Microstructures and Nanostructures for Environmental Carbon Nanotubes and Nanoparticulate Soots
Abstract
:Introduction
Experimental Methodologies
Results and Discussion
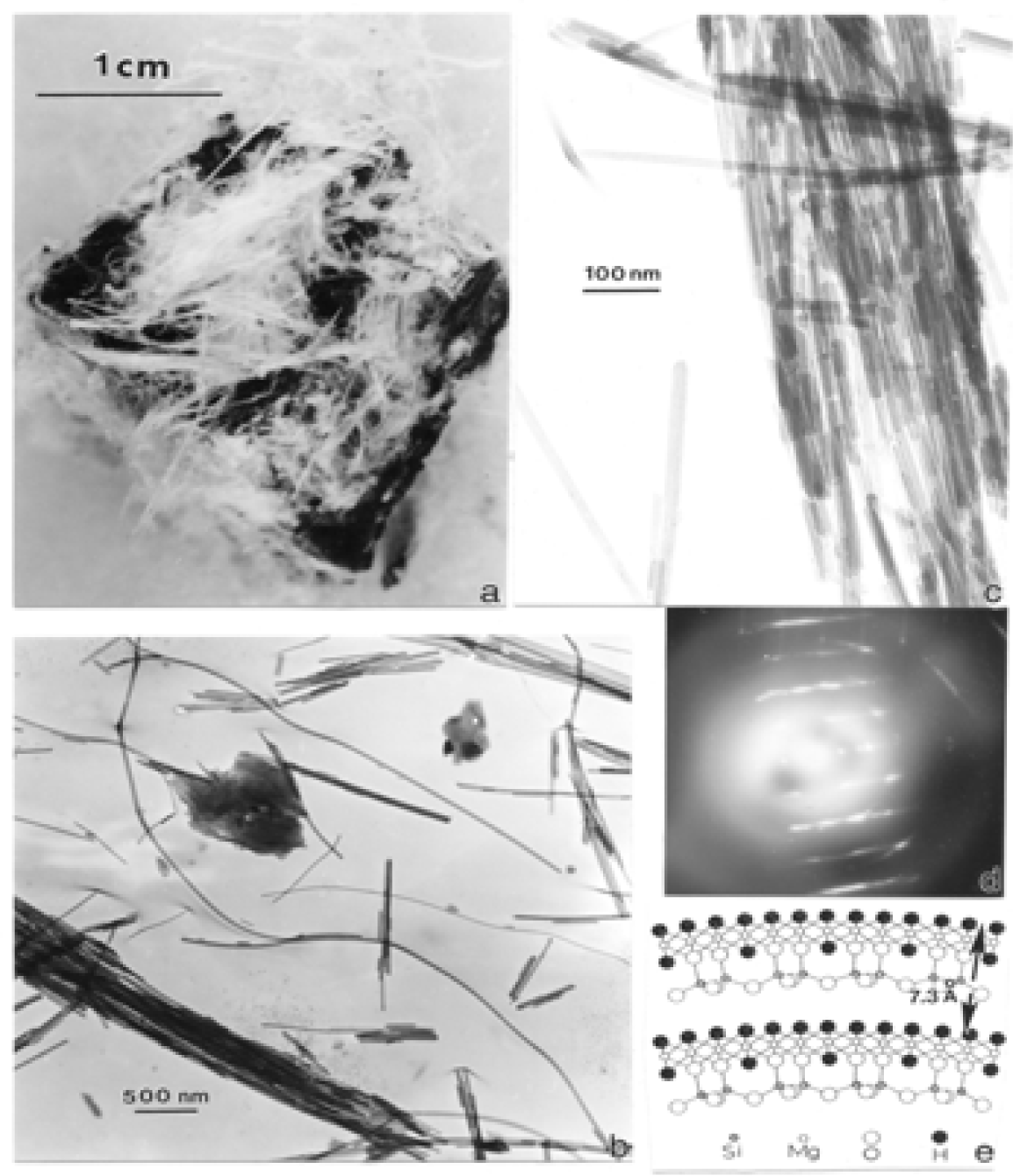
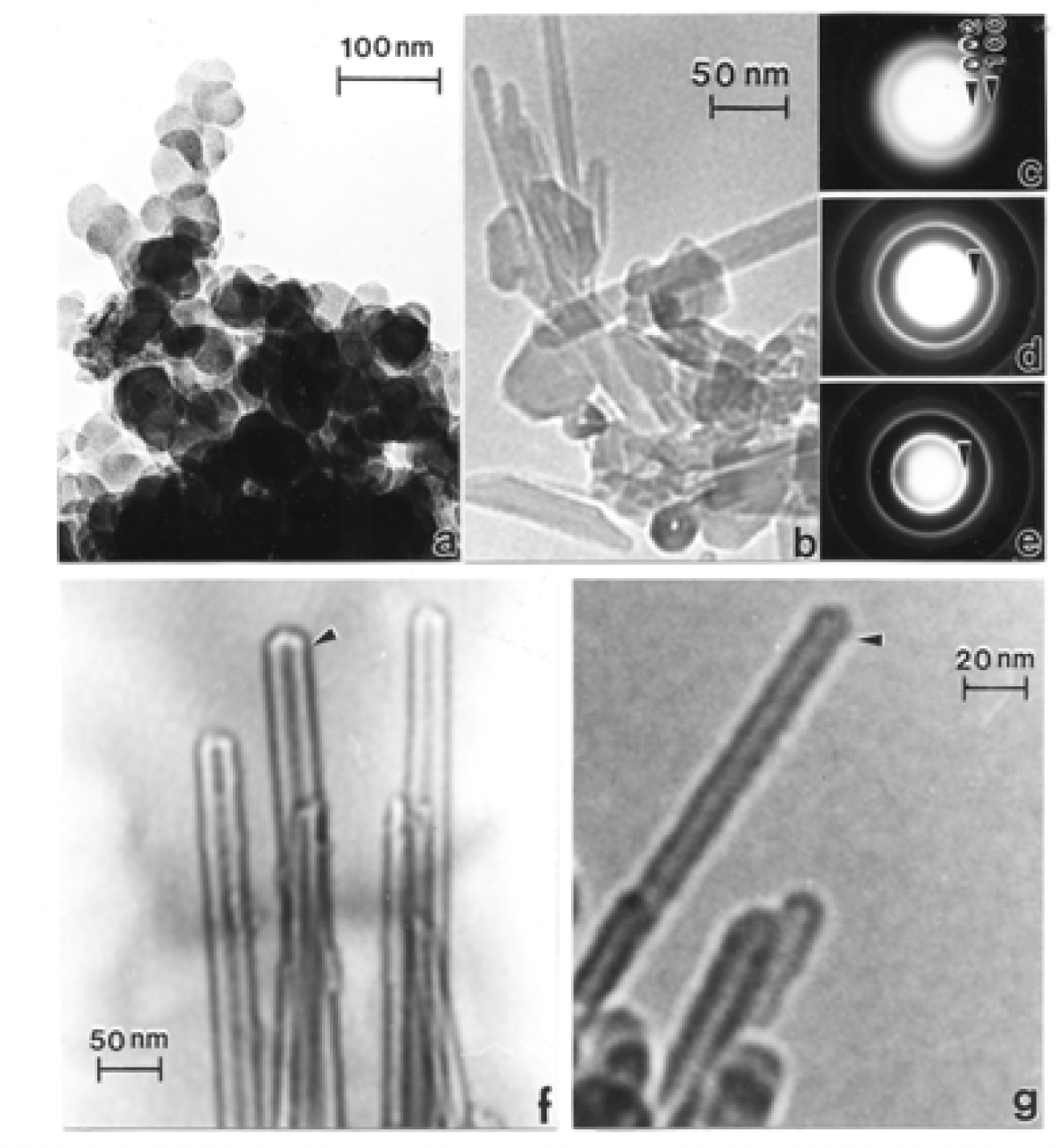
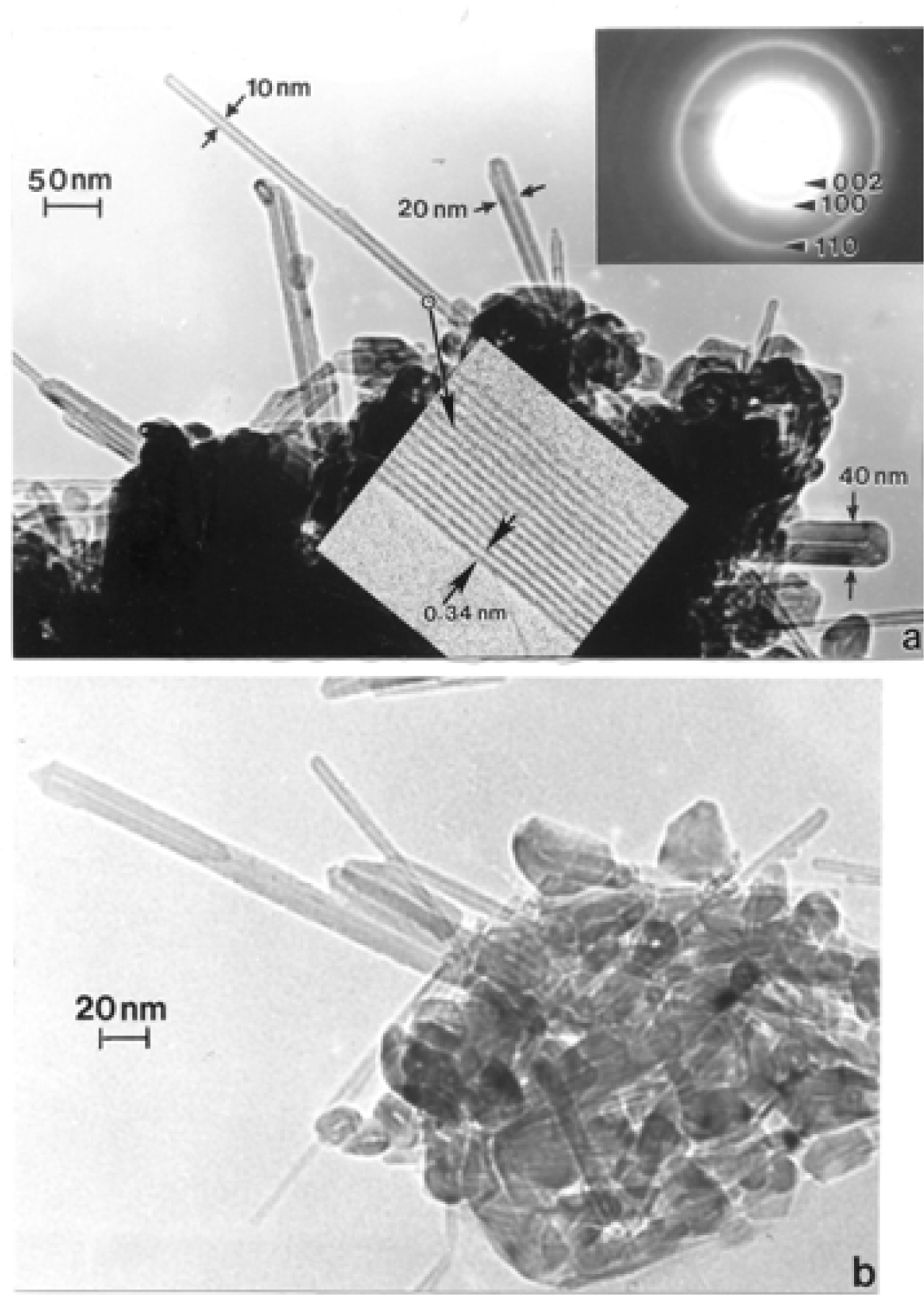
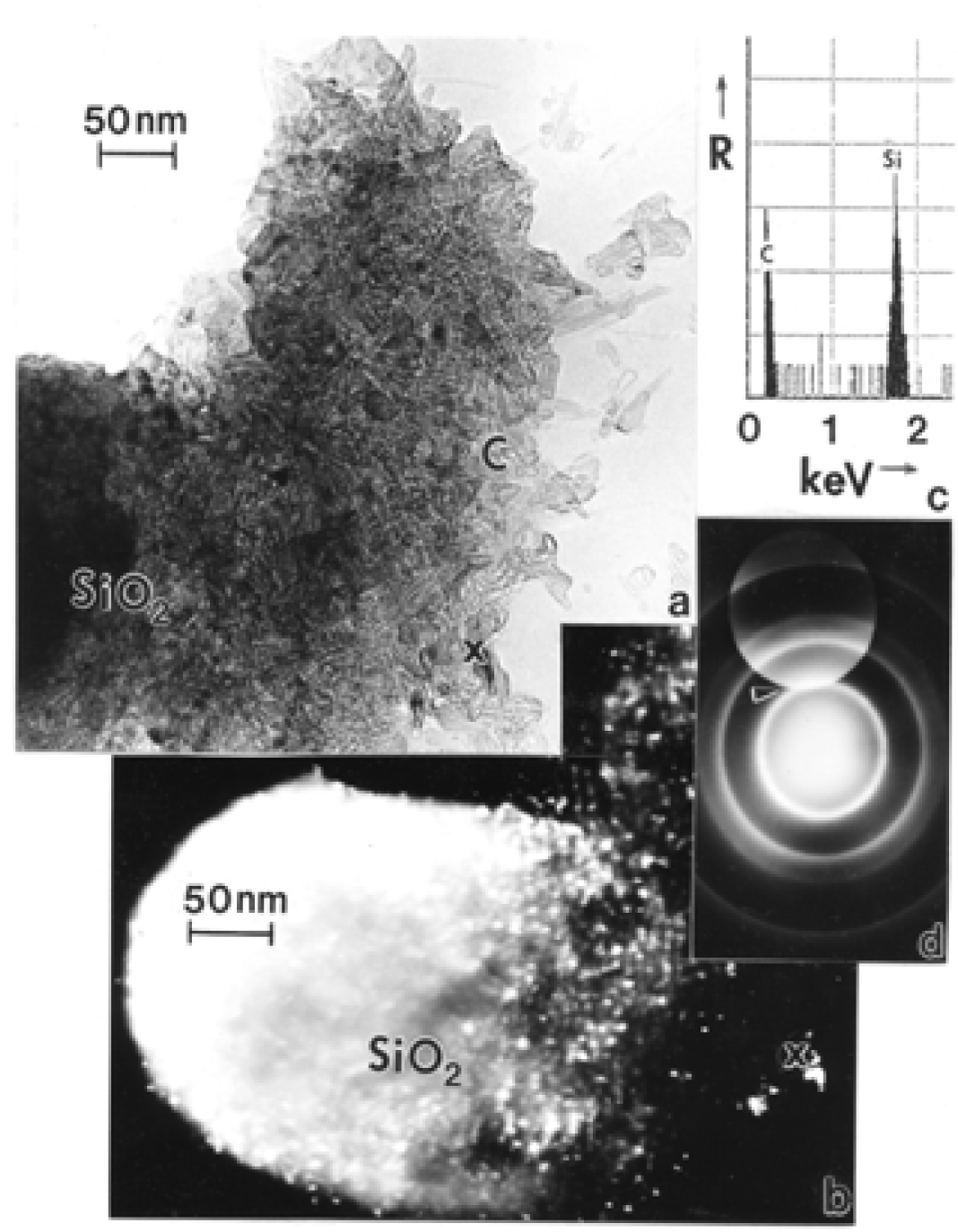
Chrysotile Asbestos Microstructure and Nanostructure
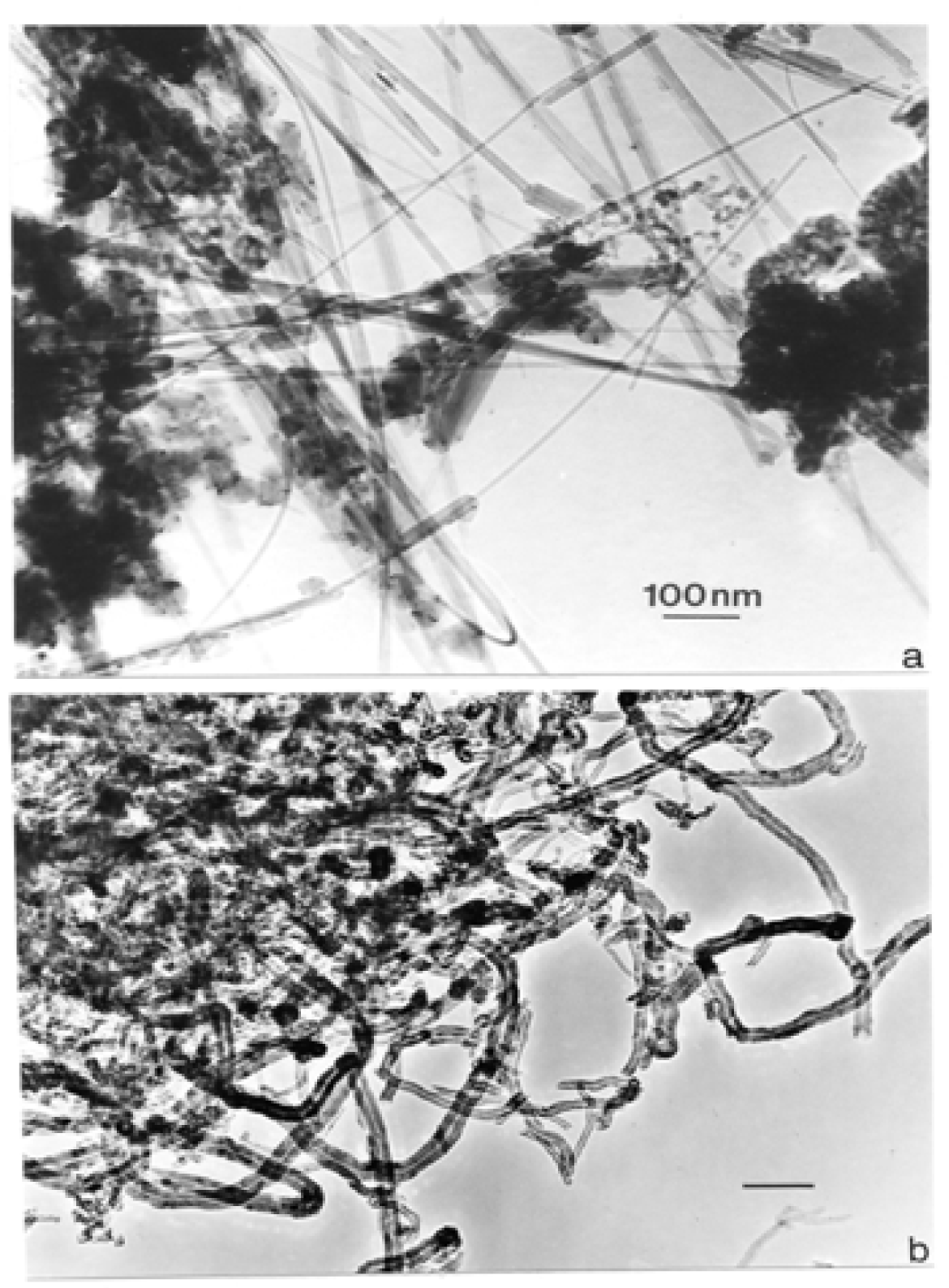
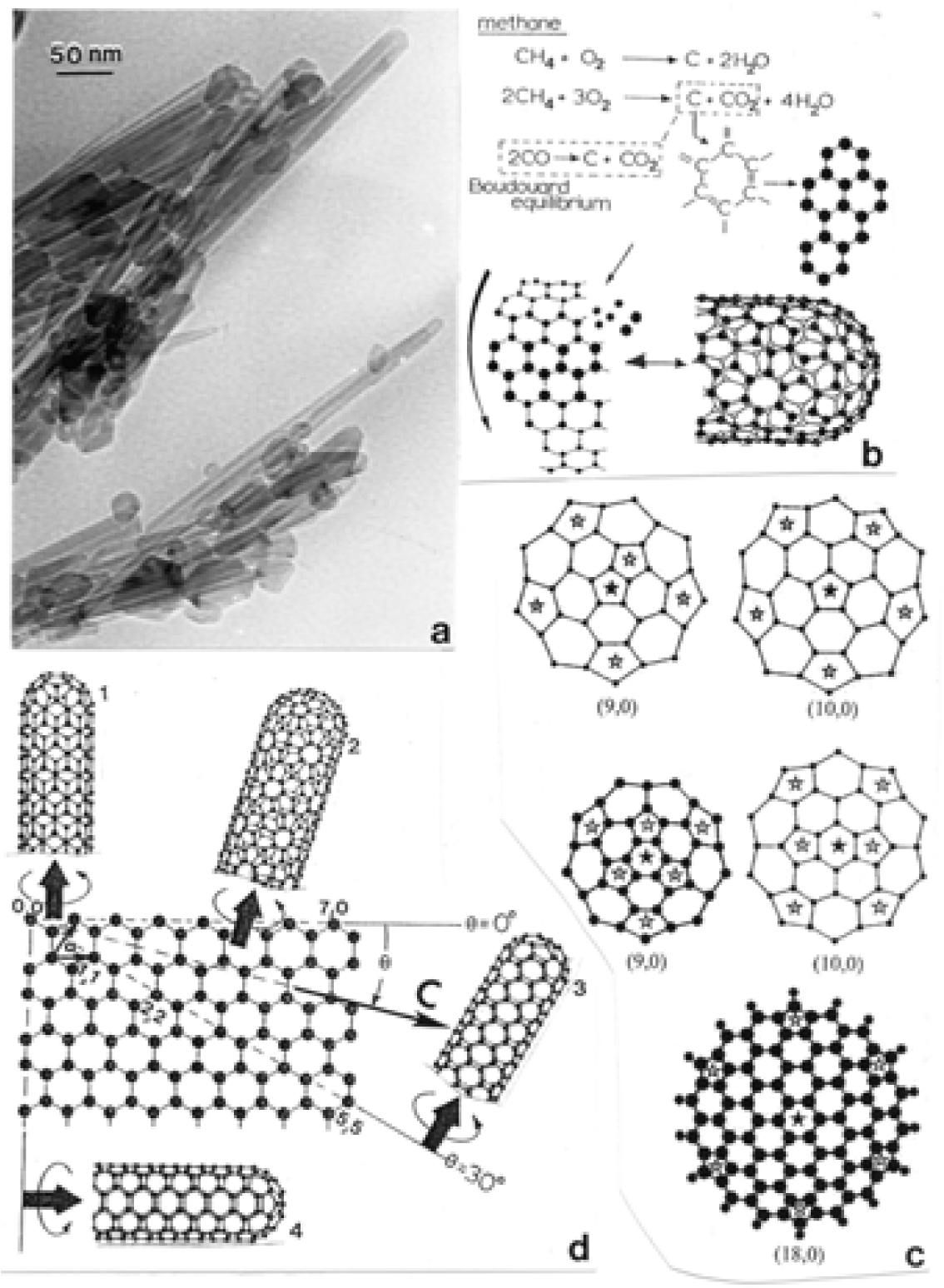
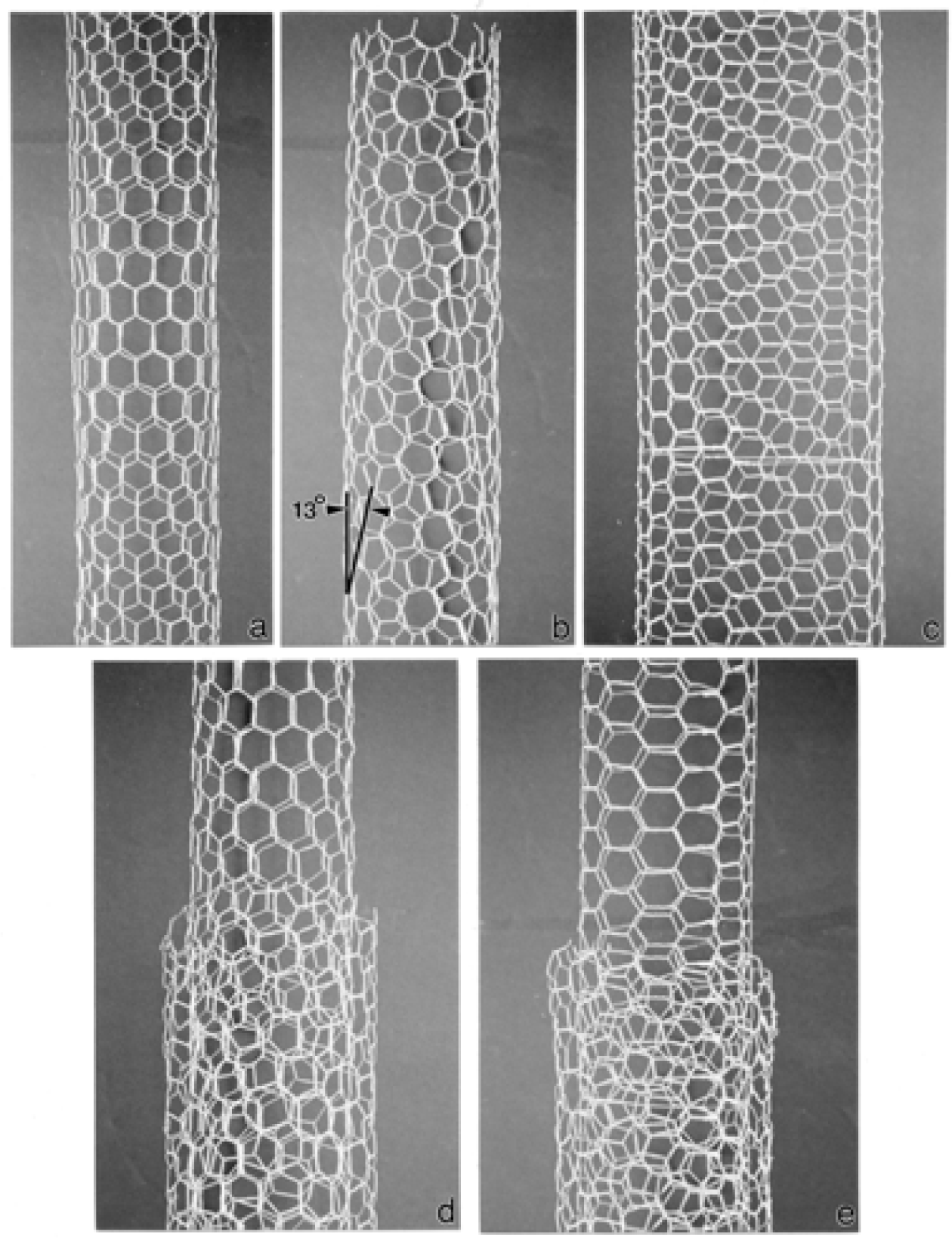
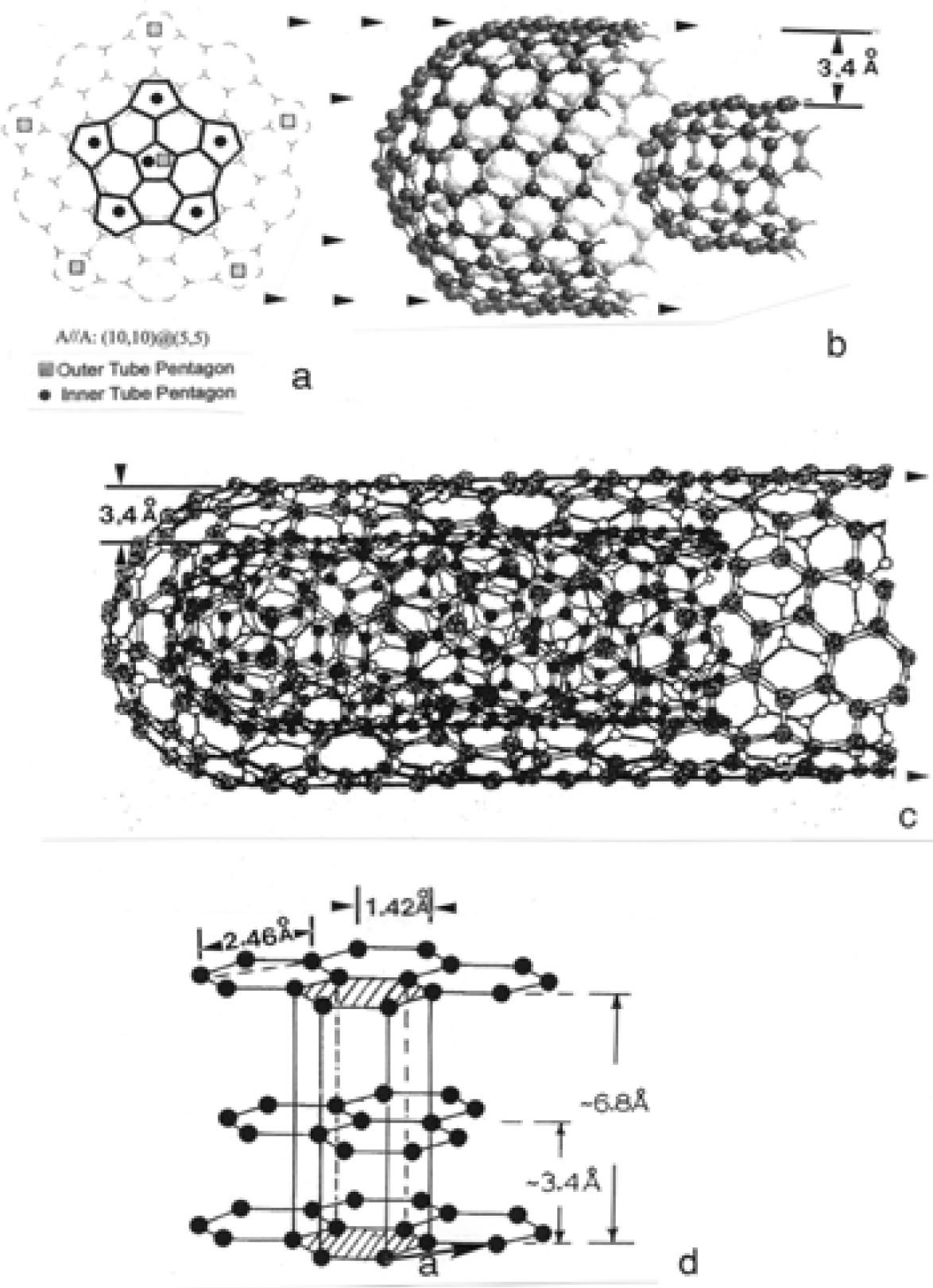
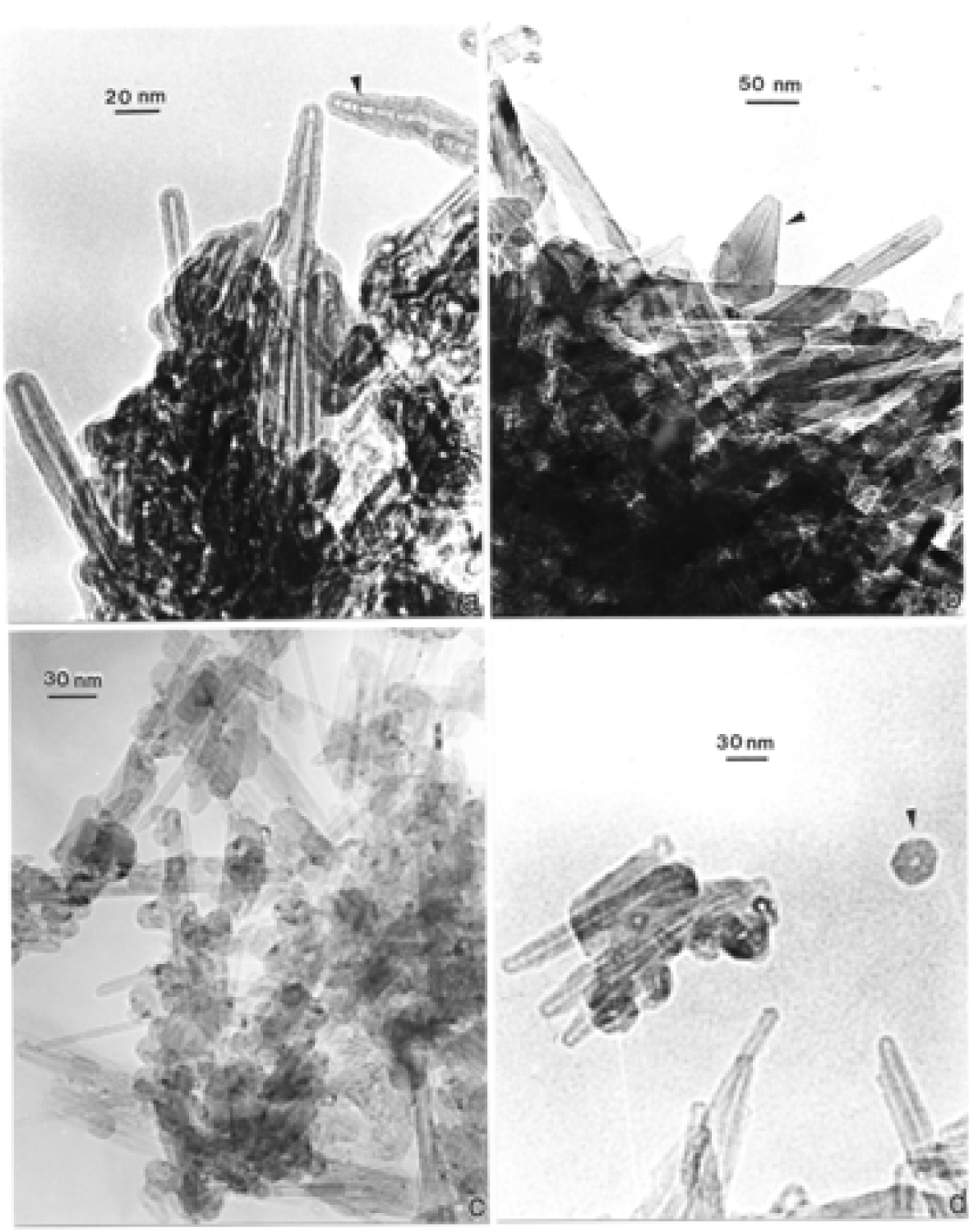
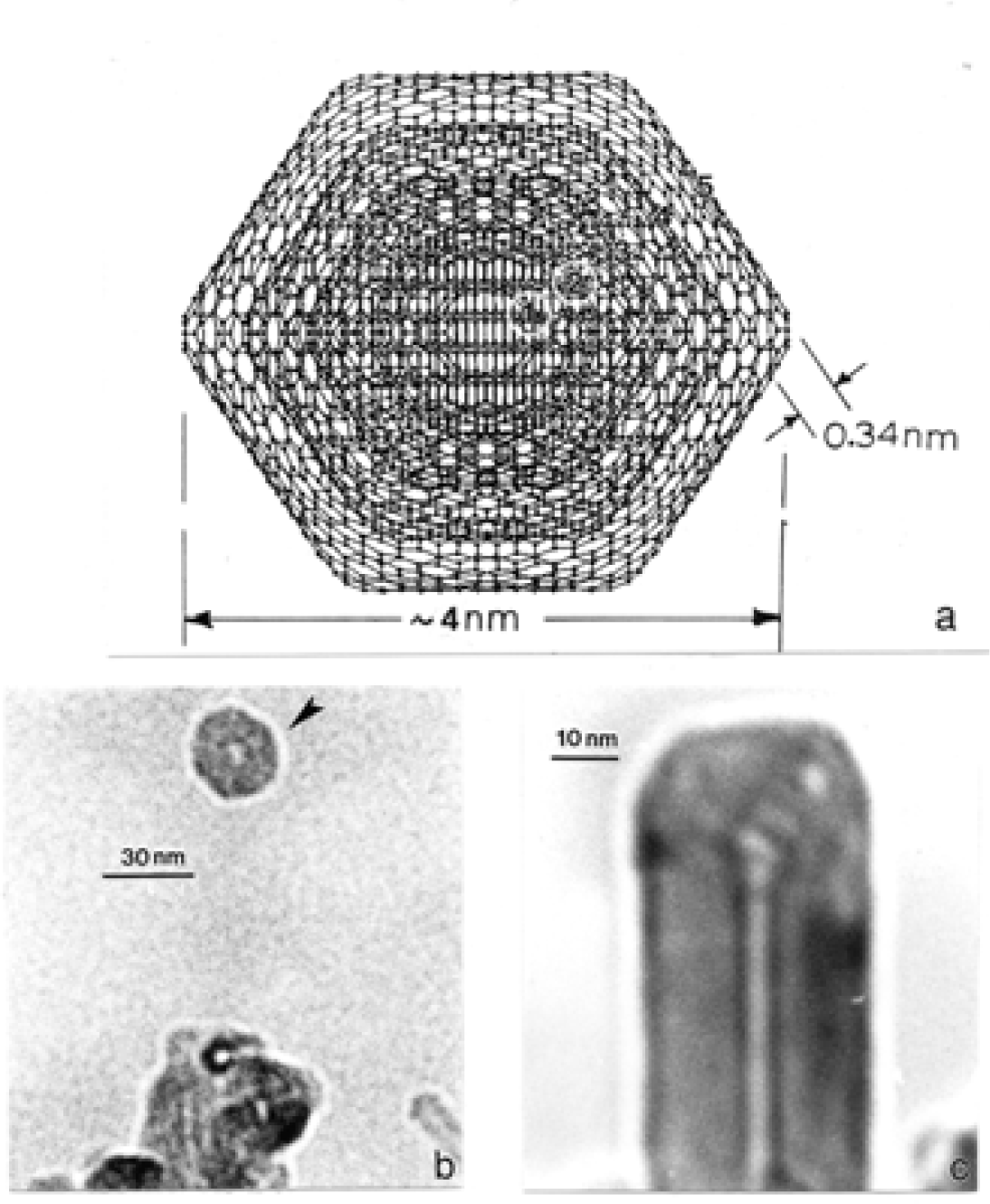
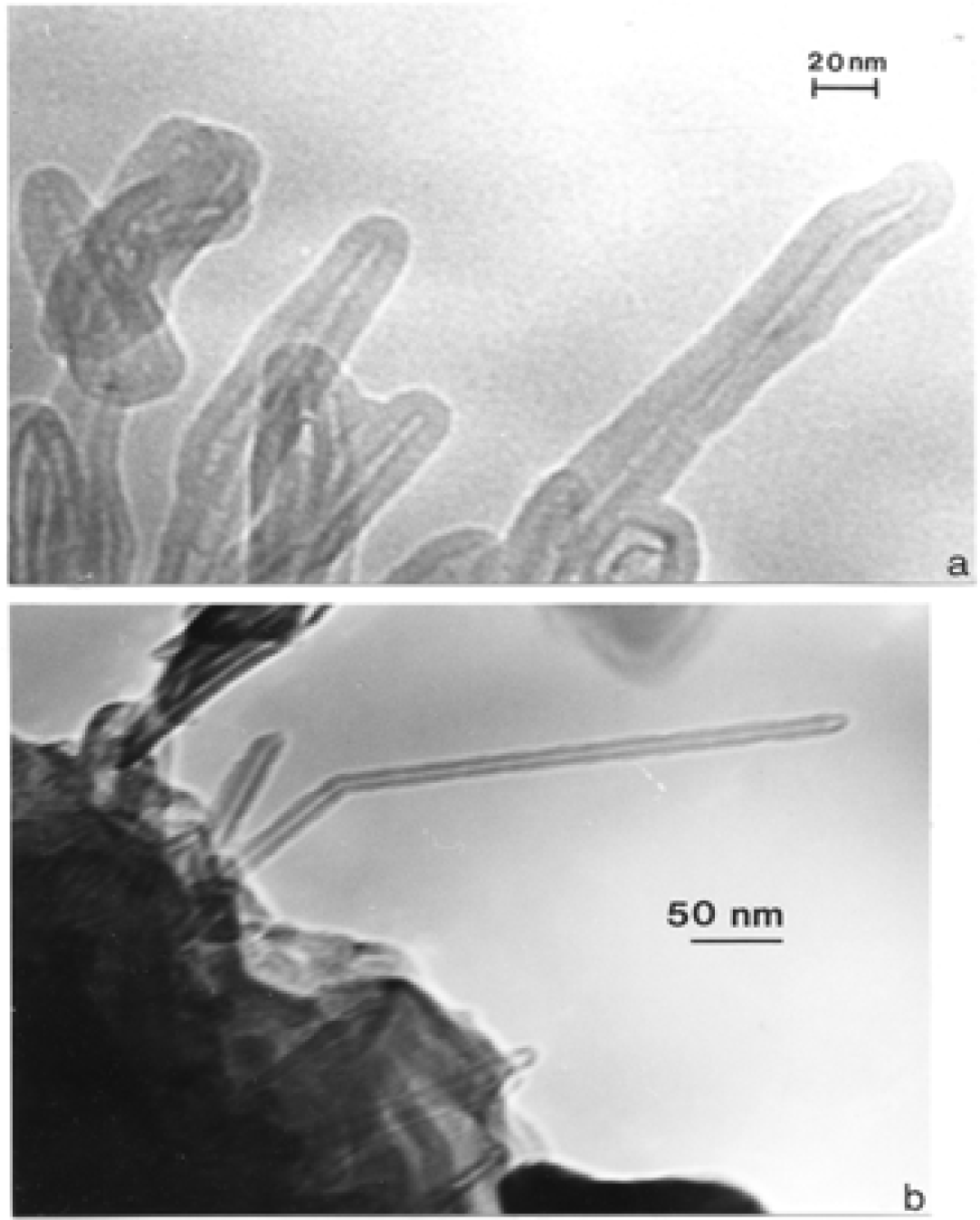
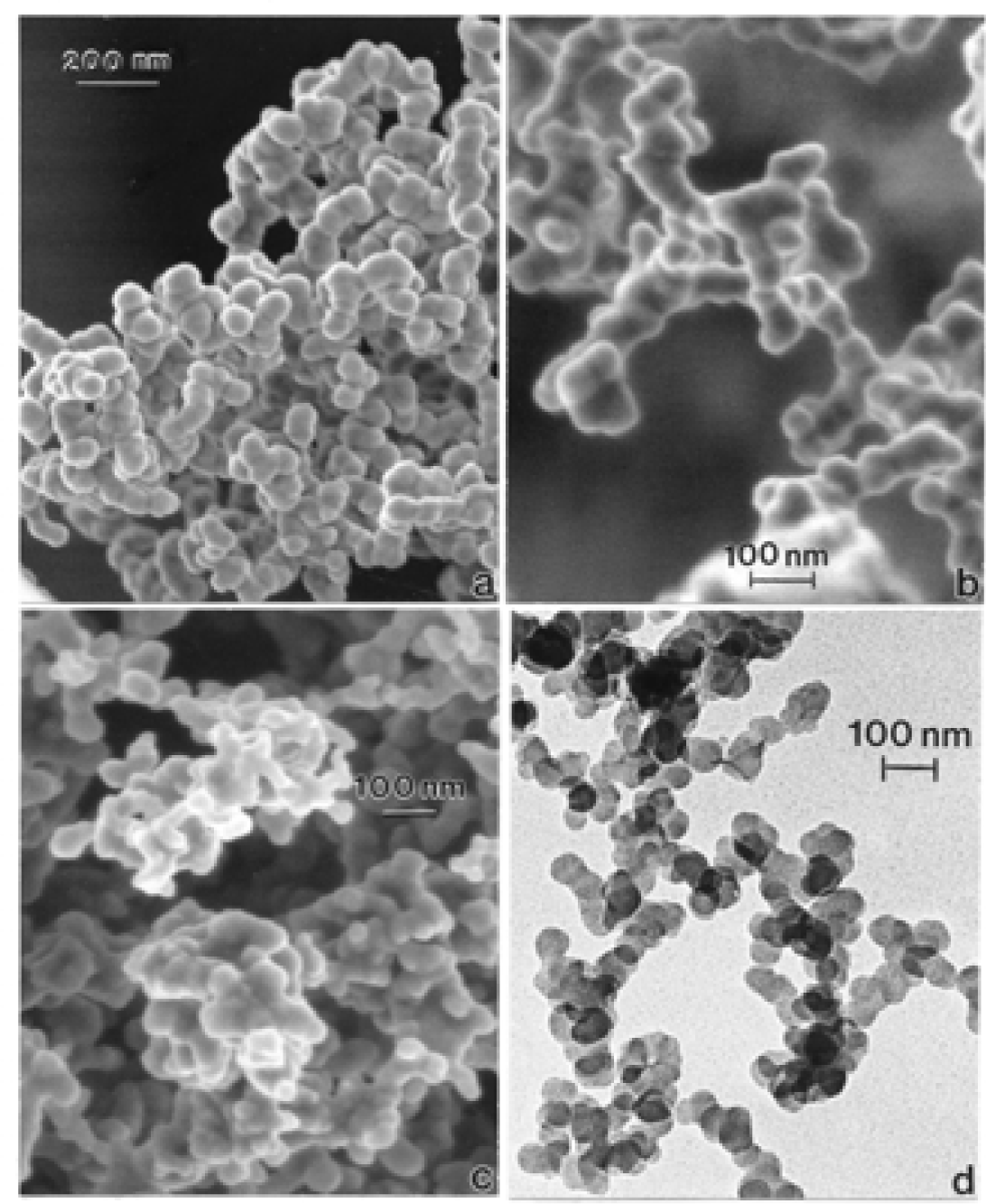
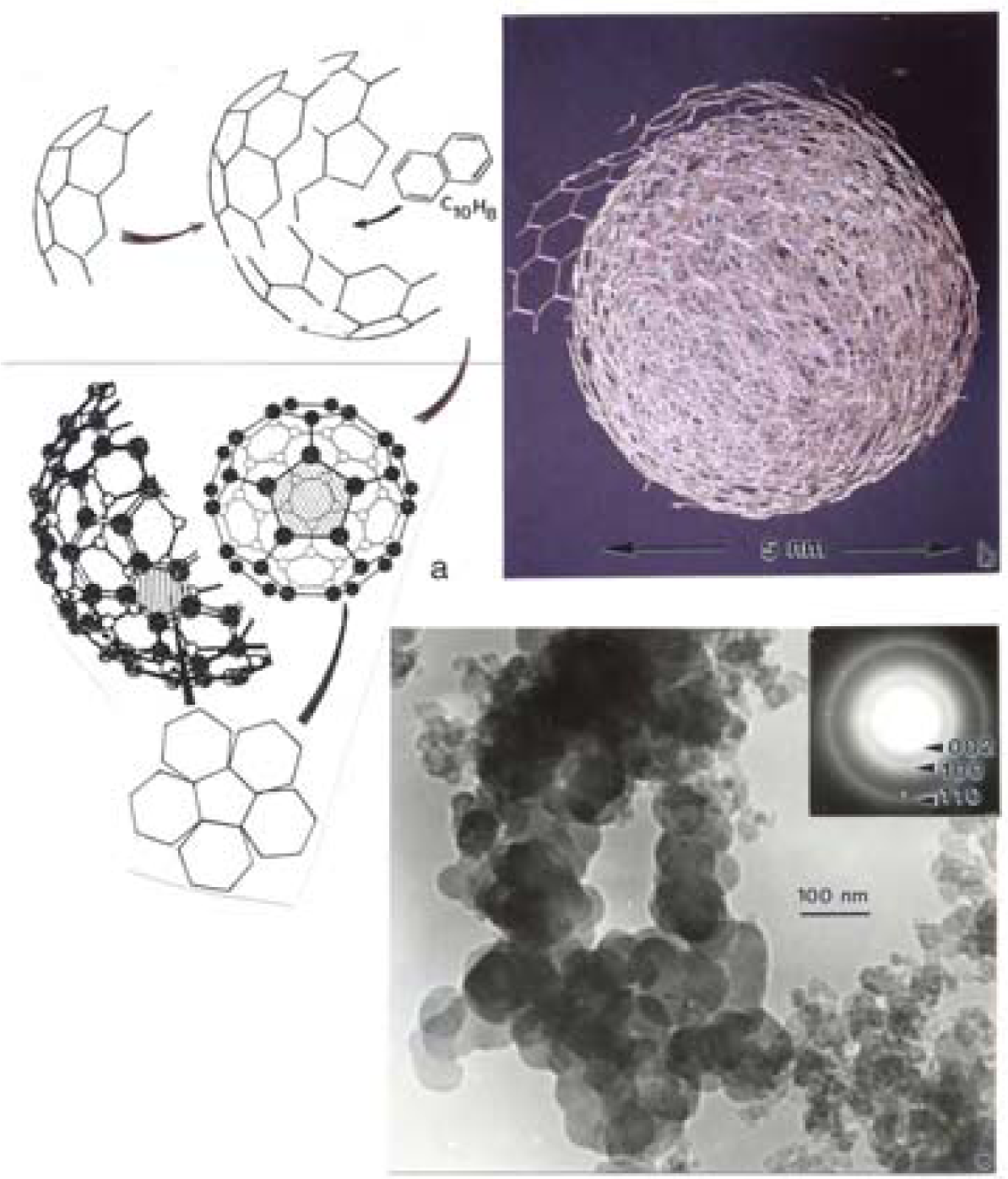
Nanostructure of Commercially Produced Multiwall Carbon Nanotubes
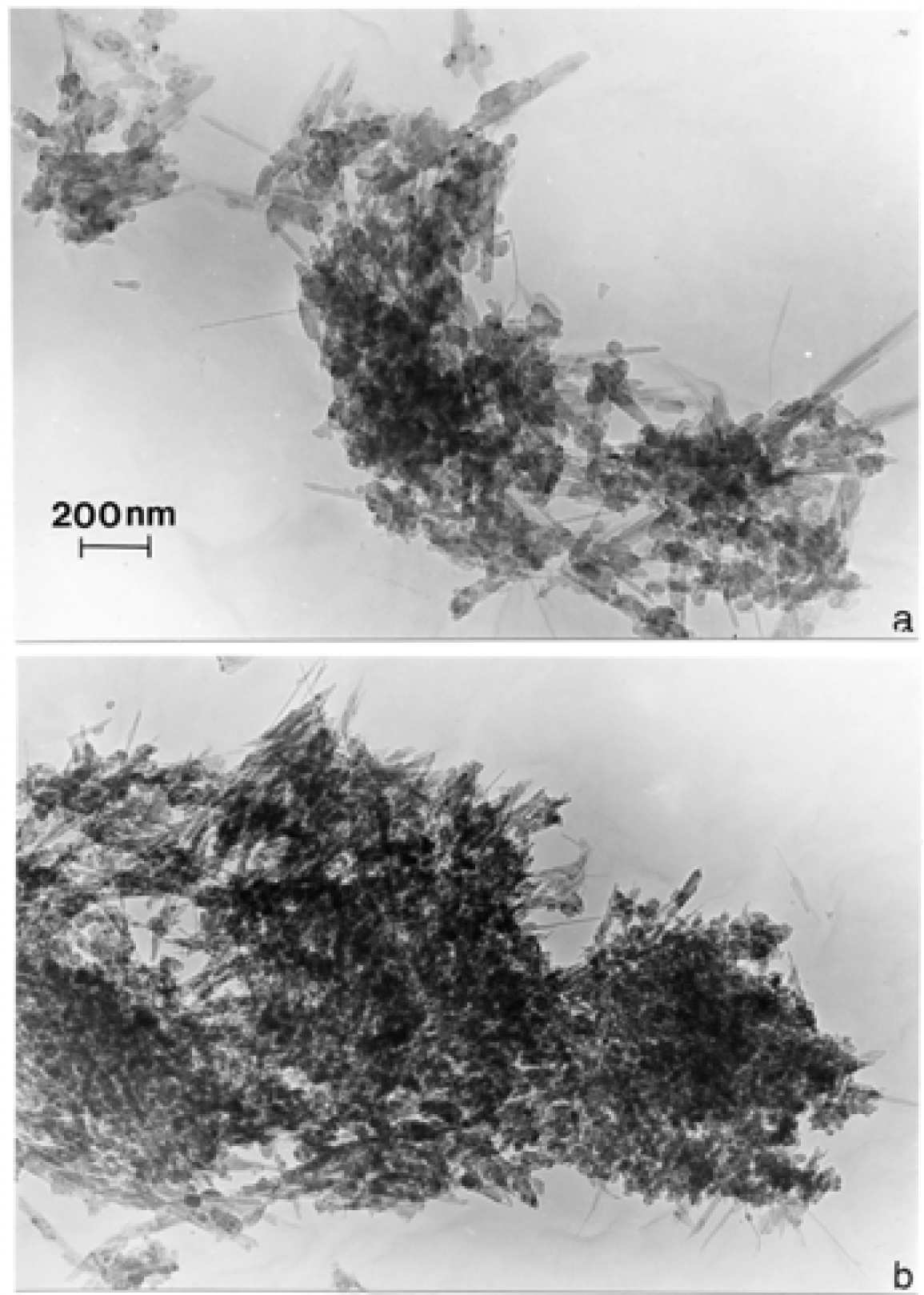
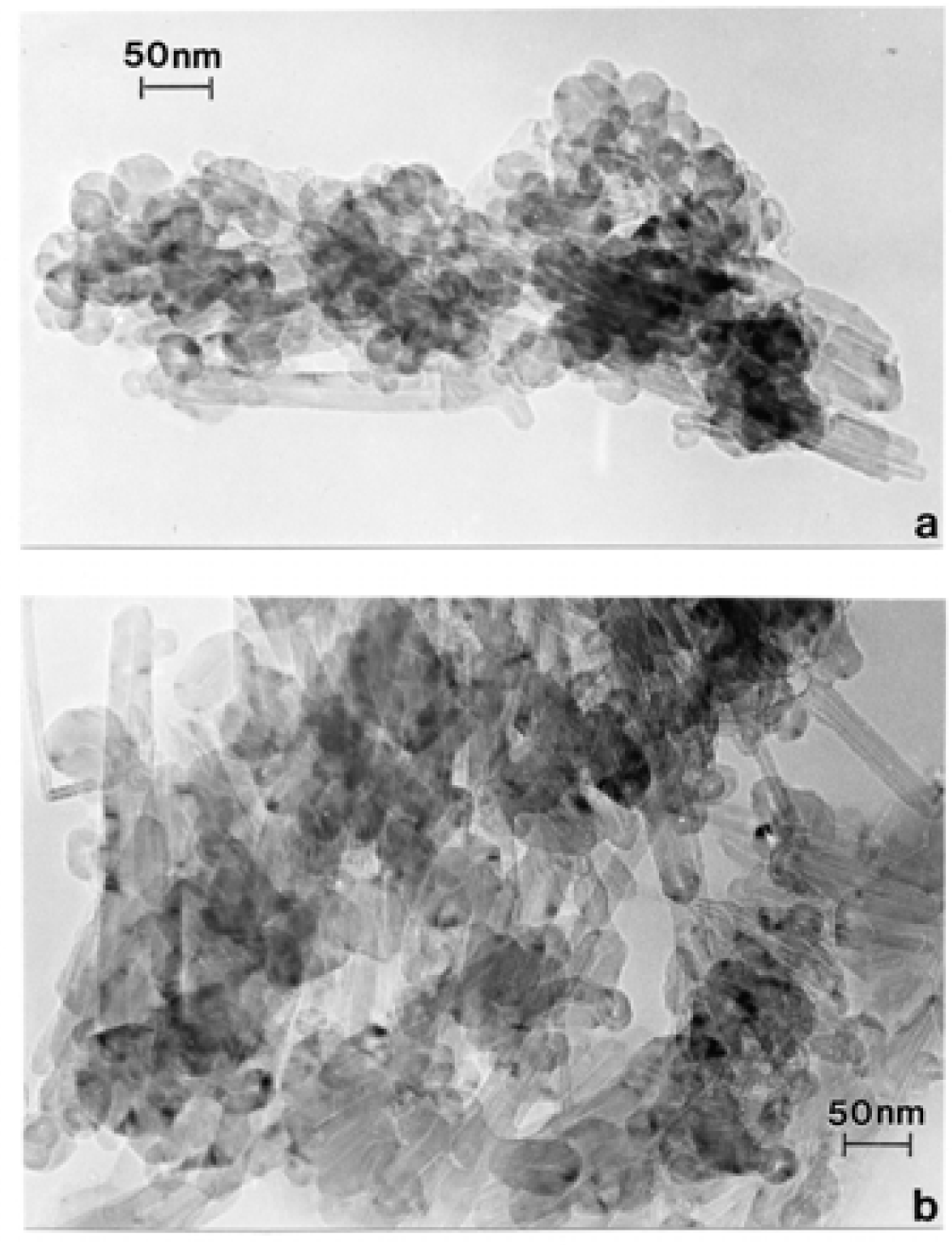
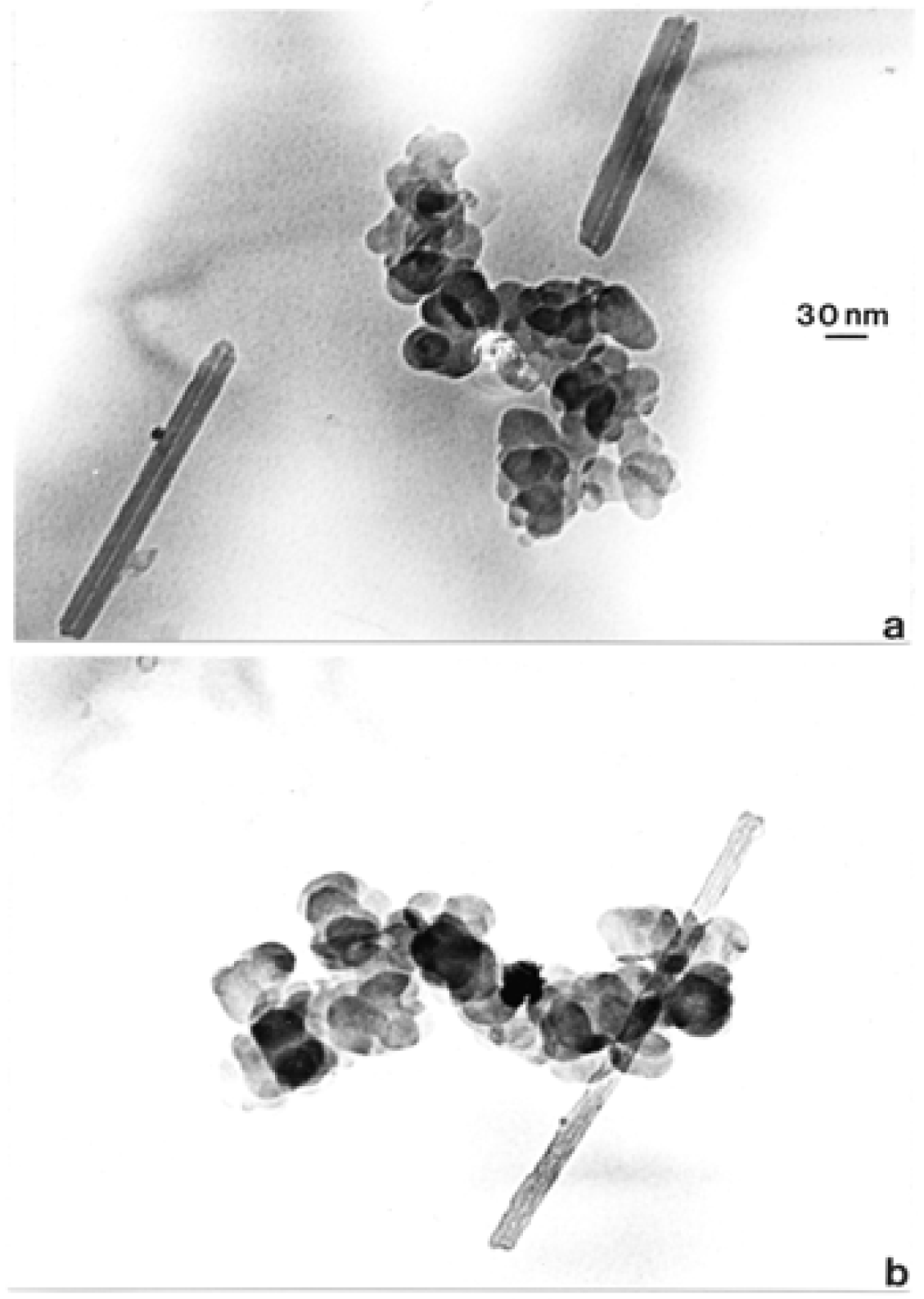
Nanostructure of Anthropogenic and Combustion Generated MWCNT Aggregates
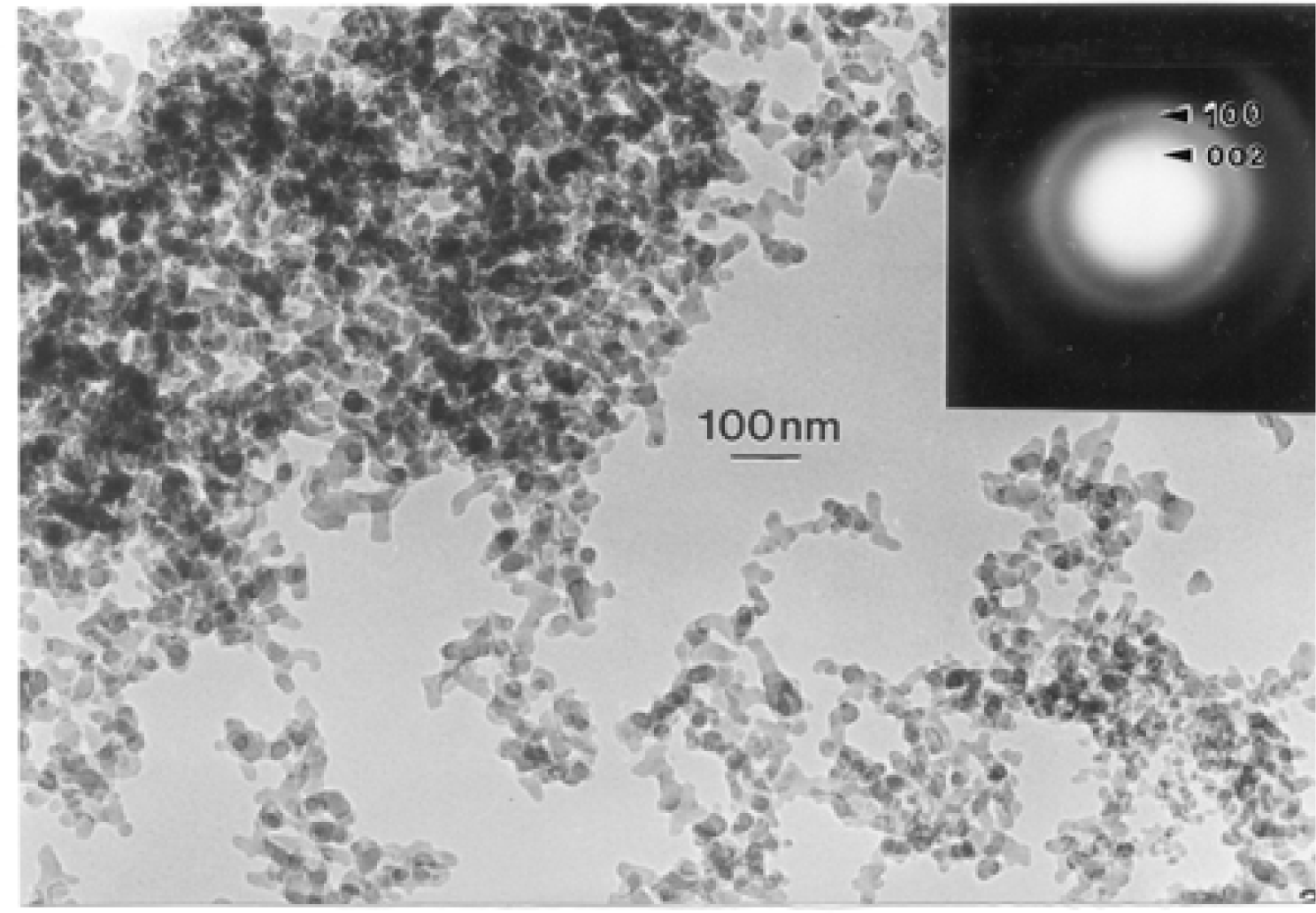
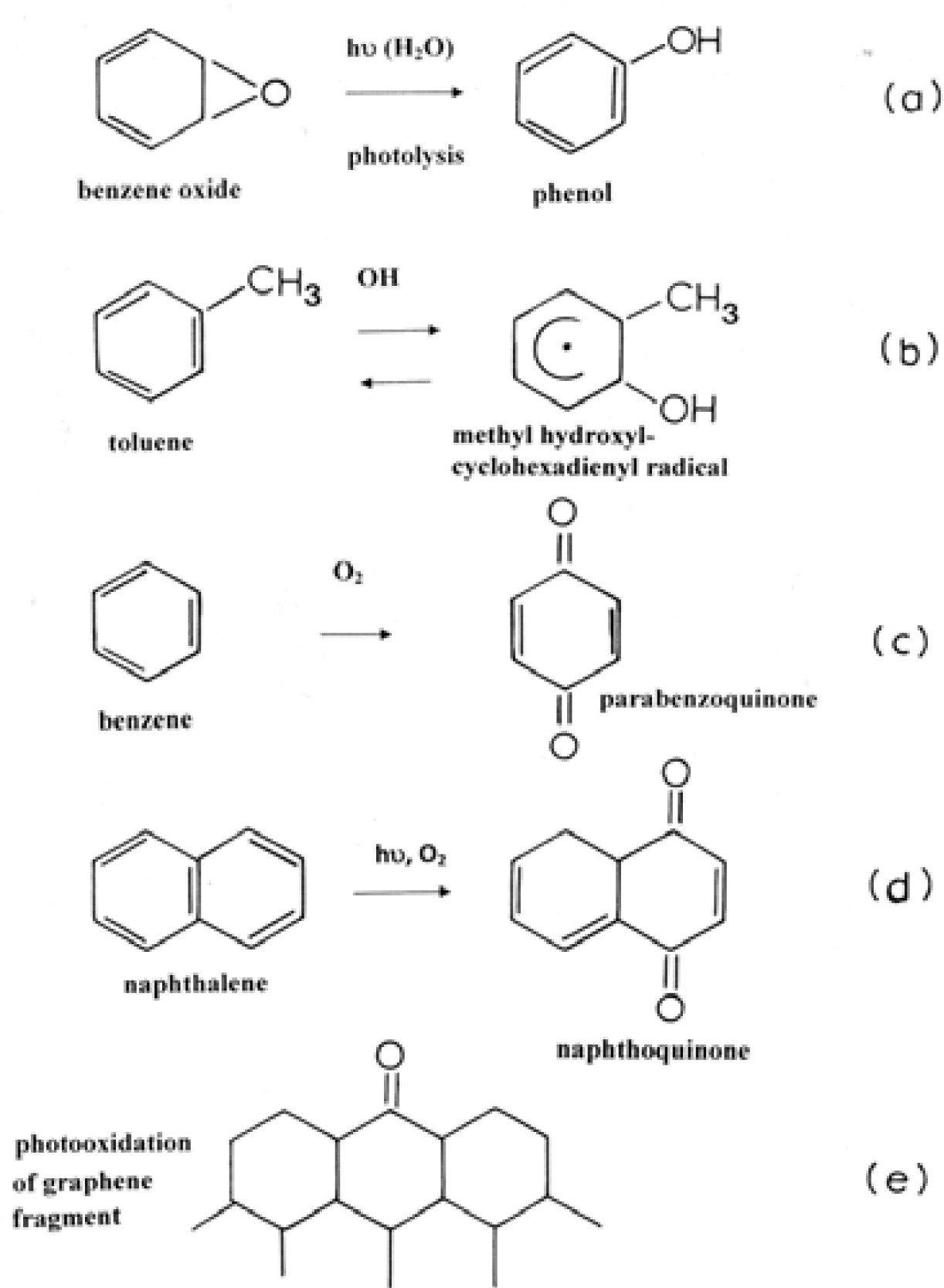
Microstructure and Nanostructure for Combustion Soots and Black Carbon
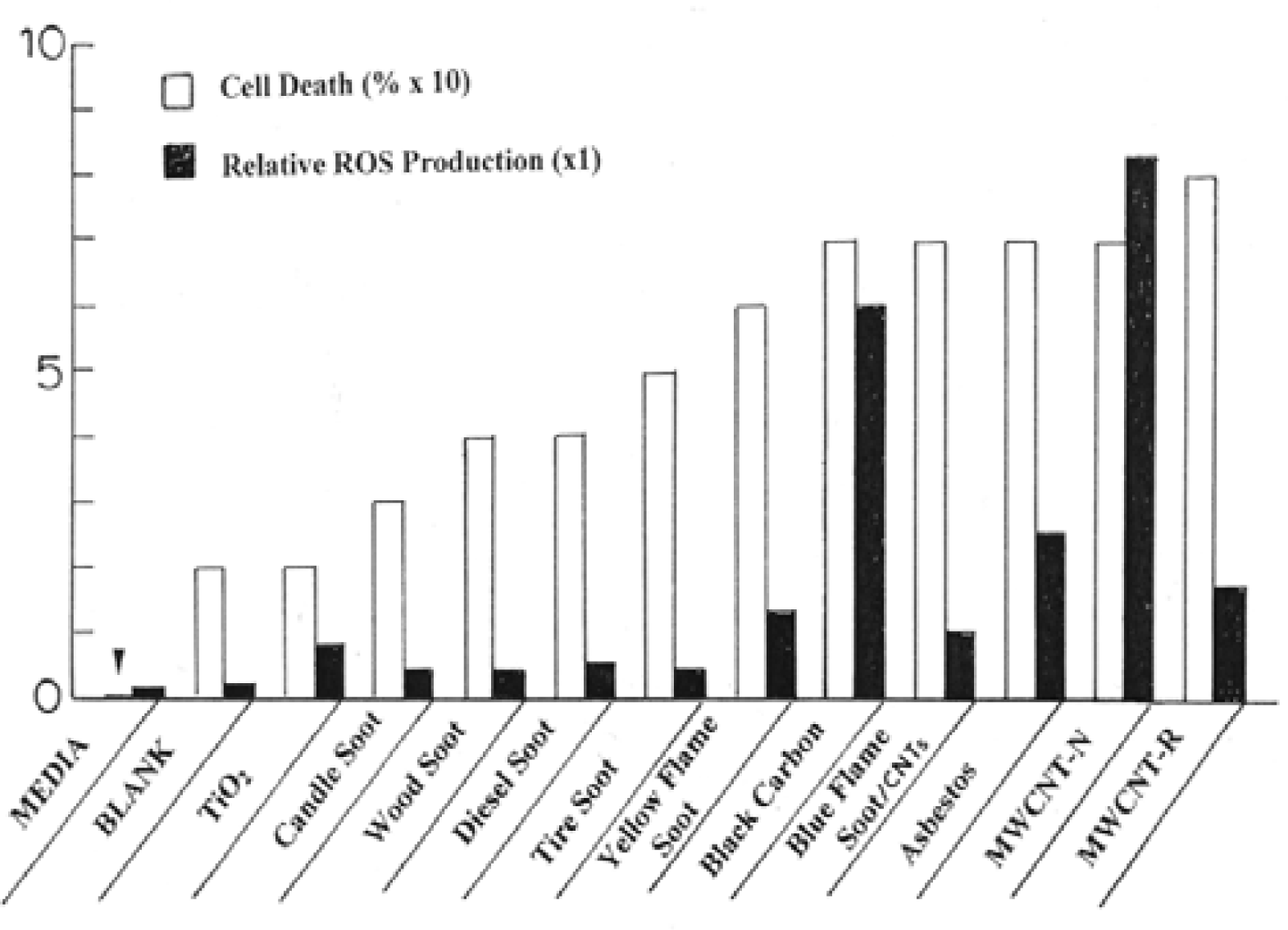
Comparative Cytotoxicities and ROS Generation for Carbonaceous Nano-PM: Respiratory Health Issues
Conclusions


| PAH | Structure | Formula | MW | TPM* | DPM | WPM | CPM | Blue Flame | Yellow Flame | BC | MW CNTR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Naphthalene |  | C10H8 | 128 | 4.6 | 1.6 | <0.1 | 6.3 | <0.3 | 2.9 | -- | 5.9 |
| Acenaphthylene | 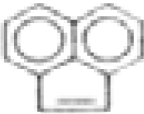 | C12H10 | 152 | 13.0 | 4.7 | <0.1 | -- | <0.3 | <0.2 | -- | 4.0 |
| Acenaphthene | 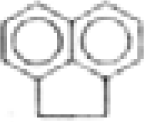 | C12H10 | 154 | 0.7 | 5.3 | 0.1 | --- | <0.3 | <0.2 | -- | -- |
| Fluorene | 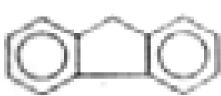 | C13H10 | 165 | 44.6 | 55.6 | 1.3 | --- | <0.3 | 0.4 | --- | 2.5 |
| Phenanthrene | 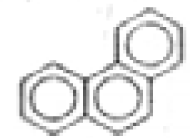 | C14H10 | 178 | 693.5 | 208.3 | 9.5 | --- | 3.8 | <0.1 | 1.5 | 1.4 |
| Anthracene |  | C14H10 | 178 | 195.5 | 245.9 | 11.5 | --- | --- | 3.2 | --- | 2.4 |
| Fluoranthene | 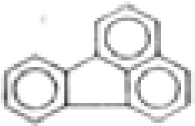 | C16H10 | 202 | 737.1 | 41.8 | 75.0 | --- | 10.4 | 1.7 | --- | <0.1 |
| Pyrene | 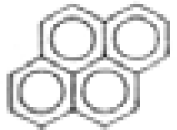 | C16H10 | 202 | 640.4 | 42.1 | 71.5 | --- | 74.7 | 113.6 | 2.4 | 1.0 |
