Induction of Cell Death, DNA Strand Breaks, and Cell Cycle Arrest in DU145 Human Prostate Carcinoma Cell Line by Benzo[a]pyrene and Benzo[a]pyrene-7,8-diol-9,10-epoxide
Abstract
:Introduction
Materials and Methods
Materials
Cell Culture
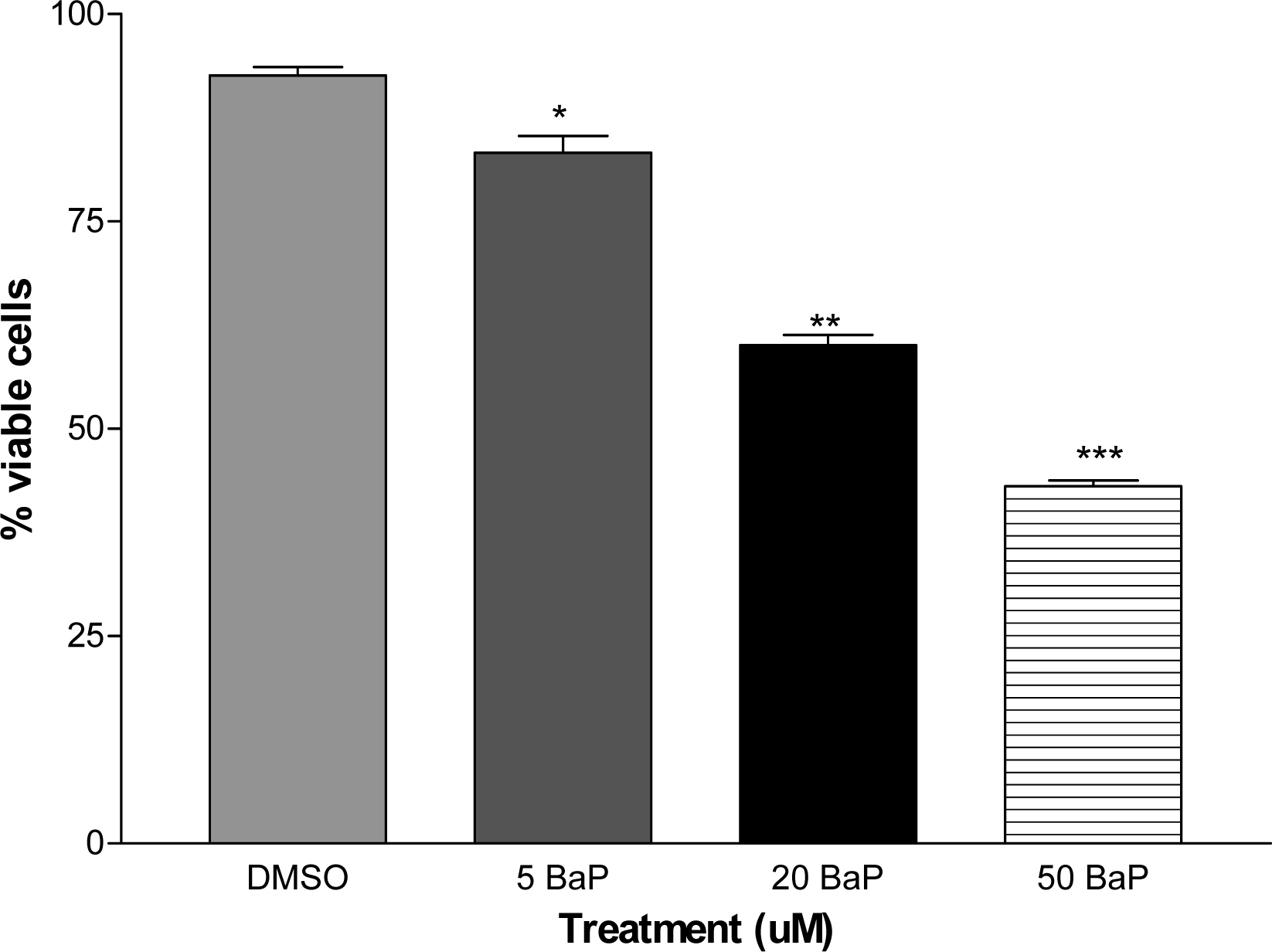
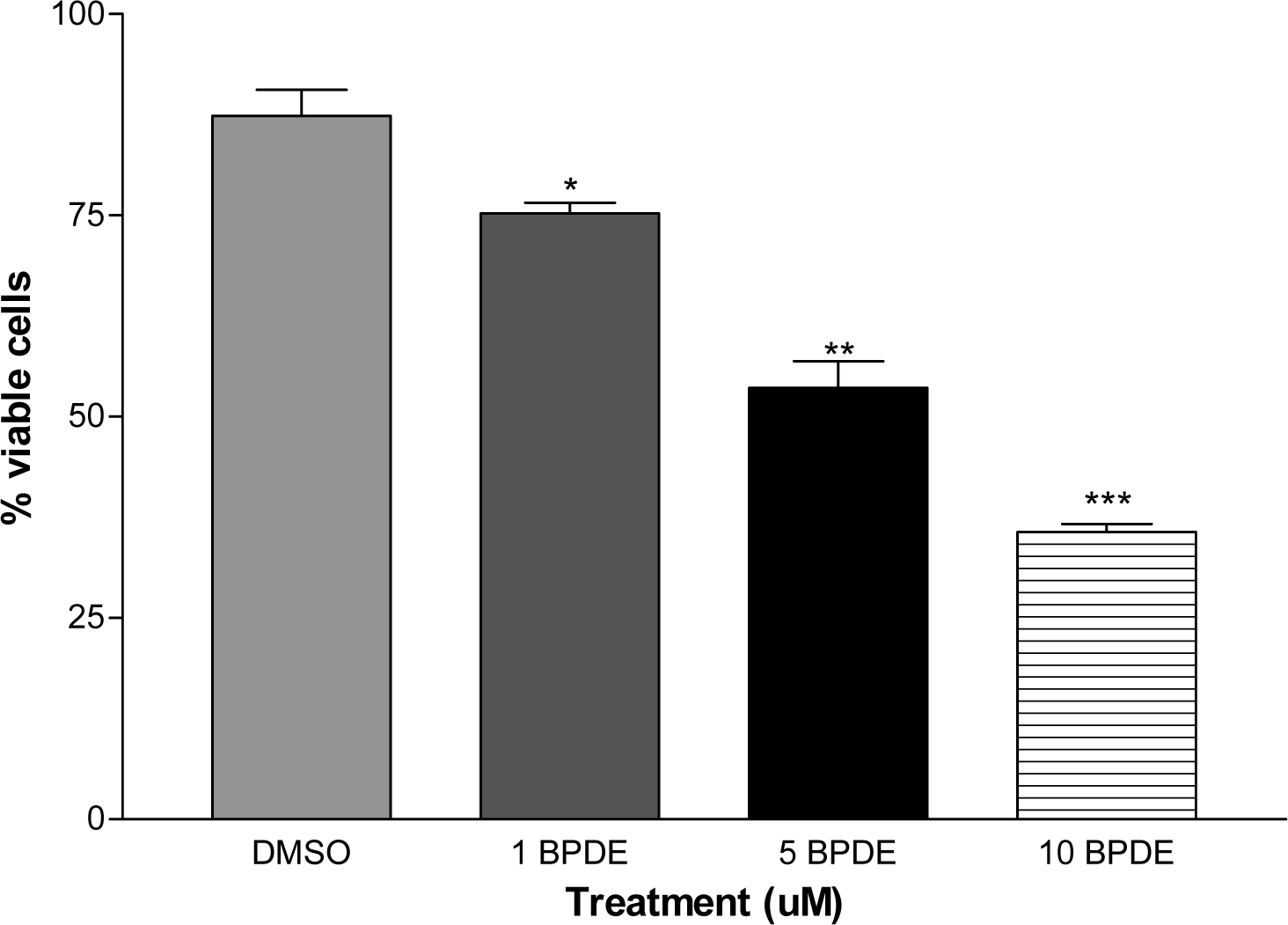
Cell Viability
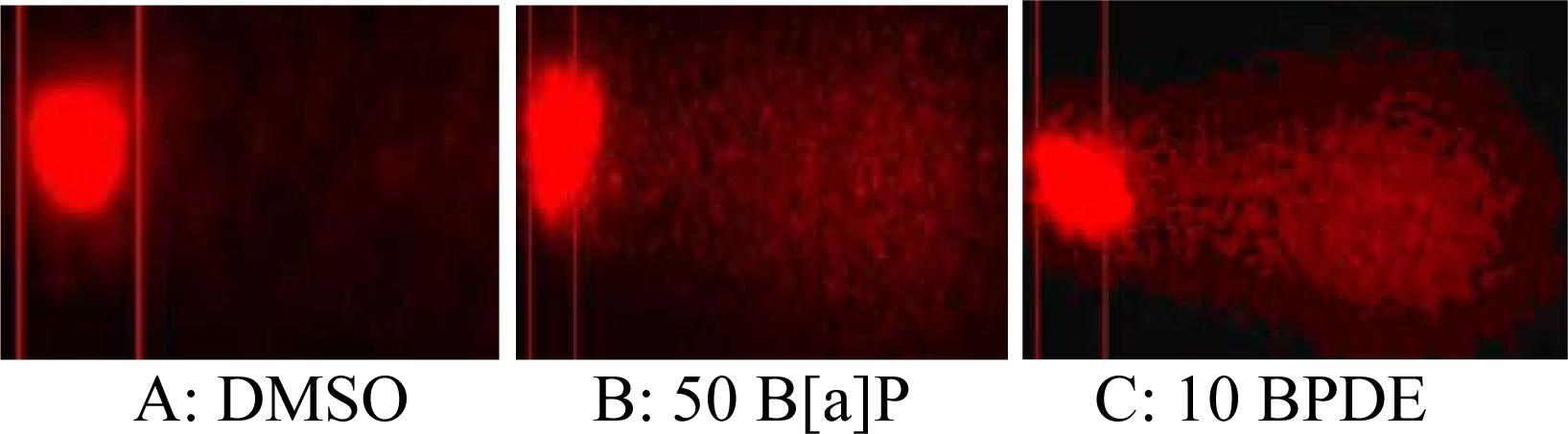
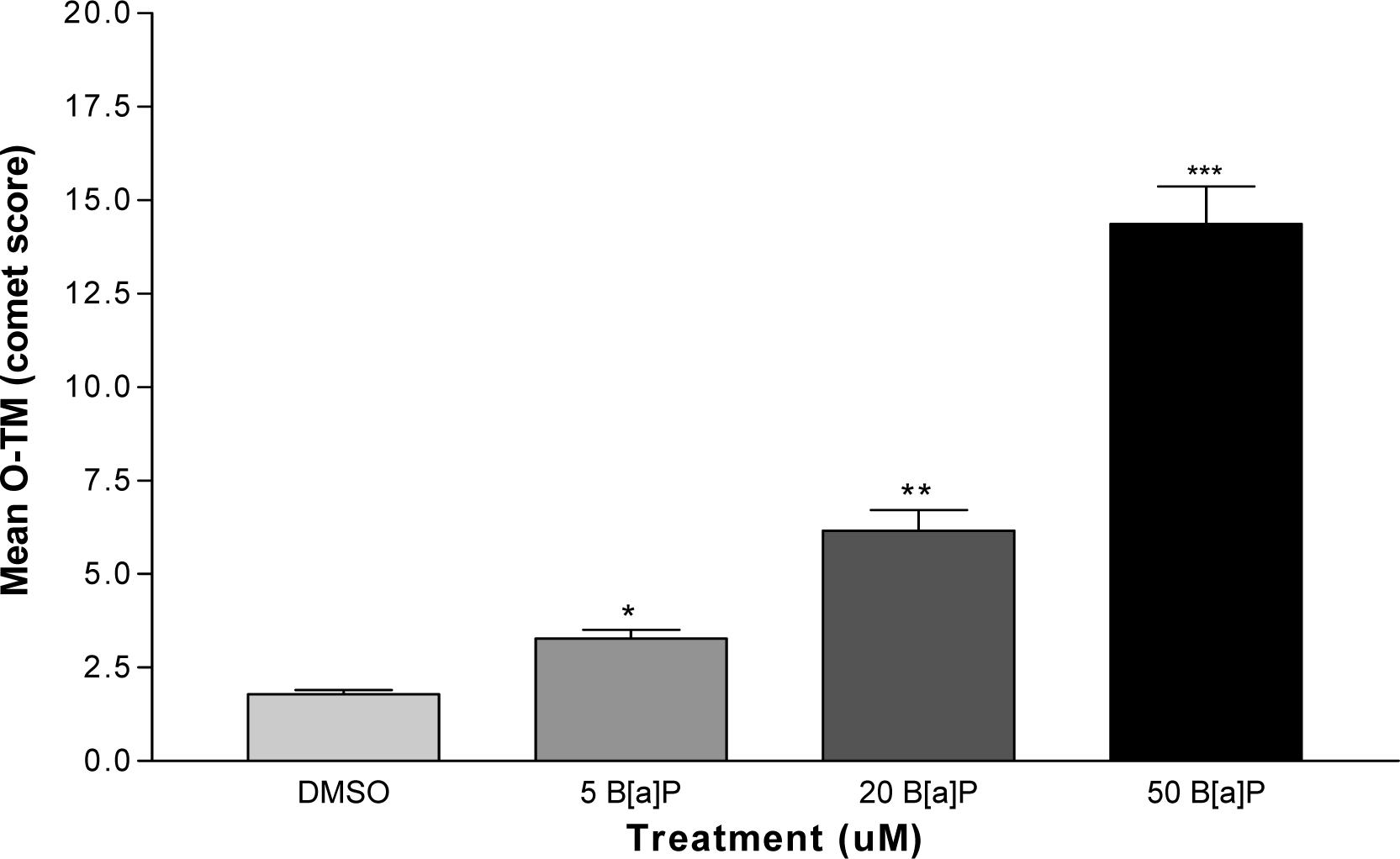
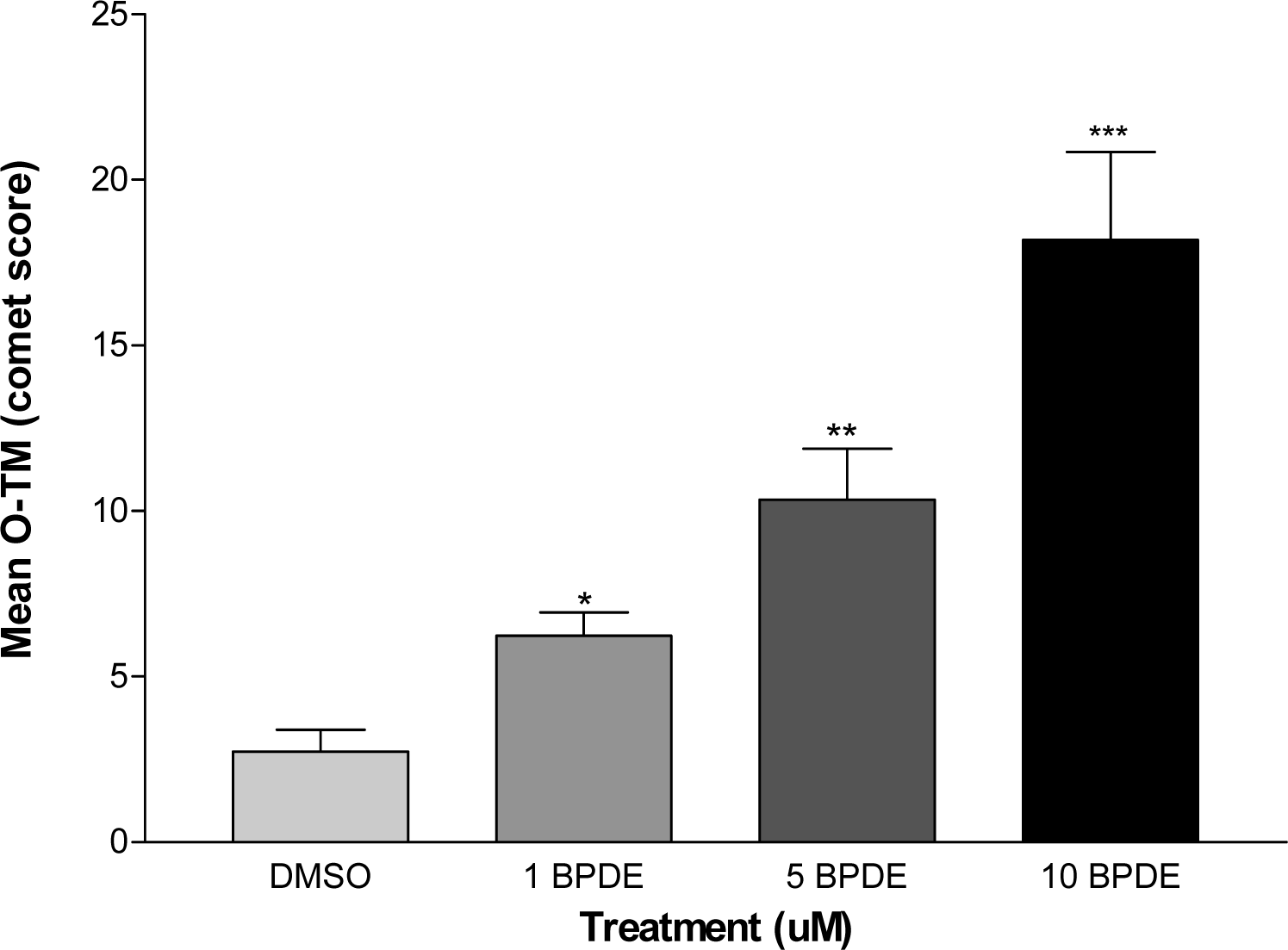
DNA Strand Breaks
Cell Cycle Analysis
Results and Discussions
Cell Cycle Analyses

| Cell line | B[a]P (μM) | % Distribution at cell cycle phase | ||
|---|---|---|---|---|
| G1 | S | G2/M | ||
| Control (0.1% DMSO) | 64.39 ± 0.03 | 14.18 ± 0.04 | 20.55 ± 0.08 | |
| 5 | 68.54 ± 0.36* | 10.86 ± 0.29 | 20.07 ± 0.42 | |
| DU145 | 20 | 69.12 ± 0.15* | 10.04 ± 0.21 | 20.47 ± 0.48 |
