Combustion-Generated Nanoparticulates in the El Paso, TX, USA / Juarez, Mexico Metroplex: Their Comparative Characterization and Potential for Adverse Health Effects
Abstract
:Introduction
The Nanoparticulate Paradigm
Experimental Methods and Materials
Outdoor PM Collection and Analysis
Indoor (Kitchen) Carbon Nanotube Aggregate Collection and Analysis
Cytotoxicity Assays of Surrogate Nanomaterials
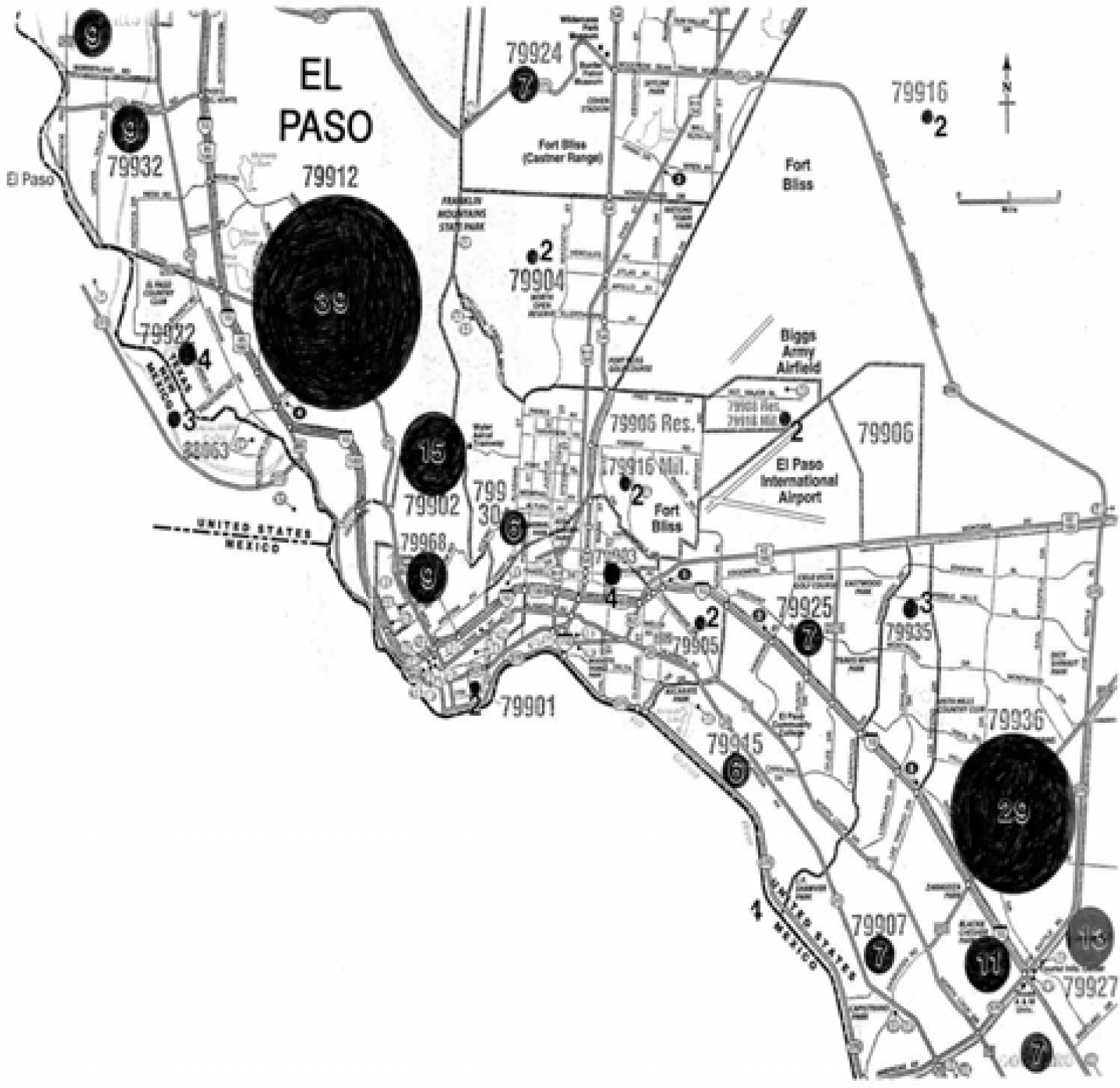
Clinical Asthma Patient Data and El Paso City-Wide Data Collection
Results
Ambient (outdoor) Nanoparticulate Analysis
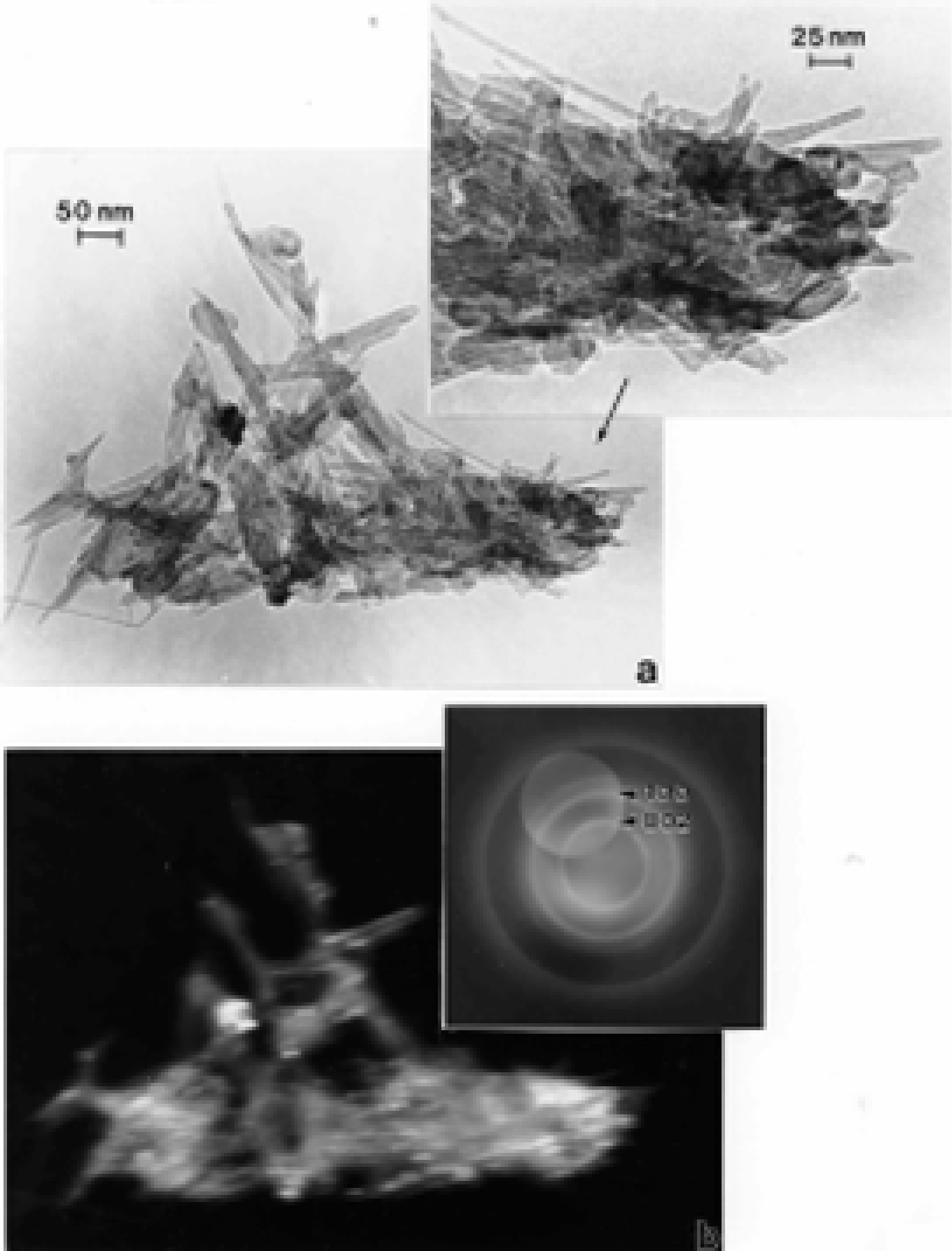
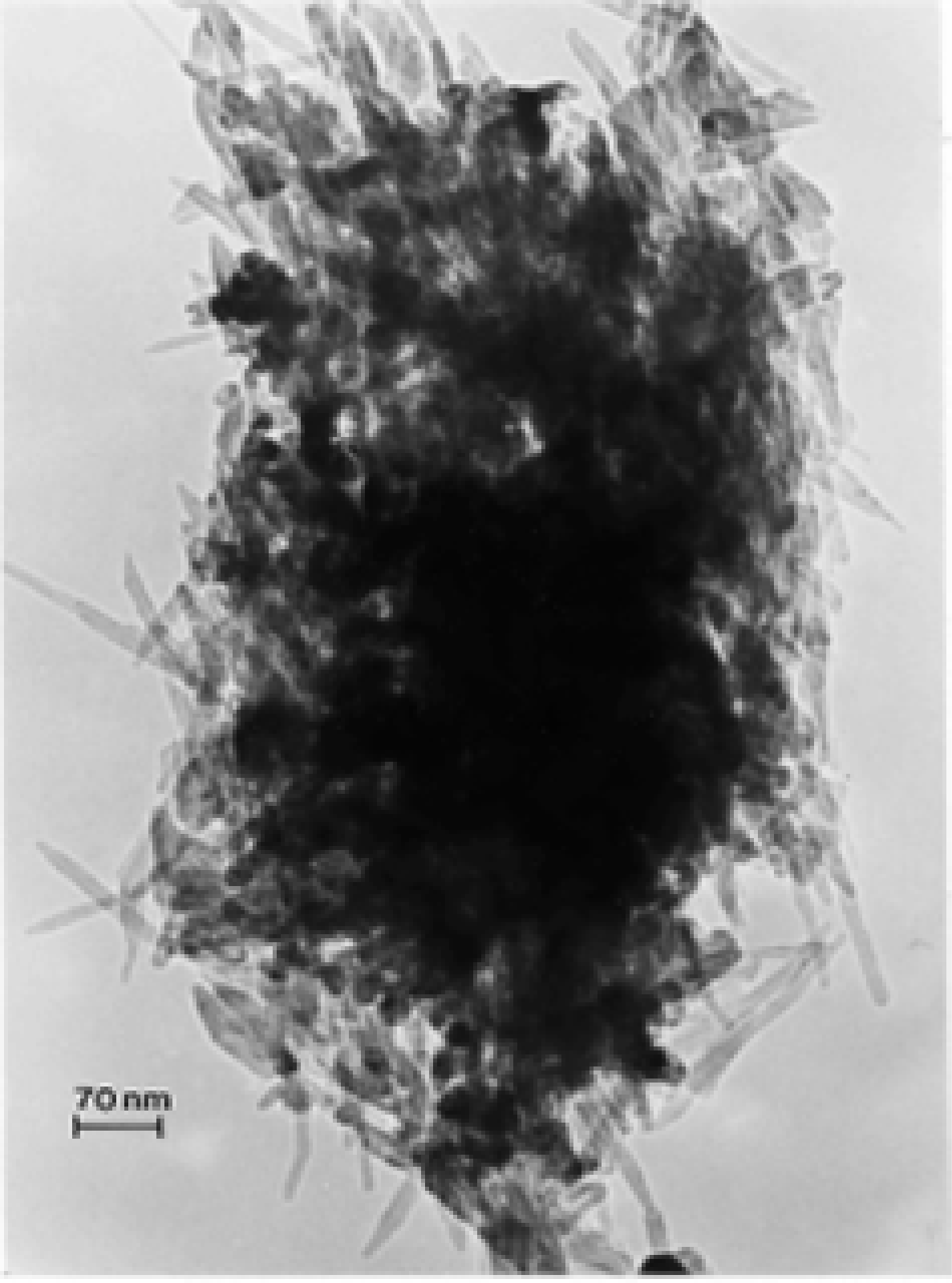
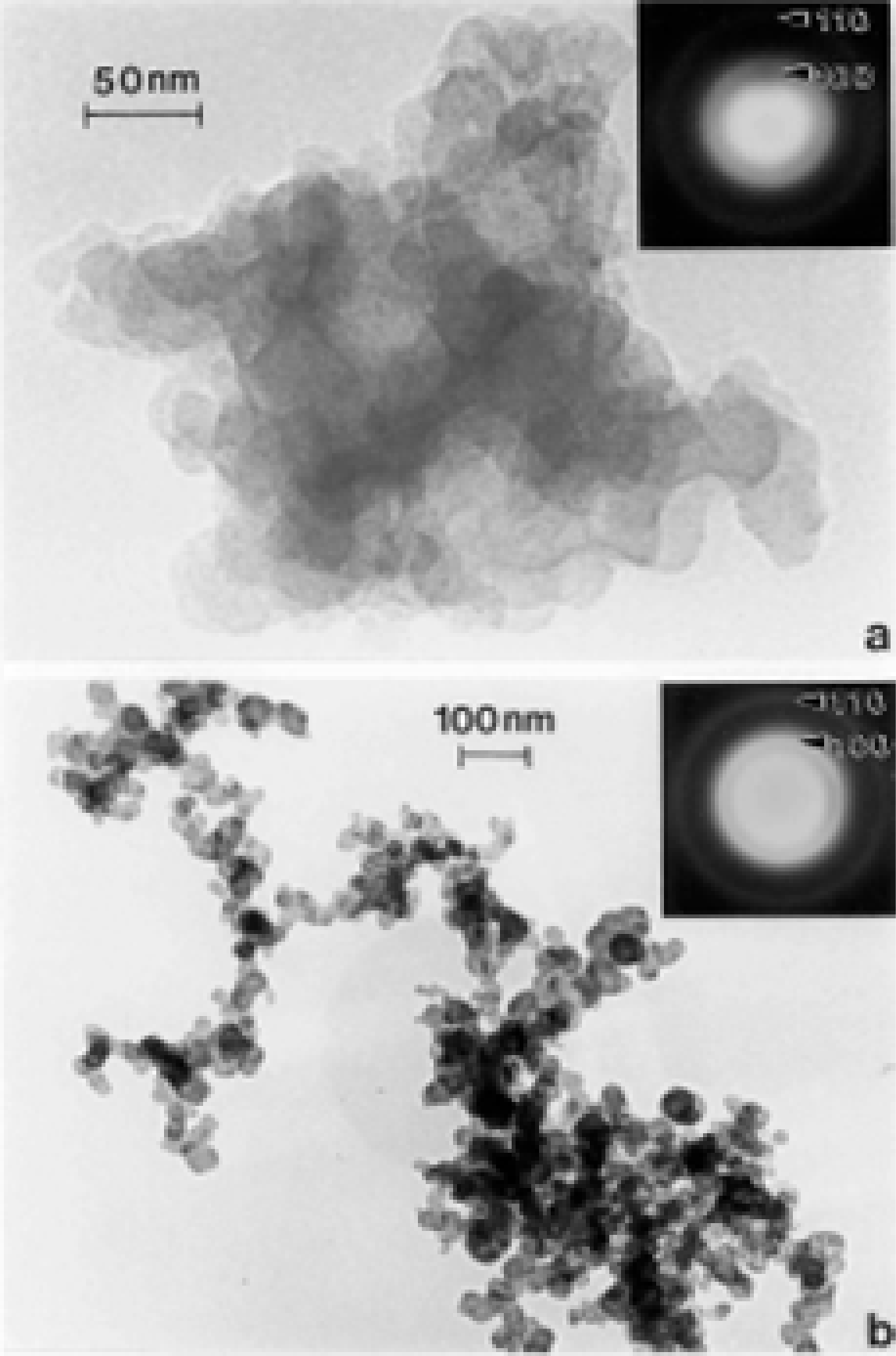