| Benz(a)anthracene | 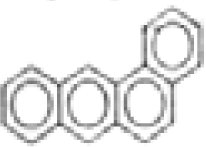 | C18H12 | 228 | 526.6 | 6.1 | 118.8 | --- | 11.7 | --- | --- | --- |
| Chrysene |  | C18H12 | 228 | 662.6 | 8.6 | 92.3 | --- | --- | --- | --- | --- |
| Benzo(b)fluoranthene | 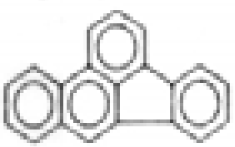 | C20H12 | 252 | 297.7 | 6.8 | 50.5 | --- | --- | --- | --- | --- |
| Benzo(k)fluoranthene | 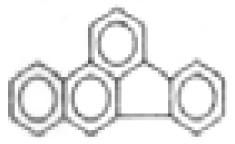 | C20H12 | 252 | 642.2 | 4.8 | 59.8 | --- | --- | --- | --- | --- |
| Benzo(a)pyrene | 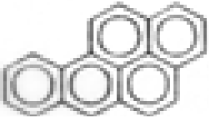 | C20H12 | 252 | 218.8 | 1.9 | 60.2 | --- | --- | --- | --- | --- |
| Indeno(1,2,3-cd)pyrene | 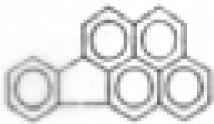 | C22H12 | 276 | 117.8 | 9.9 | 16.9 | --- | --- | --- | --- | --- |
| Benzo(ghi)perylene | 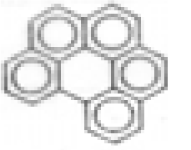 | C22H12 | 276 | 111.5 | 11.8 | 15.3 | --- | --- | --- | --- | --- |
| Dibenz(ah)anthracene | 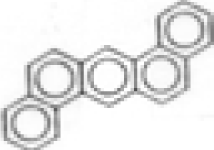 | C22H14 | 278 | --- | --- | 0.8 | --- | --- | --- | --- | --- |
| PAH | Formula | MW | PAH Content (% of Total) | |||||
|---|---|---|---|---|---|---|---|---|
| Seville[68] | Mumbai[69] | Chicago[70] | Chicago[70] | El Paso[71] | El Paso[72] | |||
| Outdoor | Outdoor | Outdoor | Indoor | Indoor | Soil | |||
| Naphthalene | C10H8 | 128 | 10 | 48 | 86 | 79 | 92 | 10 |
| Acenaphthylene | C12H10 | 152 | 9 | 21 | <1 | 1 | 1 | <1 |
| Acenaphthene | C12H10 | 154 | 5 | 10 | 2 | 7 | <1 | <1 |
| Fluorene | C13H10 | 165 | 6 | 3 | 4 | 6 | 1 | 1 |
| Phenanthrene | C14H10 | 178 | 31 | 7 | 3 | <6 | 3 | 6 |
| Anthracene | C14H10 | 178 | 4 | 2 | <1 | <1 | 3 | 2 |
| Fluoranthene | C16H10 | 202 | --- | --- | 1 | --- | --- | 22 |
| Pyrene | C16H10 | 202 | 34 | 9 | <1 | <1 | <1 | 18 |
| Benz(a)anthracene | C18H12 | 228 | --- | --- | <1 | --- | --- | 9 |
| Chrysene | C18H12 | 228 | --- | --- | <1 | --- | --- | 13 |
| Benzo(b)fluoranthene | C20H12 | 252 | --- | --- | <1 | --- | --- | 5 |
| Benzo(k)fluoranthene | C20H12 | 252 | --- | --- | <1 | --- | --- | 4 |
| Benzo(a)pyrene | C20H12 | 252 | --- | --- | <1 | --- | --- | 8 |
| Indeno(1,2,3-cd)pyrene | C22H12 | 276 | --- | --- | <1 | --- | --- | <1 |
Acknowledgments
References
- Vidal, S. Critical review – Ambient particles and health: Lines that divide. J. Air Waste Manage. Assoc 1997, 47, 551–581. [Google Scholar]
- Pope, CA, III; Dockery, DW; Schwartz, J. Review of epidemiological evidence of health effects of particulate air pollution. Inhal. Toxicol 1995, 7, 1–18. [Google Scholar]
