Vehicle-Dependent Disposition Kinetics of Fluoranthene in Fisher-344 Rats
Abstract
:Introduction
Materials and Methods
Chemicals
Animals and Exposure
Sample Collection, Extraction and Processing for FLA Parent Compound/Metabolite Analysis
Toxicokinetics
Statistical Analysis
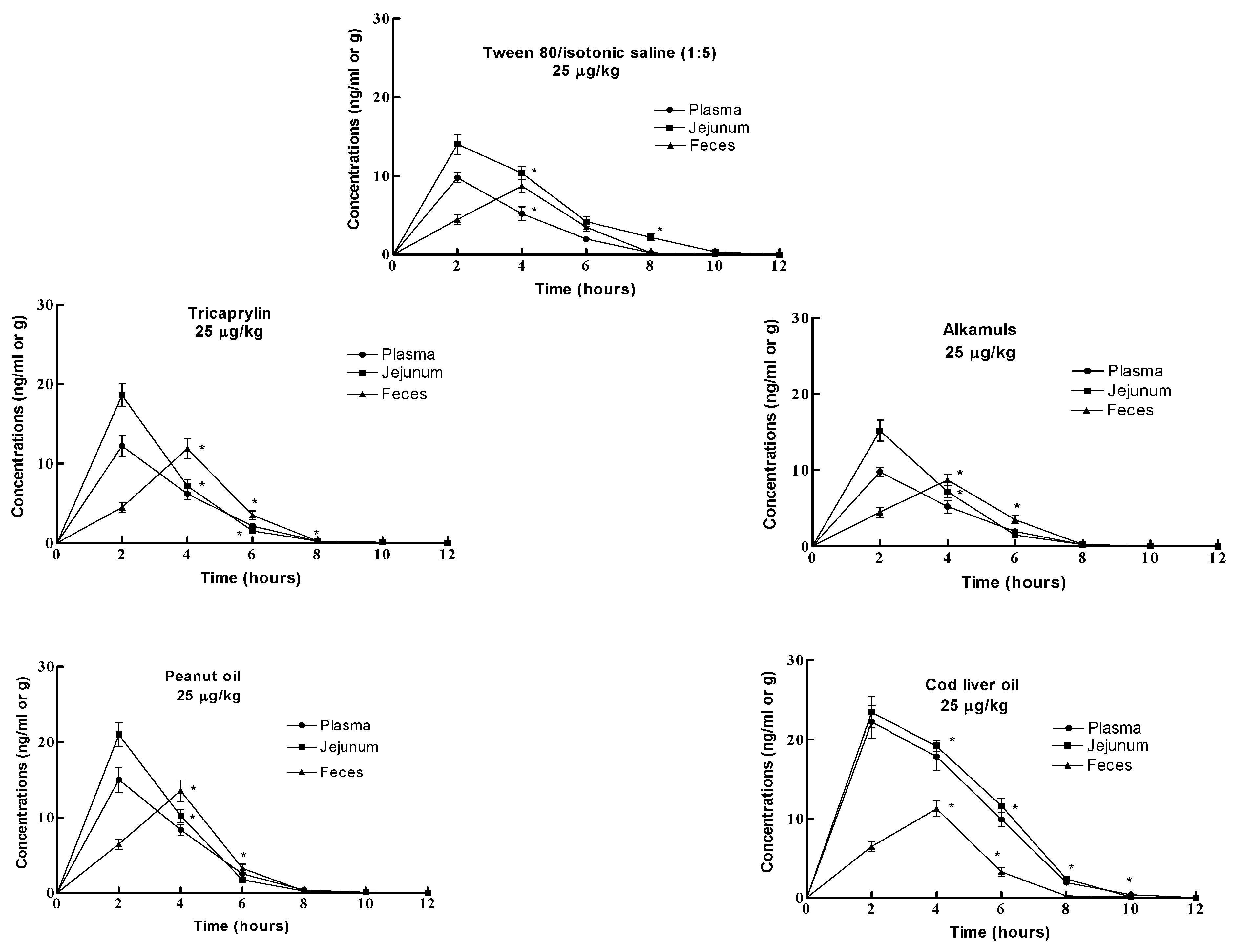
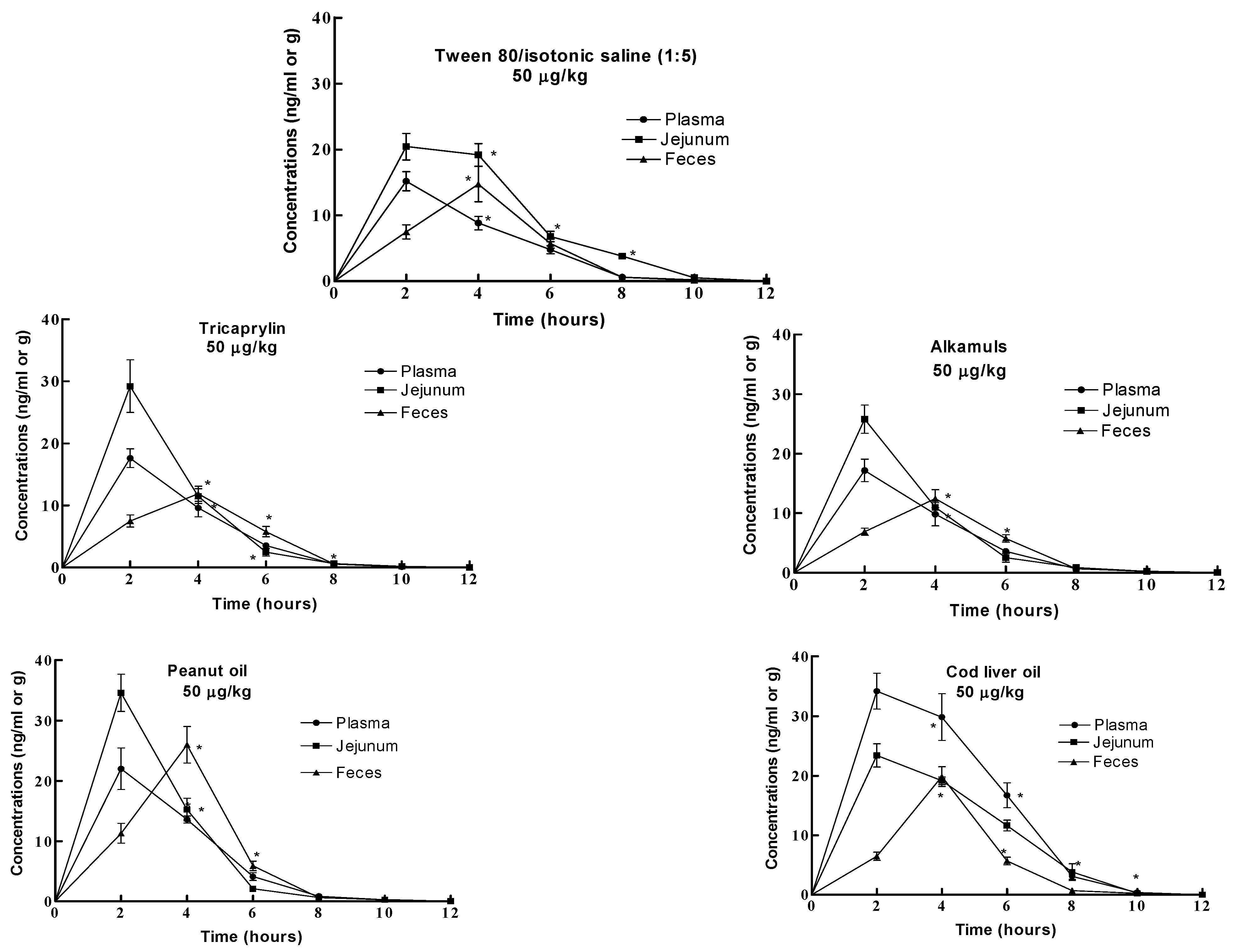
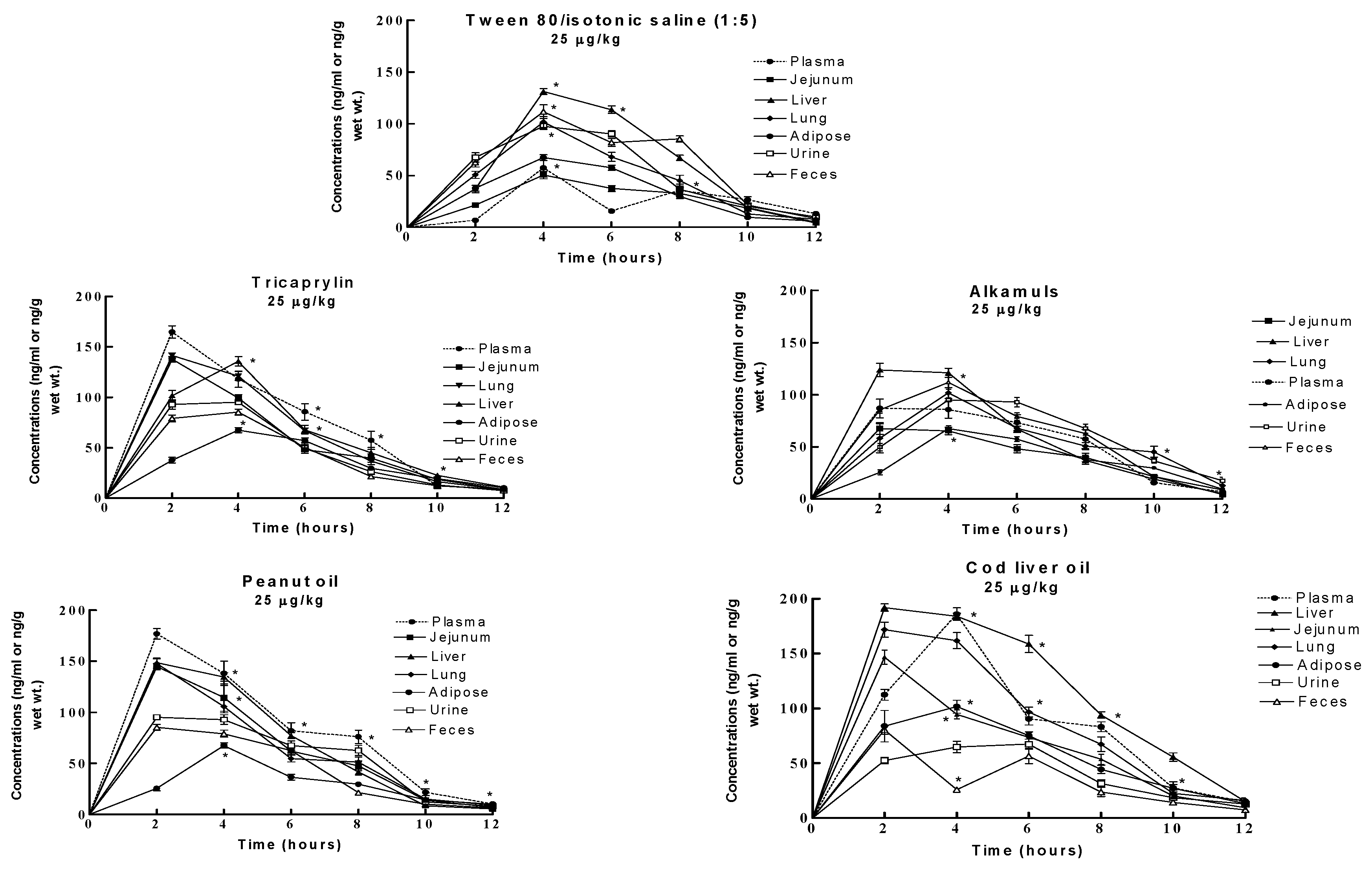
Results
| Parameter | Peanut oil | Cod liver oil | Tricaprylin | 2% Alkamuls | Twe en 80/isotonic saline (1:5) |
| Area under curve (AUC; mg/ml/hr) | 0.013 ± 0.005 | 0.018 ± 0.004* | 0.010 ± 0.005 | 0.005 ± 0.002* | 0.003 ± 0.0003* |