TEM Characterization of Outdoor Carbon Nanotube Aggregates
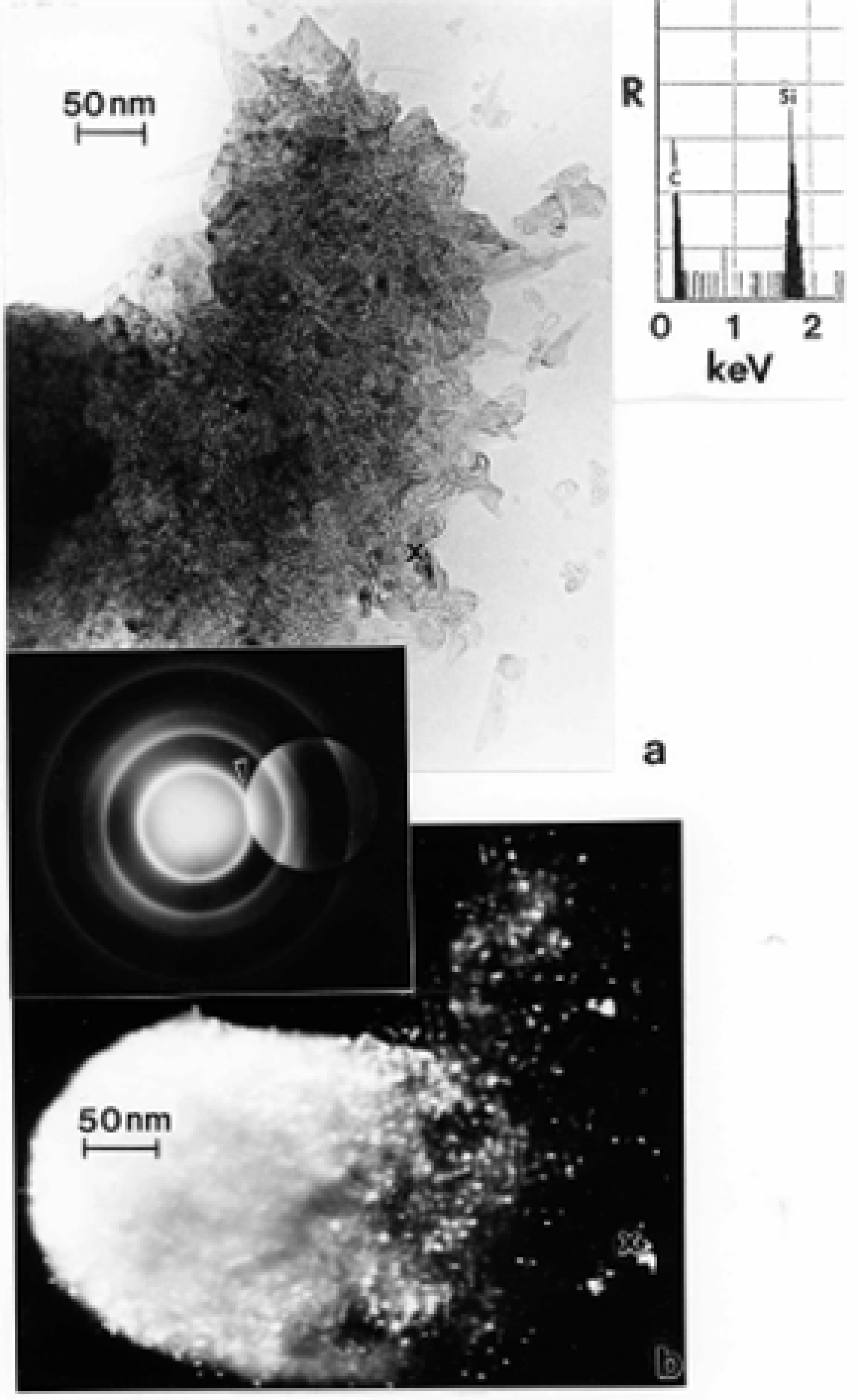
TEM Characterization of Indoor (Kitchen) Carbon Nanotube Aggregates
TEM Characterization of DPM, WPM, TPM,
Simple Microstructural Comparisons of Carbon Nanotubes and Soot PM
Cytotoxicity Assays
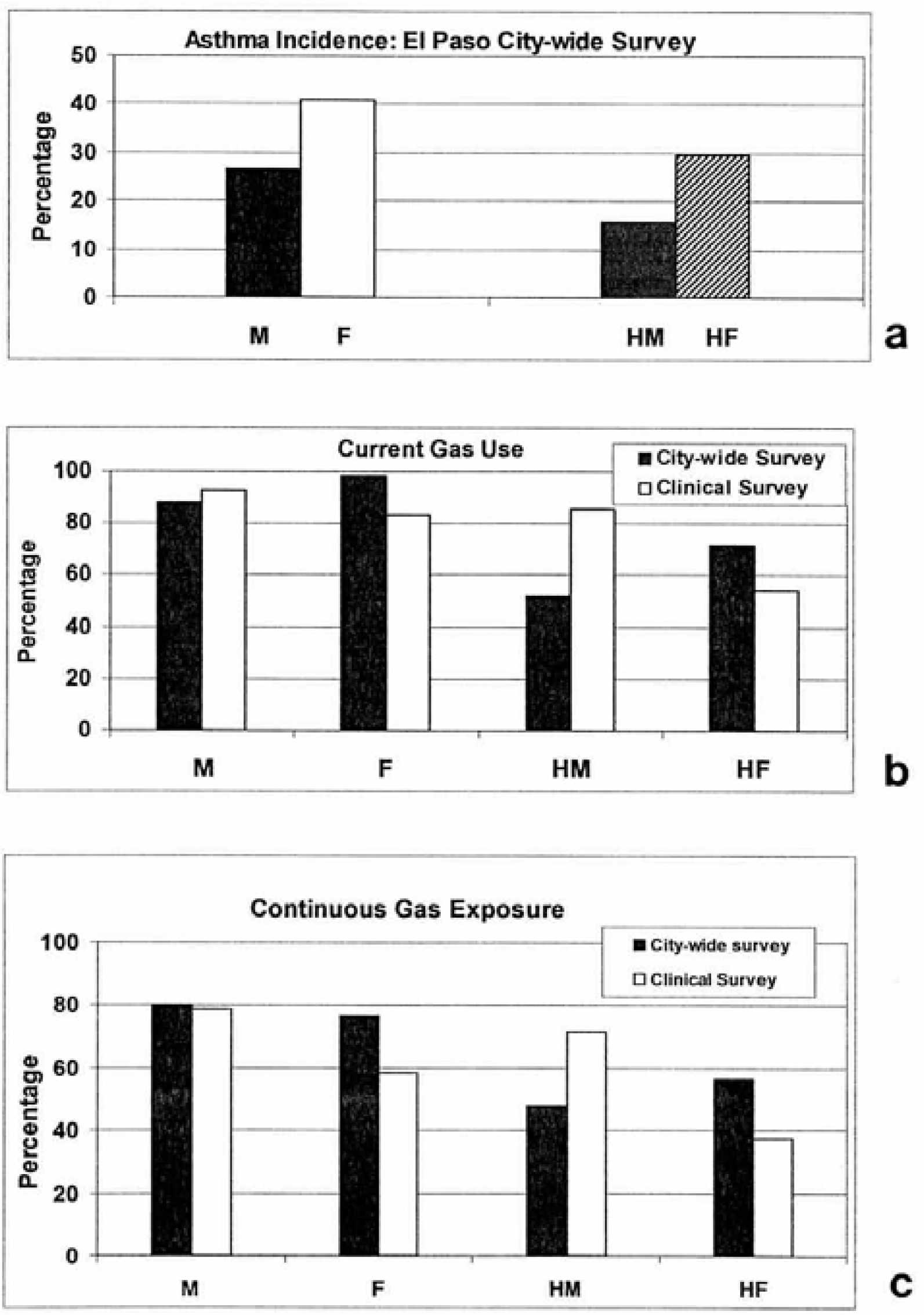