- Pope, CA, III; Dockery, DW. Health effects of fine particulate air pollution: Lines that connect. J. Air & Waste Manage. Assoc 2006, 56, 709–742. [Google Scholar]
- Burnett, RT; Smith-Doiron, M; Stieb, D; Cakmak, S; Brook, JR. Effects of particulate and gaseous air pollution on cardiorespiratory hospitalizations. Arch Environ. Health 1999, 54(2), 130–139. [Google Scholar]
- Peters, A; Liu, E; Verier, RL; Schwartz, J; Gold, DR; Mittleman, M; et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology 2000, 11, 11–17. [Google Scholar]
- Pope, CA, III; Burnett, RT; Thurston, GD; Thun, MJ; Callo, EE; Krewski, D; et al. Cardiovascular mortality and long term exposure to particulate air pollution. Epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109, 71–77. [Google Scholar]
- Miller, KA; Siscovick, DS; Sheppard, L; Shepherd, K; Sullivan, JH; Anderson, GL; et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 2007, 356(5), 447–458. [Google Scholar]
- Mossman, BT; Churg, A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med 1998, 157, 1666–1680. [Google Scholar]
- Trush, MA; Venster, TW. An overview of the relationship between oxidative stress and chemical carcinogenesis. Free Radical. Biol. Med 1991, 10, 201–210. [Google Scholar]
- Marnett, LJ. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar]
- Lippman, M. Effects of fiber characteristics on lung deposition, retention and disease. Environ. Health Perspect 1990, 88, 311–317. [Google Scholar]
- Lippman, M. Biophysical factors affecting fiber toxicity. Warheit, DB, Ed.; In Fiber Toxicology; Academic press: San Diego, CA, 1993; pp. 259–303. [Google Scholar]
- Hill, IM; Beswick, PH; Donaldson, K. Differential release of superoxide onions by macrophages treated with long and short fibre amosite asbestos is a consequence of differential affinity for opsonin. Occup. Environ. Med 1995, 52, 92–95. [Google Scholar]
- Fubini, B. Use of physico-chemical and cell-free assay to evaluate the potential carcinogenesis of fibers. In Mechanisms of Fibre Carcinogenesis; Volume 140, IARC Scientific Publications: Lyon France, 1996; pp. 35–54. [Google Scholar]
- Everitt, JI. Mechanisms of fiber-induced diseases: implications for safety evaluation of synthetic vitreous fiber. Regul. Toxicol. Pharmacol. 1994, 20, 568–575. [Google Scholar]
- Brown, DM; Fisher, C; Donaldson, K. Free radical activity of synthetic vitreous fibers: iron chelation inhibits hydroxyl radical generation by refractory ceramic fiber. J. Toxicol. Environ. Health 1998, 53, 545–561. [Google Scholar]
