Ultraviolet Radiation Increases the Toxicity of Pyrene, 1-Aminopyrene and 1-Hydroxypyrene to Human Keratinocytes
Abstract
:Introduction
Materials and Methods
Chemicals and Reagents
Preparation of Growth Media
Human Keratinocyte Cell Line, HaCaT
Culturing HaCaT Cells
DNA Synthesis – [3H]Thymidine Incorporation Assay
HaCaT Exposure to Ultraviolet Radiation
Treatment of HaCaT Cells with PAH
Effect of UV-A irradiated PAH on HaCaT Cells
Results
Effect of UV-A Alone Exposure on HaCaT Cells
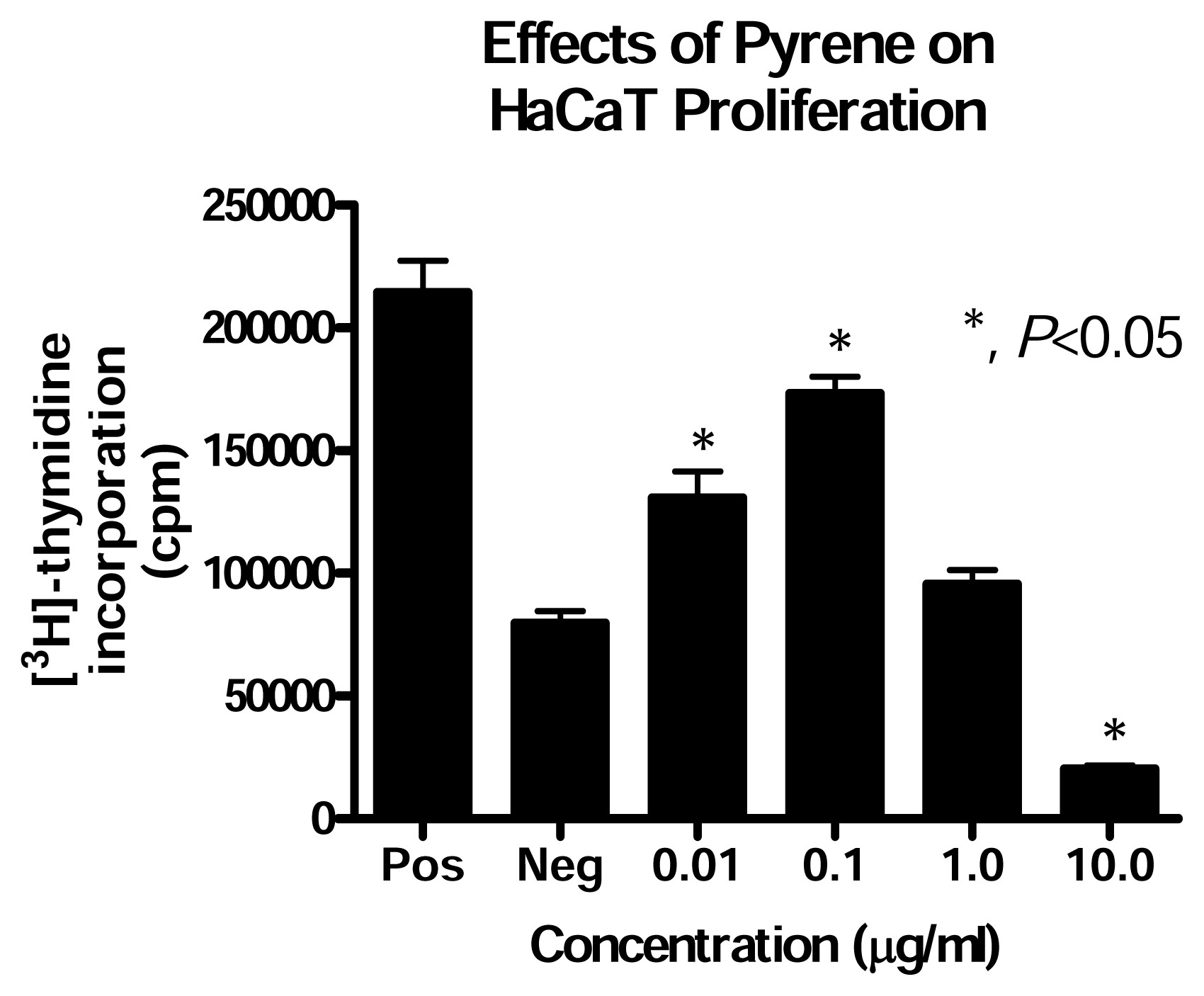
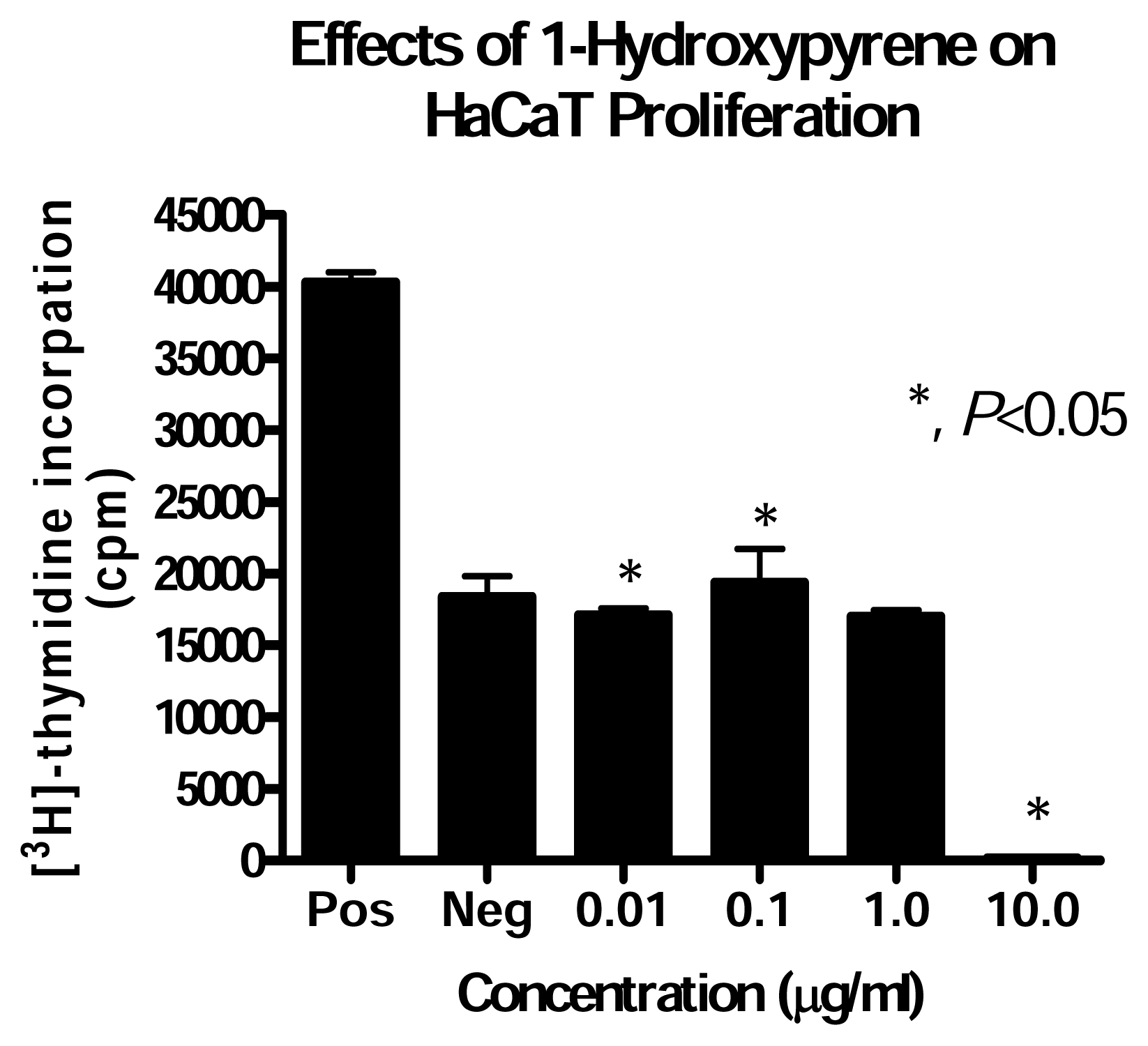
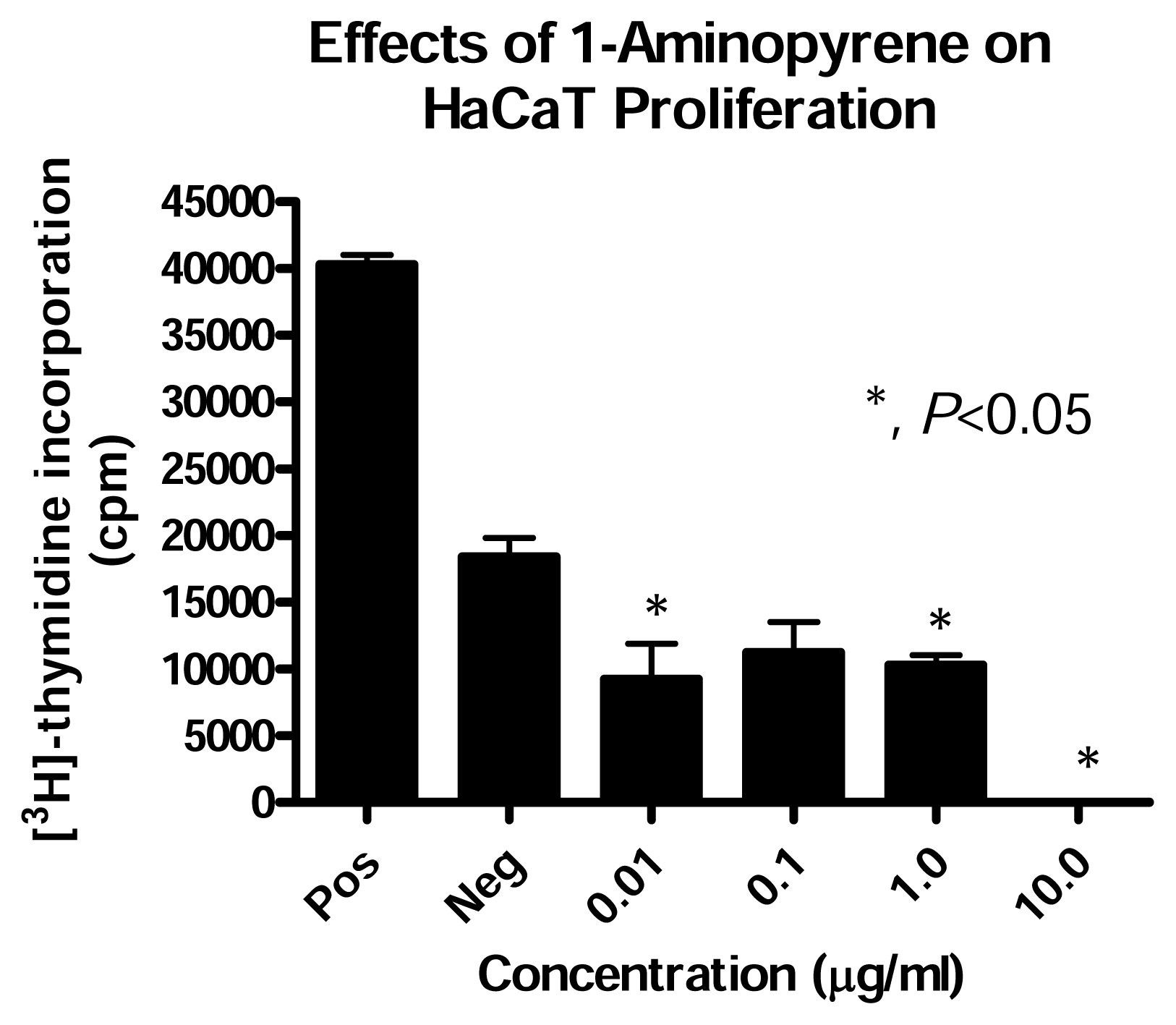
Effect of PAH Treatment on HaCaT Cell Proliferation