Clinical and El Paso City-Wide Survey Data Analysis
Discussion
Conclusions
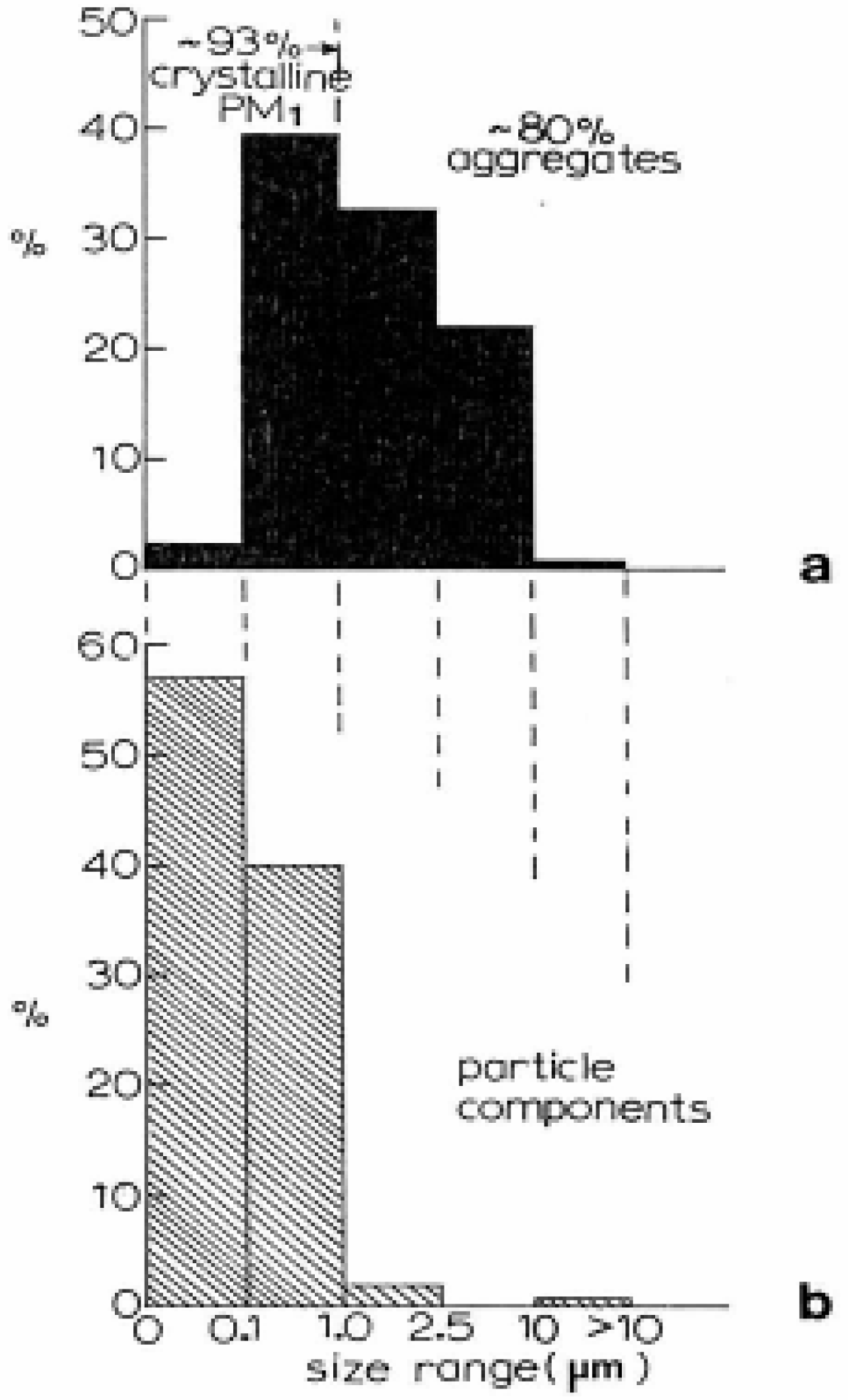
- 93% of the PM, fraction in the El Paso, TX air is crystalline while 80% of this PM is aggregated. 57% of the aggregated PM is composed of primary particulates with mean diameters less than 100 nm.