| Biological half-life (tr1/2; hrs) | 2.5 ± 0.027 | 3.0 ± 0.035 | 2.1 ± 0.028 | 1.5 ± 0.020* | 0.7 ± 0019* |
| Volume of distribution (Vd; ml/kg) | 0.12 ± 0.016 | 0.19 ± 0013 | 0.10 ± 0.014 | 0.07 ± 0.011* | 0.03 ± 0.010* |
| Clearance (Cl; ml/hr/kg) | 0.013 ± 0.008 | 0.022 ± 0.003* | 0.009 ± 0.001 | 0.005 ± 0.004* | 0.002 ± 0.004* |
| Mean residence time (MRT; hrs) (MRT; hrs) | 2.5 ± 0.015 | 3.7 ± 0.015* | 2.3 ± 0.019 | 2.0 ± 0.017* | 1.4 ± 0.015* |
| Elimination rate (Kd; hrs) | 0.055 ± 0.006 | 0.08 ± 0.002 | 0.04 ± 0.003 | 0.04 ± 0.006 | 0.01 ± 0.0002 |
| Parameter | Peanut oil | Cod liver oil | Tricaprylin | 2% Alkamuls | Tween 80/isotonic saline (1:5) |
| Area under curve (AUC; mg/ml/hr) | 0.018 ± 0.002 | 0.025 ± 0.004* | 0.015 ± 0.005 | 0.009 ± 0.003* | 0.005 ± 0.0003* |
| Biological half-life (t1/2; hrs) | 3.2 ± 0.031 | 3.7 ± 0.035 | 2.8 ± 0.028 | 2.1 ± 0.020* | 1.3 ± 0.015* |
| Volume of distribution (Vd; ml/kg) | 0.22 ± 0.016 | 0.27 ± 0.014 | 0.19 ± 0.014 | 0.13 ± 0.011* | 0.09 ± 0.010* |
| Clearance (Cl; ml/hr/kg) | 0.020 ± 0.008 | 0.031 ± 0.002* | 0.013 ± 0.004 | 0.009 ± 0.004* | 0.006 ± 0.004* |
| Mean residence time (MRT; hrs) (MRT; hrs) | 5.5 ± 0.015 | 6.5 ± 0.013* | 4.8 ± 0.015 | 3.8 ± 0.017* | 2.6 ± 0.015* |
| Elimination rate (Kd; hrs) | 0.095 ± 0.006 | 0.11 ± 0.002 | 0.08 ± 0.003 | 0.07 ± 0.006 | 0.03 ± 0.002 |
Discussion
Acknowledgements
References
- Dolusio, J. T.; Tan, G. H.; Billups, N. F.; Diamond, L. : Drug absorption. II. Effect of fasting on intestinal drug absorption. J. Pharmacol. Sci., 1969, 58, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Balimane, P. V; Chong, S; Morrison, R. A.: Current methodologies used for evaluation of intestinal permeability and absorption. Journal of Pharmacol. and Toxicol. Methods, 2000, 44, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Gad, S. C.; Chengelis, C. P. : Acute toxicology of testing perspectives and horizons. Caldwell, N. J.: Telford Press, 1988.