| 50 | 70.39 ± 0.45* | 7.22 ± 0.56 | 22.41 ± 0.62 | |
| Cell line | BPDE (μM) | % Distribution at cell cycle phase | ||
|---|---|---|---|---|
| G1 | S | G2/M | ||
| Control (0.1% DMSO) | 64.39 ± 0.03 | 14.18± 0.04 | 20.55 ± 0.08 | |
| 1 | 69.26 ± 0.57* | 9.03 ± 0.65 | 20.89 ± 0.88 | |
| DU145 | 5 | 74.63 ± 0.42* | 6.08 ± 0.26 | 18.49 ± 0.34 |
| 10 | 76.88 ± 0.35* | 5.48 ± 0.28 | 17.04 ± 0.74 | |
Acknowledgments
References
- Solhaug, A; Øvrebø, S; Mollerup, M; Låg, PE; Schwarze, S; Nesnow, JA; Holme, A. Role of cell signaling in B[a]P-induced apoptosis: Characterization of unspecific effects of cell signaling inhibitors and apoptotic effects of B[a]P metabolites. Chemico-Biological Interactions 2005, 151, 101–119. [Google Scholar]
- Mahadevan, B; Luch, A; Bravo, CF; Atkin, J; Steppan, LB; Pereira, C; Kerkvliet, NI; Baird, WM. Dibenzo[a,l]pyrene induced DNA adduct formation in lung tissue in vivo. Cancer Letters 2005, 227, 25–32. [Google Scholar]
- IACR, Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Human. Polynuclear Aromatic Compounds. Part 1: Chemical, Environmental and Experimental Data; Volume 32, International Agency for Research on Cancer: Lyon, France, 1983. [Google Scholar]
- Dipple, A. Reactions of polycyclic aromatic hydrocarbons with DNA. IARC Sci. Publ 1994, 107–129. [Google Scholar]
- Rojas, M; Marie, B; Vignaud, JM; Martinet, N; Siat, J; Grosdidier, G; Cascorbi, I; Alexandrov, K. High DNA damage by benzo[a]pyrene 7,8-diol-9,10-epoxide in bronchial epithelial cells from patients with lung cancer: comparison with lung parenchyma. Cancer Letters 2004, 207, 157–163. [Google Scholar]