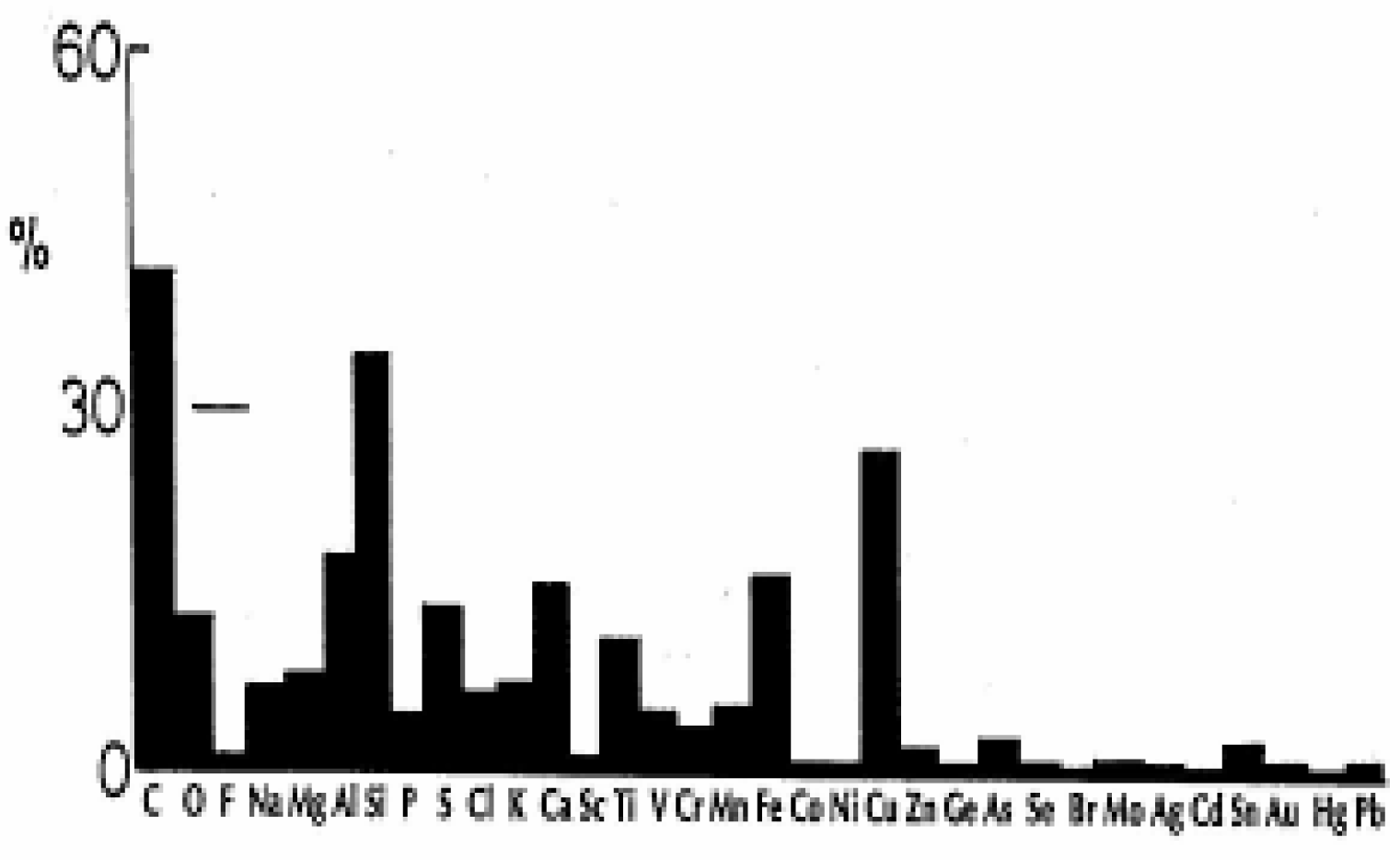
- 42% of the El Paso, TX PM2.5 contains carbon and of this PM roughly half represents anthropogenic soot: diesel, wood and tire combustion products and brake lining debris. The other half is essentially carbon nanotube aggregates (∼10%) which are ubiquitous in the ambient (outdoor) air even at nominal concentrations of 102 to 103/m3, and carbonates (∼5%). These nanoparticle aggregates originate from natural gas and other gas combustion sources venting to the atmosphere: water heaters, furnaces (both residential and industrial), power generation plants, etc. Aggregates of carbon nanotubes, fullerene polyhedra, and nanoparticulate silica (SO2) are a common occurrence in the ambient air.
- The indoor (kitchen) number concentration of carbon nanotube aggregates is approximately 103/m3 to 105/m3 during gas use (above the gas burners). Carbon spherule aggregates or soot, appearing like DPM or other complex, branched structures also occur at similar number concentrations.
- The fractile-like, branched, aggregated spherules of turbostratic or more regular (even) fullerenic graphene fragments are indistinguishable structures characteristic of diesel, wood, or tire combustion soot.
- In contrast to the irregular or turbostratic graphene fragment structure characteristic of anthropogenic soots, with primary spherule particle sizes ranging from 20 to 80 nm, carbon nanotube aggregates are variously mixed multiwall carbon nanotubes (ranging in diameters from 10 to 40 nm) and concentric fullerene polyhedra having similar diameters.
- Black carbon (BC) and a multi-wall carbon nanotube aggregate surrogate were demonstrated to be cytotoxic for a murine macrophage cell line for exposures ranging from 2 days to 2 weeks. Both a chrysotile asbestos control and the MWCNT surrogate effectively killed the macrophage cells but the MWCNT surrogate demonstrated both apoptosis and necrosis in contrast to the asbestos-induced apoptosis.
- Analysis of El Paso City-wide survey data indicated that 34% of all respondents were asthmatic; nearly 6 times the U.S. national average of 6%. Nearly an equal number of respondents were smokers but amongst asthmatics 51% were smokers. Correspondingly, only 28% of clinical asthma respondents were smokers. Approximately 78% of City-wide respondents were continuously exposed to gas stove use; slightly less for clinical respondents. The current gas use is considerably higher amongst Hispanic females in contrast to Hispanic males for both the City-wide survey data and the clinical survey data. Considering asthma migration and hereditary factors, gas use or exposure and asthma may be circumstantial rather than problematic.
- Because of the ability of various particulates, especially nanoparticulates, to induce reactive oxygen species as well as transition metal ions, adsorbed PAHs, and other gas-phase species, including CO, NOx, ozone, etc., respiratory adversities are characterized by intermittent and long-term exposures to a complex synergy of inflammatory agents, especially in a complex region like the Paso del Norte air shed.









| City** | Asthma Prevalence U.S. Rank (2002) | Worst Asthma*† Cities Rank (2005) | % Hispanic (1990) | Homes with Gas (1990) | City Population (1990) | % Estimated Gas Exposure* Population: 1990 (%) | Clear Days Ave. (1997) | Fugitive Air† TCR 1996 (lbs.) | SO2 (1996) (lbs) | NOX (96) (lbs) | VOC†† (96) (lbs) | CO (96) (lbs) | PM10 (96) (lbs) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ELP | 6 | 67 | 74 | 136138 | 602951 | 90 | 192 | 134328 | 9026 | 32670 | 33929 | 142337 | 15509 |
| ABQ | 7 | 77 | 38 | 133838 | 426736 | 100 | 168 | 4848 | 4365 | 37505 | 36799 | 223214 | 86547 |
| PHX | 3 | 14 | 20 | 150326 | 1139793 | 53 | 210 | 641475 | 5971 | 154710 | 139195 | 536117 | 68244 |
| TUC | 1 | 61 | 28 | 105824 | 472385 | 90 | 194 | 105970 | 9805 | 49424 | 43436 | 218623 | 35286 |
| LA | -- | 42 | 38 | 957619 | 3498138 | 100 | 143 | 4268754 | 27047 | 328380 | 415073 | 1726306 | 105181 |
| SDG | -- | 82 | 25 | 110982 | 1168364 | 38 | 146 | 402010 | 5391 | 92144 | 113722 | 497027 | 88532 |
| HON | -- | -- | 7 | 7381 | 878044 | 3 | 90 | 318432 | 21380 | 32069 | 23994 | 166518 | 24239 |
| OMA | 24 | 45 | 4 | 117630 | 350602 | 100 | 111 | 615710 | 23477 | 42233 | 36334 | 166820 | 30239 |
| BOS | -- | 66 | 5 | 93179 | 552519 | 67 | 98 | 23556 | 8495 | 28363 | 18606 | 140723 | 15957 |
Acknowledgments
References
- D’Amato, G. Urban air pollution and plant-derived respiratory allergy. Clin. and Exper. Allergy 2000, 30, 628–636. [Google Scholar]
- Salvi, SS; Holgate, ST. Is diesel exhaust a cause for increasing allergies? Clin. and Exper. Allergy 1999, 29(9), 1187–1194. [Google Scholar]
- Zhiqiang, Q; Siegmann, K; Keller, A; Matter, U; Scherrer, L; Siegmann, HC. Nanoparticle air pollution in major cities and its origin. Atmos. Environ 2000, 34, 443–451. [Google Scholar]
- Renwick, LC; Donaldson, K; Clouter, A. Impairment of alveolar macrophage/phagocytosis by ultrafine particles. Toxicol. and Appl. Pharmacol 2001, 172, 119–127. [Google Scholar]
- Samet, JM; Dominici, F; Curriero, FC; Coursac, I; Zeger, SC. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N. Eng. J. of Med 2000, 343(24), 1742–1749. [Google Scholar]
- Krewski, P; Burnett, R; Goldberg, MS; Hoover, K; Siemiatycki, J; Jerrett, M; Abramowicz, M; White, W. 22 others:; Reanalysis of the Harvard six cities study and the American cancer society study of particulate air pollution and mortality: Investigators Report, Part 1: Replication and validation; Part II; 2000; Sensitivity analysis health effects institute: Boston, MA. [Google Scholar]
- Glousky, MM; Miguel, AG; Cass, GR. Particulate air pollution: possible relevance in asthma. Allergy Asthma Proc. 1997, 18(3), 163–166. [Google Scholar]
- Mauderly, JL. Diesel emissions: Is more health research still needed? Toxicol. Sci. 2001, 62(1), 6–12. [Google Scholar]