- O’Hara, T. M.; Borzelleca, J. F.; Clarke, E. C.; Sheppard, M. A.; Condie, L. W. A. : CCl4/CHCl3 interaction study in isolated hepatocytes: selection of a vehicle. Fundam. Appl. Toxicol., 1989, 13, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Braak, K.; Frey, H.-H. : Effects of solvents and detergents on the contractions of isolated smooth muscle preparations. J. Pharm. Pharmacol., 1990, 42, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Gad, S. C.; Cassidy, C. D.; Aubert, N.; Spainhour, B.; Robbe, H. : Nonclinical vehicle use in studies by multiple routes in multiple species. Int. J. Toxicol., 2006, 25, 499–521. [Google Scholar] [CrossRef]
- Kim, K. B.; Anand, S. S.; Muralidhara, S.; Kim, H. J.; Bruckner, J. V. : Formulation-dependent toxicokinetics explains differences in the GI absorption, bioavailability and acute neurotoxicity of deltamethrin in rats. Toxicology, 2007, 234, 194–202. [Google Scholar] [CrossRef] [PubMed]
- IPCS. Environmental Health Criteria 202: Selected non-heterocyclic polycyclic aromatic hydrocarbons. International Programme on Chemical Safety, Lyon, France: World Health Organization, 1998.
- Wang, J. S.; Busby, W.F. Jr. : Induction of lung and liver tumors by fluoranthene in a preweanling CD-1 mouse bioassay. Carcinogenesis, 1993, 14, 1871–1874. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Near, R.; Shneider, A.; Cui, H.; Ju, S.- T.; Sherr, D. H. : Fluoranthene-induced apoptosis in murine T cell hybridomas is independent of the aromatic hydrocarbon receptor. Toxicol. Appl. Pharmacol., 1996, 139, 144–152. [Google Scholar] [CrossRef]
- Knuckles, M.; Inyang, F.; Ramesh, A. : Acute and subchronic oral toxicity of fluoranthene in F-344 rats. Ecotoxicol. Environ. Saf., 2004, 59, 102–108. [Google Scholar] [CrossRef]
- Walker, S. A. : Effects of dietary fat on the metabolism of fluoranthene: an environmental toxicant. Ph.D. Thesis, Meharry Medical College. 2005, 138pp.
- NIH. Guidelines for the laboratory use of chemical carcinogens. NIH Publication No. 81-2385, Washington, DC: National Institutes of Health, U.S. Government Printing Office; 1981.
- Ramesh, A.; Walker, S. A.; Hood, D. B.; Guillén, M. D.; Schneider, K.; Weyand, E. H. : Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int. J. Toxicol,, 2004, 23, 301–33. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, G.; Liu, C.-Q. : Pilot study of polycyclic aromatic hydrocarbons in surface soils of Guiyang city, People’s Republic of China. Bull. Environ. Contam. Toxicol., 2006, 76, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Walker, S. A.; Addai, A. B.; Mathis, M.; Ramesh, A. : Effect of dietary fat on metabolism and DNA adduct formation after acute oral exposure of F-344 rats to fluoranthene. J Nutr Biochem., 2007, 18, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Inyang, F.; Hood, D. B.; Archibong, A. E.; Knuckles, M. E.; Nyanda, A. M. ; Metabolism, bioavailability, and toxicokinetics of benzo[a]pyrene [B(a)P] in F-344 rats following oral administration. Exp Toxic Pathol., 2001, 53, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, G.; Brune, H.; Dettbarn, G.; Heinrich, U.; Jacob, J.; Mohatashamipur, E. , Norpoth, K.; Pott, F.; Wenzel-Hartung, R.: Urinary and faecal excretion of chrysene and chrysene metabolites by rats after oral, intraperitoneal, intratracheal or intrapulmonary application. Arch. Toxicol., 1988, 62, 401–405. [Google Scholar] [CrossRef]
- Jacob, J.; Brune, H.; Dettbarn, G.; Grimmer, D.; Heinrich, U.; Mohatashamipur, E.; Norpoth, K.; Pott, F.; Wenzel-Hartung, R. : Urinary and faecal excretion of pyrene and hydroxypyrene by rats after oral, intraperitoneal, intratracheal or intrapulmonary application. Cancer Lett., 1989, 46, 15–20. [Google Scholar] [CrossRef]
- Rahman, A.; Barrowman, J. A.; Rahimtula, A. : The influence of bile on the bioavailability of polynuclear aromatic hydrocarbons from the rat intestine. Can. J. Physiol. Pharmacol., 1986, 64, 1214–1218. [Google Scholar] [CrossRef]
- Laher, J. M.; Rigler, M. W.; Vetter, R. D.; Barrowman, J. A.; Patton, J. S. : Similar bioavailability and lymphatic transport of benzo(a)pyrene when administered to rats in different amounts of dietary fat. Journal of Lipid Research, 1984, 25, 1337–1342. [Google Scholar] [CrossRef]
- Holland, B.; Welch, A. A.; Unwin, I. D.; Buss, D. H.; Paul, A. A.; Southgate, D. A. T. : McCance and Widdowson’s – The Composition of Foods, Cambridge (England), The Royal Society of Chemistry, 1992, p. 462.