- Liu, W; Ernst, JD; Broaddus, CVO. Phagocytosis of crocidolite asbestos induces oxidative stress, DNA damage, and apoptosis in mesothelial cells. Am. J. Respir. Cell. Mol. Biol 2000, 23, 371–378. [Google Scholar]
- Hansen, K; Mossman, BT. Generation of superoxide (O2–.) from alveolar macrophages exposed to asbestiform and nonfibrous particles. Cancer Res. 1987, 47(6), 1681–1686. [Google Scholar]
- Ziqiang, Q; Siegmann, HC; Keller, A; Matter, U; Scherrer, L; Siegmann, HC. Nanoparticle air pollution in major cities and its origin. Atmos. Environ 2000, 34, 443–451. [Google Scholar]
- Oberdörster, G. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Health 2001, 74, 1–8. [Google Scholar]
- Donaldson, K; Stone, V; Clouter, A; Renwick, L; Mac Nee, W. Ultrafine particles. Occup. Environ. Med. 2001, 58, 211–216. [Google Scholar]
- Li, N; Sioutas, C; Cho, A; Schmitz, P; Misra, C; Sempf, J; Chag, M; Oberley, T; Froines, J; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspectives 2004, 111, 455–460. [Google Scholar]
- Oberdörster, G; Oberdörster, E; Oberdörster, J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspectives 2005, 113, 823–839. [Google Scholar]
- Murr, LE; Soto, KF; Garza, KM; Guerrero, PA; Martinez, F; Esquivel, EV; Ramirez, DA; Shi, Y; Bang, JJ; Venzor, J, III. Combustion-generated nanoparticulates in the El Paso, TX, USA/Juarez, Mexico metroplex: Their comparative characterization and potential for adverse health effects. Int. J. Environ. Res. Public Health 2006, 3(1), 48–66. [Google Scholar]
- Gerhardt, P; Löffler, S; Homann, K-H. Polyhedral carbon ions in hydrocarbon flames. Chem. Phys. Lett 1987, 137, 306–309. [Google Scholar]
- Homann, K-H. Fullerenes and soot formation – New pathways to large particles in flames. Angew. Chem. Int. Ed. 1998, 37, 2434–2451. [Google Scholar]
- Murr, LE; Bang, JJ; Esquivel, EV; Guerrero, PA; Lopez, D. Carbon nanotubes, nanocrystal forms, and complex nanoparticle aggregates in common fuel-gas combustion sources and the ambient air. J. Nanoparticle Res. 2004, 6, 241–251. [Google Scholar]
- Murr, LE; Garza, KM; Soto, KF; Carrasco, A; Powell, TG; Ramirez, DA; Guerrero, PA; Lopez, DA; Venzor, J, III. Cytotoxicity assessment of some carbon nanotubes and related carbon nanoparticle aggregates and the implications for anthropogenic carbon nanotube aggregates in the environment. Int. J. Environ. Res. Public Health 2005, 2(1), 31–42. [Google Scholar]