- Denissenko, MF; Cahill, J; Koudriakova, TB; Gerber, N; Pfeifer, GP. Quantitation and mapping of aflatoxin B1-induced DNA damage in genomic DNA using aflatoxin B1-8,9-epoxide and microsomal activation systems. Mutat. Res 1999, 425, 205–211. [Google Scholar]
- Oh, S; Im, H; Oh, E; Lee, J; Khim, J-Y; Mun, J; Kim, Y; Lee, E; J. Kim, J; Sul, D. Effects of benzo[a]pyrene on protein expression in Jurkat T-cells. Proteomics 2004, 4, 3514–3526. [Google Scholar]
- A. Jemal, A; Murray, T; Ward, E; Samuels, A; R.C. Tiwari, RC; A. Ghafoor, A. Cancer statistics, 2005. CA Cancer J. Clin 2005, 55, 10–30. [Google Scholar]
- Fairbrain, DW; Olive, PL; O’Neill, KL. The comet assay: a comprehensive review. Mutat Res 1995, 339, 37–59. [Google Scholar]
- Olive, PL. The comet assay. An overview of techniques. Methods Mol Biol 2002, 203, 179–194. [Google Scholar]
- Dehon, DP; Dubois, GJ. Validation of raw data measurements in the comet assay. Tanata 2004, 63, 879–886. [Google Scholar]
- Kaina, B. DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem. Pharmacol 2003, 66, 1547–1554. [Google Scholar]
- Jackson, KM; DeLeon, M; Verret, CR; Harris, WB. Dibenzoylmethane induces cell cycle arrest deregulation in human prostate cancer sell. Caner Letters 2002, 178, 161–165. [Google Scholar]
© 2007 MDPI All rights reserved.
Share and Cite
Nwagbara, O.; Darling-Reed, S.F.; Tucker, A.; Harris, C.; Abazinge, M.; Thomas, R.D.; Gragg, R.D. Induction of Cell Death, DNA Strand Breaks, and Cell Cycle Arrest in DU145 Human Prostate Carcinoma Cell Line by Benzo[a]pyrene and Benzo[a]pyrene-7,8-diol-9,10-epoxide. Int. J. Environ. Res. Public Health 2007, 4, 10-14. https://doi.org/10.3390/ijerph2007010002
Nwagbara O, Darling-Reed SF, Tucker A, Harris C, Abazinge M, Thomas RD, Gragg RD. Induction of Cell Death, DNA Strand Breaks, and Cell Cycle Arrest in DU145 Human Prostate Carcinoma Cell Line by Benzo[a]pyrene and Benzo[a]pyrene-7,8-diol-9,10-epoxide. International Journal of Environmental Research and Public Health. 2007; 4(1):10-14. https://doi.org/10.3390/ijerph2007010002
Chicago/Turabian StyleNwagbara, Onyinye, Selina F. Darling-Reed, Alicia Tucker, Cynthia Harris, Michael Abazinge, Ronald D. Thomas, and Richard D. Gragg. 2007. "Induction of Cell Death, DNA Strand Breaks, and Cell Cycle Arrest in DU145 Human Prostate Carcinoma Cell Line by Benzo[a]pyrene and Benzo[a]pyrene-7,8-diol-9,10-epoxide" International Journal of Environmental Research and Public Health 4, no. 1: 10-14. https://doi.org/10.3390/ijerph2007010002