- van den Berg, M.; Sinke, M.; Wever, H. : Vehicle- dependent bioavailability of polychlorinated dibenzo- p-dioxins (PCDDs) and –dibenzofurans (PCDFs) in the rat. Chemosphere, 1987, 16, 1193–1203. [Google Scholar] [CrossRef]
- Farooqui, M. Y.; Ybarra, B.; Piper, J.; Tamez, A. : Effect of dosing vehicle on the toxicity and metabolism of unsaturated aliphatic nitriles. J. Appl. Toxicol., 1995, 15, 411–420. [Google Scholar] [CrossRef]
- Palin, K. J.; Whalley, D. R.; Wilson, C. G.; Davis, S. S.; Phillips, A. J. : Determination of gastric-emptying profiles in the rat: Influence of oil structure and volume. Int. J. Pharmacol., 1982, 12, 315–322. [Google Scholar] [CrossRef]
- Kim, H. J.; Bruckner, J. V.; Dallas, C. E.; Gallo, J. M. : Effect of dosing vehicles on the pharmacokinetics of orally administered carbon tetrachloride in rats. Toxicol. Appl. Pharmacol., 1990, 102, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Gershkovich, P.; Hoffman, A. : Uptake of lipophilic drugs by plasma derived isolated chylomicrons: linear correlation with intestinal lymphatic bioavailability. Eur J Pharm Sci., 2005, 26, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Withey, J. R.; Collins, B. T.; Collins, P. G. : Effect of vehicle on the pharmacokinetics and uptake of four halogenated hydrocarbons from the gastrointestinal tract of the rat. J. Appl. Toxicol., 1983, 3, 249–253. [Google Scholar] [CrossRef]
- Dix, K. J.; Kedderis, G. L.; Borghoff, S. J. : Vehicle- dependent oral absorption and target tissue dosimetry of chloroform in male rats and female mice. Toxicol. Lett., 1997, 91, 197–209. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2008 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, D.L.; Hood, D.B.; Ramesh, A. Vehicle-Dependent Disposition Kinetics of Fluoranthene in Fisher-344 Rats. Int. J. Environ. Res. Public Health 2008, 5, 41-48. https://doi.org/10.3390/ijerph5010041
Harris DL, Hood DB, Ramesh A. Vehicle-Dependent Disposition Kinetics of Fluoranthene in Fisher-344 Rats. International Journal of Environmental Research and Public Health. 2008; 5(1):41-48. https://doi.org/10.3390/ijerph5010041
Chicago/Turabian StyleHarris, Deacqunita L., Darryl B. Hood, and Aramandla Ramesh. 2008. "Vehicle-Dependent Disposition Kinetics of Fluoranthene in Fisher-344 Rats" International Journal of Environmental Research and Public Health 5, no. 1: 41-48. https://doi.org/10.3390/ijerph5010041