- Soto, KF; Murr, LE; Garza, KM. Cytotoxic responses and potential respiratory health effects of carbon and carbonaceous nanoparticulates in the Paso del Norte airshed environment. Int. J. Environ Res. Public Health, 2008, 5(1). [Google Scholar]
- Arieta, DE; Ontiveras, CC; Li, W-W; Garcia, CH; Denison, MS. Aryl hydrocarbon receptor-mediated activity of particulate organic matter from the Paso del Norte airshed along the U.S.-Mexico border. Environ. Health Perspective 2003, 111(10), 1299–1305. [Google Scholar]
- Nikula, KJ; Snipes, MB; Barr, EB; Griffith, WC; Henderson, RF; Mauderly, JL. Comparative pulmonary toxicities and carcinogenicities of chronically inhaled diesel exhaust and black carbon in F344 rats. Fund. Appl. Toxicol. 1995, 25(1), 80–94. [Google Scholar]
- Jung, H; Guo, B; Anastasio, C; Kennedy, IM. Quantitative measurements of the generation of hydroxyl radicals by soot particles in a surrogate lung fluid. Atmos. Environ. 2006. [Google Scholar]
- Garza, KM; Soto, KF; Murr, LE. Cytotoxicity and reactive oxygen species generation from aggregated carbon and carbonaceous nanoparticulate materials. Int. J. Nanomedicine 2008, 3(1), 1–12. [Google Scholar]
- Manning, CB; Vallyathan, V; Mossman, BT. Diseases caused by asbestos: mechanisms of injury and disease development. Int. Immunopharmacol 2002, 2, 191–200. [Google Scholar]
- Murr, LE; Soto, KF. TEM comparison of chrysotile (asbestos) nanotubes and carbon nanotubes. J. Mater. Sci. Lett 2004, 39, 4941–4947. [Google Scholar]
- Hall, EJ. From chimney sweeps to oncogenes: the quest for the causes of cancer. Radiology 1991, 179, 297–306. [Google Scholar]
- Shi, Y; Murr, LE; Soto, KF; Lee, W-Y; Guerrero, PA; Ramirez, DA. Characterization and comparison of speciated atmospheric carbonaceous particulates and their polycyclic aromatic hydrocarbon contents in the context of the Paso del Norte airshed along the U.S.-Mexico border. Polycyclic Aromatic Compounds 2007, 27, 361–400. [Google Scholar]
- Bang, JJ; Trillo, EA; Murr, LE. Utilization of selected area electron diffraction (SAED) patterns for characterization of air submicron particulate matter collected by a thermophoretic precipitator. J. Air & Waste Managmt. Assoc. 2003, 53, 227–236. [Google Scholar]
- DeSouza Santos, P. Tecnologia de Argilas, vol. 1, Fundamentus; Editora Edgard Blücher Ltda: São Paulo, Brazil, 1975. [Google Scholar]
