | −0.09 | −0.25 |
| Feb | 0.21 | 0.25 | −0.32 | −0.17 | −0.16 | −0.08 | |
| Mar | 0.04 | 0.04 | −0.33 | −0.11 | −0.03 | −0.21 | |
| Apr | 0.20 | 0.24 | −0.61*** | −0.26 | −0.22 | −0.17 | |
| May | 0.22 | 0.18 | −0.07 | 0.04 | 0.02 | 0.00 | |
| FMA1 | 0.20 | 0.24 | −0.48** | −0.27 | −0.22 | −0.20 | |
| FMAM2 | 0.24 | 0.27 | −0.46** | −0.24 | −0.21 | −0.19 | |
| Annual3 | −0.04 | 0.02 | −0.43* | −0.33 | −0.33 | −0.06 | |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yli-Panula, E.; Fekedulegn, D.B.; Green, B.J.; Ranta, H. Analysis of Airborne Betula Pollen in Finland; a 31-Year Perspective. Int. J. Environ. Res. Public Health 2009, 6, 1706-1723. https://doi.org/10.3390/ijerph6061706
Yli-Panula E, Fekedulegn DB, Green BJ, Ranta H. Analysis of Airborne Betula Pollen in Finland; a 31-Year Perspective. International Journal of Environmental Research and Public Health. 2009; 6(6):1706-1723. https://doi.org/10.3390/ijerph6061706
Chicago/Turabian StyleYli-Panula, Eija, Desta Bey Fekedulegn, Brett James Green, and Hanna Ranta. 2009. "Analysis of Airborne Betula Pollen in Finland; a 31-Year Perspective" International Journal of Environmental Research and Public Health 6, no. 6: 1706-1723. https://doi.org/10.3390/ijerph6061706