- Buseck, PR; Posfai, M. Airborne mineral and related aerosol particles: Effects on climate and the environment. Proc. Nat. Acad. Sci 1999, 96, 3372–3379. [Google Scholar]
- Michaels, R. A: Particulate matter policy. Science 1997, 278, 1696–1620. [Google Scholar]
- Andreae, MO. In Future climates of the world: A modeling perspective, world survey of climatology; Henderson-Sellers, A, Ed.; Elsevier Science: Amsterdam, 1995; Volume 16, pp. 341–392. [Google Scholar]
- Chameides, WL; Bergin, M. Soot takes center stage. Science 2002, 297, 2214–2215. [Google Scholar]
- Murr, LE; Bang, JJ. Electron microscope comparisons of fine and ultra-fine carbonaceous and non-carbonaceous, airborne particulates. Atmos. Environ 2003, 37, 4795–4806. [Google Scholar]
- Warheit, DB. Nanoparticles: Health impacts? Materials Today 2004, 32–35. [Google Scholar]
- Momarca, S; Creberlli, R; Feretti, D; Zanardini, A; Fuselli, J; Fillinin, L. Mutagenes and Carcinogenesis in size-classified air particulates of a northern Italian town. Sci. Total Environ 1997, 205(2–3), 137–144. [Google Scholar]
- Nikula, KJ; Snipes, MB; Barr, EB; Griffith, WC; Henderson, RF; Mauderly, JL. Comparative pulmonary toxicities and carcinogenicities of chronically inhaled diesel exhaust and carbon black in F344 rats. Fund. Appli. Toxicol 1995, 25(1), 80–94. [Google Scholar]
- Heinrich, U; Muhle, H; Takenaka, S; Ernst, E; Fahst, R; Mohr, U; Pott, F; Stöber, W. Chronic effects on the respiratory tract of hamsters, mice, and rats after long-term inhalation of high concentration of filtered and unfiltered diesel engine emissions. J .Appl. Toxicol 1986, 6, 383–395. [Google Scholar]
- Brightwell, J; Fouillet, X; Cassano-Zoppi, AL; Bernstein, D; Crawley, F; Duchostal, F; Gatz, R; Perezel, S; Pfeifer, H. Tumors of the respiratory tact in rats and hamsters following chronic inhalation of engine exhaust emissions. J. Appl. Toxicol 1989, 9, 23–31. [Google Scholar]
- Heinrich, U; Fuhst, R; Rittinghausen, S; Creutzenberg, O; Bellman, B; Koch, W; Lessen, K. Chronic inhalation exposure of Wistar rats and two different strains of mice to diesel engine exhaust, carbon black, and titanium dioxide. Inhalation Toxicol. 1995, 7(4), 533–556. [Google Scholar]
- Gerde, P; Muggenburg, BA; Lundborg, M; Dahl, AR. The rapid alveolar absorption of diesel soot-adsorbed benzo[a]pyrene: Bioavailabiity, metabolism and dosimetry of an inhaled particle-borne carcinogen. Carcinogenesis 2001, 22(5), 741–749. [Google Scholar]
- Sato, H; Sone, H; Sagai, M; Suzuki, KT; Yasunobu, A. Increase in mutation frequency in lung of big blue rat by exposure to diesel exhaust. Carcinogenesis 2000, 21(4), 653–661. [Google Scholar]
- Katrinak, KA; Rez, P; Perkes, PR; Buseck, PR. Fractal geometry of carbonaceous aggregates from an urban aerosol. Environ. Sci. Technol 1993, 27, 539–547. [Google Scholar]
- Katrinak, KA; Anderson, JR; Buseck, PR. Individual particle types in the aerosol of Phoenix, Arizona. Environ. Sci. Technol 1995, 29, 321–329. [Google Scholar]
- Huntzicker, JJ; Heyerdahl, EK; McDaw, SR; Rau, JA; Griest, WH; MacDougall, CS. Combustion as the principal source of carbonaceous aerosol in the Ohio river valley. J. Air Pollution Control Assoc 1986, 36, 705–709. [Google Scholar]
- Kleeman, MJ; Eldering, A; Cass, GR. Modeling the airborne particulate complex as a source-oriented external mixture. J. Geophys. Res. 1997, 102(D17), 2803–2816. [Google Scholar]
- Murr, LE; Esquivel, EV; Bang, JJ. Characterisation of nanostructure phenomena in airborne particulate aggregates and their potential for respiratory health effects. J. Mater. Sci.: Materials in Medicine 2004, 15, 237–247. [Google Scholar]
- Murr, LE; Bang, JJ; Lopez, DA; Guerrero, PA; Esquivel, EV; Choudhuri, AR; Subramanya, M; Morandi, M; Holian, A. Carbon nanotubes and nanocrystals in methane combustion and the environmental implications. J. Mater. Sci. (Letters) 2004, 39, 2199–2204. [Google Scholar]
- Murr, LE; Bang, JJ; Esquivel, EV; Guerrero, PA; Lopez, DA. Carbon nanotubes, nanocrystal forms, and complex nanoparticle aggregates in common fuel gas combustion streams. J. Nanoparticle Res 2004, 6, 241–251. [Google Scholar]
- Nikula, KJ; Vallyathan, V; Green, FH; Hahn, EF. Influence of exposure concentration or dose on the distribution of particulate material in rat and human lungs. Environ. Health Perspect. 2001, 109(4), 311–328. [Google Scholar]
- Seaton, A; Macnee, W; Donaldson, K; Godden, D. Particulate air pollution and acute health effects. Lancet 1995, 345, 176–178. [Google Scholar]
- Lam, C; James, JT; McCluskey, R; Hunter, RL. Pulmonary toxicity of carbon nanotubes in mice 7 and 90 days after intratrachael instillation. Toxicologist 2004, 77(1), 126. [Google Scholar]
- Shvedova, AA; Castranova, V; Kisin, ER; Murray, AR; Schwegler-Berry, D; Gandelsman, VZ; Maynard, A; Baron, P. Exposure to nanotube material: Assessment of nanotube cytotoxicity using human keratinocyte cells. J. Toxicol. Environ. Health 2003, 66, 1901–1918. [Google Scholar]
- Warheit, DB; Lawrence, BR; Reed, KL; Roach, DH; Reynolds, GAM; Webb, TR. Comparative pulmonary toxicity assessment of singlewall carbon nanotube in rats. Toxicol. Sci 2004, 77, 117–125. [Google Scholar]
- Murr, LE; Garza, KM; Soto, KF; Carrasco, A; Powell, TG; Ramirez, DA; Guerrero, PA; Lopez, DA; Venzor, J, III. Cytotoxicity assessment of some carbon nanotubes and related carbon nanoparticle aggregates and the implications for anthropogenic carbon nanotube aggregates in the environment. Int. J. Environ. Res. Public Health 2005, 2, 31–42. [Google Scholar]
- Soto, KF; Carrasco, A; Powell, TG; Garza, KM; Murr, LE. Comparative in vitro cytotoxicity assessment of some manufactured nanoparticulate materials characterized by transmission electron microscopy. J. Nanoparticle Res 2005, 7, 145–169. [Google Scholar]
- Murr, LE; Soto, KF; Esquivel, EV; Bang, JJ; Guerrero, PA; Lopez, DA; Ramirez, DA. Carbon nanotubes an d other fullerene-related nanocrystals in the environment: A TEM study. JOM 2004, 56(6), 28–31. [Google Scholar]