- Alleman, JE; Mossman, BT. Asbestos revisited. Sci. American 1997, 70–79. [Google Scholar]
- Soto, KF; Carrasco, A; Powell, TG; Garza, KM; Murr, LE. Comparative in-vitro cytotoxicity assessment of some manufactured nanoparticulate materials characterized by transmission electron microscopy. J. Nanoparticle Res. 2005, 7, 145–169. [Google Scholar]
- Lair, SL; Herndon, WC; Murr, LE; Quinones, SA. End cap nucleation of carbon nanotubes. Carbon 2006, 44, 447–455. [Google Scholar]
- Lair, SL; Herndon, WC; Murr, LE. Energetic trends of single-walled carbon nanotube ab initio calculations. J. Mater. Sci. 2007, 42, 1819–1827. [Google Scholar]
- Lair, SL; Herndon, WC; Murr, LE. Energetic comparison of double-walled carbon nanotube systems. Carbon 2008, in press.. [Google Scholar]
- Guo, T; Nikolaes, P; Rinzler, AG; Tomanek, D; Colbert, DT; Smalley, RE. Self-assembly of tubular fullerenes. J. Phys. Chem. 1995, 99, 10694–10697. [Google Scholar]
- Murr, LE; Soto, KF. A TEM study of soot, carbon nanotubes, and related fullerene nanopolyhedra in common fuel-gas combustion sources. Mater. Characterization 2005, 554, 50–61. [Google Scholar]
- Murr, LE; Soto, KF; Garza, KM. Health hazards of manufactured, natural environmental and anthropogenic atmospheric nanoparticulate materials: past, present and future, Chap. 1 in Biomaterials and Biomedical Engineering; Ahmed, W, Ali, N, Öchsner, Eds.; Trans Tech Publishers: Switzerland, 2008; pp. 1–54. [Google Scholar]
- Dresselhaus, MS; Dresselhaus, G; Eklund, PC. Science of Fullerenes and Carbon Nanotubes; Academic Press: New York, 1996. [Google Scholar]
- Murr, LE; Bang, JJ; Esquivel, EV; Guerrero, PA; Lopez, DA. Carbon nanotubes, nanocrystal forms, and complex nanoparticle aggregates in common fuel-gas combustion sources and ambient air. J. Nanoparticle Res 2004, 6, 241–251. [Google Scholar]
- Murr, LE; Guerrero, PA. Carbon nanotubes in wood soot. Atmos. Sci. Lett 2006, 7, 93–95. [Google Scholar]
- Esquivel, EV; Murr, LE. A TEM analysis of nanoparticulates in a Polar ice cave. Mater. Characterization 2004, 52, 15–25. [Google Scholar]
- Pratsinis, SE. Flame aerosol synthesis of ceramic powders. Prog. Energy Combust. Sci 1998, 24(1), 197–219. [Google Scholar]
- Woolridge, MS. Gas-phase combustion synthesis of particles. Prog. Energy Combust. Sci. 1998, 24(1), 63–87. [Google Scholar]
- Katrinak, KA; Rez, P; Perkes, PR; Buseck, PR. Fractal geometry of carbonaceous aggregates forms an urban aerosol. Environ. Sci. Technol 1993, 27, 539–547. [Google Scholar]
- Skillas, G; Konzel, S; Burtscher, H; Baltensperger, U; Siegman, K. High fractal-like dimension of diesel soot aggregates. J. Aerosol. Sci 1998, 29(4), 411–419. [Google Scholar]
- Grieco, WJ; Howard, JB; Rainey, LC; Vander Sande, JB. Fullerenic carbon in combustion-generated soot. Carbon 2000, 38, 597–614. [Google Scholar]
- Clague, ADH; Donnet, JB; Wang, TK; Peng, JCM. A comparison of diesel engine soot with carbon black. Carbon 1999, 37, 1553–1565. [Google Scholar]
- Vander Wal, RL; Tomasek, AJ. Soot nanostructure: dependence upon synthesis conditions. Combustion & Flame 2004, 136, 129–140. [Google Scholar]
- Li, Y-Y; Hsieh, C-C. synthesis of carbon nanotubes by combustion of a paraffin wax candle. Micro & Nano Lett 2007, 3, 63–66. [Google Scholar]
- Soto, KF; Garza, KM; Shi, Y; Murr, LE. Direct contact cytotoxicity assays for filter-collected, carbonaceous (soot) nanoparticulate material and observations of lung cell response. Atmos. Environ. 2008, 42, 1970–1982. [Google Scholar]
- Garza, KM; Soto, KF; Murr, LE. Cytotoxicity and reactive oxygen species generation from aggregated carbon and carbonaceous nanoparticulate materials. Int. J. Nanomedicine 2008, 3(1), 1–12. [Google Scholar]
- Herndon, WC. Quantum theory of aromatic hydrocarbon carcinogenesis. Int. J. Quantum Chem. Quantum Biology Supplement No. 1.. 1974, 123–134. [Google Scholar]
- Herndon, WC; Chen, H-T; Zhang, Y; Rum, G. QSAR study of PAH carcinogenicity activities: test of a general model for molecular similarity analysis in. In Molecular Modeling and Prediction of Bioactivity; Gunderfotte, Jorgensen, Eds.; Kluwer Academic Press: New York, 2000. [Google Scholar]
- Nel, A; Xia, T; Mädler, L; Ning, L. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar]
- Millette, JR; Boltin, R; Few, P; Turner, W, Jr. Microscopical studies of World Trade Center disaster dust particles. Microscopy Today 2003, 32–36. [Google Scholar]
- Dahl, A; Gharibi, A; Swietlicki, E; Gudmondsson, A; Bohgard, M; Ljungman, A; Blomquist, G; Gustafsson, M. Traffic-related emissions of ultrafine particles from pavement-tire interface. Atmos. Environ. 2006. [Google Scholar]
- Veranth, JM; Moss, TA; Chow, JC; Labban, R; Nichols, WK; Walton, JC; Watson, JG; Yost, GS. Correlation of in-vitro cytokine responses with the chemical composition of soil-derived particulate matter. Environ. Health Perspect. 2006, 114(3), 341–349. [Google Scholar]