- Oberdörster, G; Gelein, R-M; Ferin, J; Loeiss, B. Association of particle air pollution and acute mortality: involvement of ultrafine particles? Inhal. Toxicol 1995, 7, 111–124. [Google Scholar]
- Oberdörster, G. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Health 2001, 74, 1–8. [Google Scholar]
- Lightly, JS; Veranth, JM; Sarofim, AF. Combustion aerosols: factors governing their size and composition and implications to human health. J. Air & Waste Manage. Assoc 2000, 50, 1565–1618. [Google Scholar]
- Salvi, S; Holgate, ST. Mechanisms of particulate matter toxicity. Clinical and Exp. Allergy 1999, 29, 1187–1194. [Google Scholar]
- Abdul-Khalek, IS; Kittelson, DB; Graskow, BR; Wei, Q; Brear, F. Diesel Exhaust Particle Size: Measuremet Issues and Trends; Soc. Automotive Engineers: Warrendale, PA, 1998; p. 980525. [Google Scholar]
- Hughes, LS; Cass, GR; Jones, J; Ames, M; Olmec, L. Physical and chemical characterization of atmospheric ultrafine particles in the Los Angeles area. Environ, Sci. Technol 1998, 32, 1153–1161. [Google Scholar]
- Wilson, WE; Suh, HH. Fine particles and coarse particles. Concentration relationships relevant to epidemiological studies. J. Air Waste Mangag. Assoc 1997, 47, 1238–1249. [Google Scholar]
- Bang, JJ; Murr, LE. Collecting and characterizing atmospheric nanoparticles. J. of Metals 2002, 54(2), 28–30. [Google Scholar]
- Bang, JJ; Trillo, EA; Murr, LE. Utilization of selected area electron diffraction patterns for characterization of air submicron particulate matter collected by a thermal precipitator. J. of the Air and Waste Management Association 2003, 53, 227–236. [Google Scholar]
- Murr, LE. Electron and ion microscopy and microanalysis: Principles and Applications; Marcel Dekker, Inc.: New York, 1991. [Google Scholar]
- Baron, PA; Willeke, K. Aerosol fundamentals, Chap. 3 in Aerosol MeasurementBaron, PA, Willeke, K, Eds.; 2nd Edition; Wiley: New York, 2001; pp. 45–60. [Google Scholar]
- Hofer, F; Mitterbauer, C; Papst, I; Pabst, MA. EFTEM tells us what the Tyrolean Iceman inhaled 5300 years ago. EUREM 12, Brno, Czech Republic 2000, 9–14, B413–B414. [Google Scholar]
- Brand, P; Roub, K; Gebhart, J. Performance of mobile aerosol spectrometer for an in-situ characterization of environmental aerosols in Frankfurt City. Atmospheric Environment 1992, 26A, 2451–2457. [Google Scholar]
- Schwartz, J; Marcus, A. Motality and air pollution in London: A time series analysis. American J. of Epidemiology 1990, 131, 185–194. [Google Scholar]
- Watts, WFJ. Assessment of occupational exposure to diesel emissions. In Diesel Exhaust: A Critical analysis of emissions, exposure, and health effects; Group, DW, Ed.; Health Effects Institute: Cambridge, M. A., 1995; pp. 107–123. [Google Scholar]
- Evans, M; Vithanadurage, I; Williams, A. An investigation of the combustion of wood. J. of Fuel and Energy 1981, 179–186. [Google Scholar]
- Clague, ADH; Donnet, JB; Wang, TK; Peng, JCM. A comparison of diesel engine soot with black carbon. Carbon 1999, 37, 1553–1565. [Google Scholar]
- Grieco, WJ; Howard, JB; Rainey, LC; Vander Sande, JB. Fullerenic carbon in combustion-generated soot. Carbon 2000, 398, 597–614. [Google Scholar]
- Haynes, BS; Wanger, HG. Sooting structure in a laminar diffusion flame. Ber. Bunser-Ges. Physical Chemistry 1980, 84(5), 499–506. [Google Scholar]
- Murr, LE; Soto, KF. A TEM study of soot, carbon nanotubes, and related fullerene nanopolyhedar in common fuel-gas combustion sources. Mater. Characterization 2005, 55, 50–65. [Google Scholar]
- Weber, AP. Characterization of the geometrical properties of agglomerated aerosol particles, Paul Scherrer Inst. Labor Fair Radiochemic 1992, 129. [Google Scholar]
- Skillas, G; Kunxel, S; Burtscher, H; Baltensperger, U; Siegman, K. High fractal-like dimension of diesel soot agglomerates. J. Aerosol Sci. 1998, 29(4), 411–419. [Google Scholar]
- Guo, T; Nikolaev, P; Rinzler, AG; Tomanek, D; Colbert, DT; Smalley, RE. Self-assembly of tubular fullerenes. J. Phys. Chem 1995, 99, 10694–10697. [Google Scholar]
- Harris, PJF. Carbon nanotubes and related structures; Cambridge Univ. Press: England, 2003. [Google Scholar]
- Lair, SL; Herndon, WA; Murr, LE; Quinones, SA. End cap nucleation of carbon nanotubes. Carbon 2006, 44, 447–455. [Google Scholar]
- Chianelli, RA; Yacaman, MJ; Arenas, J; Aldape, F. Atmospheric nanoparticles in photocatalytic an d thermal production of atmospheric pollutants. J. Hazardous Sub. Res 1998, 1, 1–17. [Google Scholar]
- Jacobson, M. Reactive oxygen species and programmed cell death. Trends Biochem 1996, 21, 83–86. [Google Scholar]
- Broaddus, VC; Yang, L; Scavo, LM; Ernst, JD; Boylan, AM. Asbestos induces apoptosis of human and rabbit pleural messothelial cells via reactive oxygen species. J. Clin. Invest. 1996, 98(6), 2050–2059. [Google Scholar]
- Kamp, DW; Aljandali, A; Pollack, N. Asbestos induces apoptosis in cultured alveolar epithelial cells. Am. J. Respir. Crit. Care Med 1998, 157, 385–394. [Google Scholar]
- Jimenez, LA; Zenela, C; Fung, H; Janseen, YMW; Vacek, P; Charland, C; Goldberg, J; Mossman, BT. Role of extra cellular signal-regulated protein Kinases in apoptosis by asbestos and H2O2. Am. J. Physiol 1997, 273, 1029–1035. [Google Scholar]
- Soto, KF; Garza, KM; Murr, LE. Biological effects of nanoparticulate materials. Mater. Sci. Engng. 2005, (in press).. [Google Scholar]
- Arrieta, DE; Ontiveros, CC; Li, W- W; Garcia, JH; Denison, MS; McDonald, JP; Burchiel, SW; Washburn, BS. Aryl hydrocarbon receptor-medicated activity of particulate organic matter from the Paso del Norte air shed along the U.S.-Mexico Border. Environmental Health Perspectives 2003, 111(10), 1299–1305. [Google Scholar]
- Herndon, WC; Chen, H-T; Zhang, Y; Rum, G. QSAR study of PAH carcinogenic activities: test of a general model for molecular similarity analysis. In Mol. Modeling Predict of Bioactivity; Gundertotte, Jorgensen, Eds.; Kluwer Academic/Plenum Publishers: New York, 2000; pp. 47–52. [Google Scholar]