- Gutierrez-Daban, A; Fernandez-Espinosa, AJ; Ternero-Rodriguez, M; Fernandez- Alveraz, I. Particle size distribution of polycyclic aromatic hydrocarbons in urban air in Southern Spain. Analyt. Bioanal. Chem 2005, 381, 721–736. [Google Scholar]
- Pandit, GG; Srivastava, PK; Mahan Rao, AM. Aromatic hydrocarbon arising from kerosene cooking fuel. Sci. Total Environ 2001, 279, 159–165. [Google Scholar]
- Van Winkle, MR; Scheff, PA. Volatile organic compounds, polycyclic aromatic hydrocarbons, and elements in the air of ten urban homes. Indoor Air 2001, 11, 49–64. [Google Scholar]
- Mora, J; Gamez, J; Astorga-Bustillo, F; et al. Speciation of indoor air pollutants and associated inhalation health hazards caused by cooking and heating in Hispanic households. Report to Southwest Consortium for Environmental Research and Policy (SCERP). June 2006. [Google Scholar]
- DeLa Torre-Roche, RJ; Lee, W-Y; Campos-diaz, SI. Soil-borne polycyclic aromatic hydrocarbons in El Paso, Texas: Analysis of a potential problem in the United States/Mexico border region. J of Hazardous Materials 2008, (in press).. [Google Scholar]
- Khalili, NR; Scheff, PA; Holsen, TM. PAH source fingerprints for cake ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 532–542. [Google Scholar]
- Levendis, YA; Atal, A; Carlson, J; Durayevskiy, Y; Vouros, P. Comparative study on combustion and emissions of waste tire crumb and pulverized coal. Environ. Sci. Technol. 1996, 30, 2742–2754. [Google Scholar]
- Poland, CA; Duffin, R; Kinloch, I; Maynard, A; Wallace, WA; Seaton, A; Stone, V; Brown, S; MacNee, W; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nature Nanotechnol 2008, 111, 1–6. [Google Scholar]
© 2008 MDPI All rights reserved.
Share and Cite
Murr, L.E. Microstructures and Nanostructures for Environmental Carbon Nanotubes and Nanoparticulate Soots. Int. J. Environ. Res. Public Health 2008, 5, 321-336. https://doi.org/10.3390/ijerph5050321
Murr LE. Microstructures and Nanostructures for Environmental Carbon Nanotubes and Nanoparticulate Soots. International Journal of Environmental Research and Public Health. 2008; 5(5):321-336. https://doi.org/10.3390/ijerph5050321
Chicago/Turabian StyleMurr, L. E. 2008. "Microstructures and Nanostructures for Environmental Carbon Nanotubes and Nanoparticulate Soots" International Journal of Environmental Research and Public Health 5, no. 5: 321-336. https://doi.org/10.3390/ijerph5050321