- Kafoury, RM; Kelley, J. Ozone Enhances Diesel Exhaust Particles (DEP)-Induced Interleukin-8 (IL-8) Gene Expression in Human Airway Epithelial Cells through Activation of Nuclear Factors- κB (NF-κB) and IL-6 (NF-IL6). Int. J. Environ. Res. Public Health 2005, 2(3), 403–410. [Google Scholar]
- Manning, CB; Vallyathan, V; Mossman, BT. Diseases caused by asbestos: mechanisms of injury and disease development. Int. Immunopharmacol 2002, 2, 191–200. [Google Scholar]
- Pinnick, RG; Fernandez, G; Martinez-Andazola, E; Hinds, BD; Hansen, ADA; Fuller, K. Aerosol in the arid Southwestern United States: Measurements of mass loading, volatility, size distribution, adsorption characteristics black carbon content, and vertical structure of 7 km above sea level. J. Geophys. Res. 1993, 98(D2), 2651–2666. [Google Scholar]
- Junker, C; Sheehan, JN; Jennings, SG; O’Brien, P; Hinds, BD; Martinez-Tuary, E; Hansen, ADA; White, C; Garvey, DM; Pinnick, RG. Measurement and analysis of aerosol and black carbon in the southwestern United States and Panama and their dependence on air mass origin. J. Geophys. Res. 2004, 109(D1301), 1–23. [Google Scholar]
- Chylek, P; Seivastava, V; Cahenzli, L; Pinnick, RG; Dod, RL; Novakov, T; Cook, TL; Hinds, BD. Aerosol and graphitic carbon content of snow. J. Geophys. Res. 1987, 92(08), 9801–9809. [Google Scholar]
- Murr, LE; Esquivel, EV; Bang, JJ; de la Rosa, G; Gardea-Torresdey, JL. Chemistry and nanoparticulate compositions of a 10,000 year-old ice core melt water. Water Res 2004, 38, 4282–4296. [Google Scholar]
- Holgate, ST; Bodey, KS; Janezic, A; Frew, AJ; Kaplan, AP; Teran, LM. Release of RANTES, MIP-1 alpha, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am. J. Respir. Crit. Care Med. 1997, 156(5), 1377–1383. [Google Scholar]
- Kheradmand, F; Rishi, K; Corry, DB. Environmental Contributions to the allergic asthma epidemic. Environ. Health Perpsect. 2002, 110 Suppl. 4, 553–556. [Google Scholar]
- Frampton, M. Effects of exposure to ultrafine carbon particles in healthy subjects and subjects with asthma. Health Effects Research Institute Report (fall) 2004, 1–2. [Google Scholar]
- Umetsu, DT; McIntire, JJ; Akbari, O; Macaubas, C; Dekruyff, RH. Asthma: an epidemic of dysregulated immunity. Nat. Immunol. 2002, 3(8), 715–720. [Google Scholar]
- Holgate, ST. The epidemic of allergy and asthma. Nature 1999, 402 6760 Suppl., B2–4. [Google Scholar]
- Shukla, A; Ramos-Nino, M; Mossman, BT. Cell signaling and transcription factor activation by asbestos in lung injury and disease. Int. J. Biochem. & Cell Biol 2003, 35, 1198–1209. [Google Scholar]
- Herndon, WC. Quantum theory of aromatic hydrocarbon carcinogenesis. Int. J. Quantum Chem: Quantum Biol. Symp. 1974, (1), 123–134. [Google Scholar]
- Arbogast, JW; Darmanyan, AP; Foote, CS; Rubin, Y; Diederich, N; Alverez, MM; Anz, SJ; Whetten, RL. Photophysical properties of C60. J. Phys. Chem 1991, 95, 11–12. [Google Scholar]
- Polansky, H. Microcompetition with foreign DNA and the origin of chronic disease; CBCD Publishing: Rochester, New York, 2003. [Google Scholar]
- Salnikow, K; Weicheng, S; Blagosklanny, MW; Costa, M. Carcinogenic metals induce hypoxiainducible factor-stimulated transcription by reactive oxygen species – independent mechanism. Cancer Res 2000, 60, 3375–3378. [Google Scholar]
- Wilson, MR; Lightbody, JH; Donaldson, K; Sales, J; Stone, V. Interactions between ultrafine particles and transition metals in vivo and in vitro. Toxicol. Appl. Pharmacol. 2002, 184(3), 172–179. [Google Scholar]
- Carter, JD; Ghio, AJ; Samet, JM; Devlin, RB. Cytokine production by human airway epithelial cells after exposure to an air pollution particle is metal-dependent. Toxicol. Appl. Pharmacol. 1997, 146(2), 180–188. [Google Scholar]
- Vallyathan, V; Shi, X; Castranova, V. Reactive oxygen species: Their relation to pneumoconiosis and carcinogenesis. Envir. Health Perspectives Supplements 1998, 106(55), 1151–1155. [Google Scholar]
- Donaldson, K; Stone, V. Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann. Ist. Super Sanita 2003, 39(3), 405–410. [Google Scholar]
- Ovrevik, J; Myran, T; Refsnes, M; Lag, M; Becher, R; Hetland, RB; Schwarze, PE. Mineral particles of varying composition induce differential chemokine release from epithelial lung cells: importance of physico-chemical characteristics. Ann. Occup. Hygiene 2005, 49(3), 219–231. [Google Scholar]
- Peden, DB. Air pollution in asthma: Effect of pollutants on airway inflammation. Ann. Allergy, Asthma, & Immun 2001, 87, 12–17. [Google Scholar]
- Dennekamp, M; Howarth, S; Dick, CA; Chemic, JW; Donaldson, K; Seaton, A. Ultrafine particles and nitrogen oxides generated by gas and electric cooking. Occup. Environ. Med 2001, 58, 511–516. [Google Scholar]
© 2006 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Murr, L.E.; Soto, K.F.; Garza, K.M.; Guerrero, P.A.; Martinez, F.; Esquivel, E.V.; Ramirez, D.A.; Shi, Y.; Bang, J.J.; Venzor, III, J. Combustion-Generated Nanoparticulates in the El Paso, TX, USA / Juarez, Mexico Metroplex: Their Comparative Characterization and Potential for Adverse Health Effects. Int. J. Environ. Res. Public Health 2006, 3, 48-66. https://doi.org/10.3390/ijerph2006030007
Murr LE, Soto KF, Garza KM, Guerrero PA, Martinez F, Esquivel EV, Ramirez DA, Shi Y, Bang JJ, Venzor, III J. Combustion-Generated Nanoparticulates in the El Paso, TX, USA / Juarez, Mexico Metroplex: Their Comparative Characterization and Potential for Adverse Health Effects. International Journal of Environmental Research and Public Health. 2006; 3(1):48-66. https://doi.org/10.3390/ijerph2006030007
Chicago/Turabian StyleMurr, L. E., K. F. Soto, K. M. Garza, P. A. Guerrero, F. Martinez, E. V. Esquivel, D. A. Ramirez, Y. Shi, J. J. Bang, and J. Venzor, III. 2006. "Combustion-Generated Nanoparticulates in the El Paso, TX, USA / Juarez, Mexico Metroplex: Their Comparative Characterization and Potential for Adverse Health Effects" International Journal of Environmental Research and Public Health 3, no. 1: 48-66. https://doi.org/10.3390/ijerph2006030007