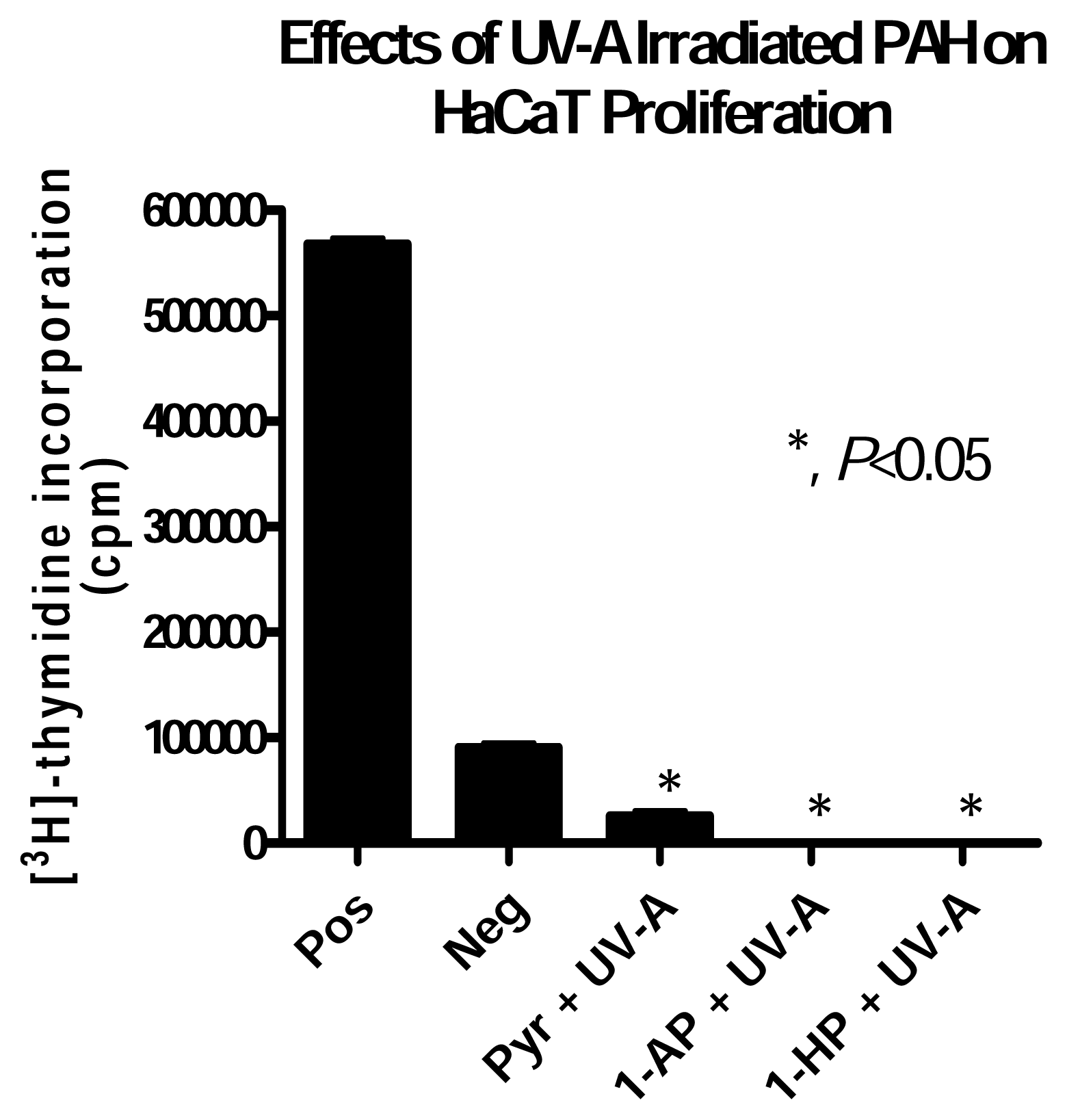
HaCaT Cell Response to UV-A irradiated PAH Exposure
Discussion
Conclusions

Acknowledgements
References
- Villemur, R.; Deziel, E.; Benachenhou, A.; Marcoux, J.; Gauthier, E.; Lepine, F.; Beaudet, R.; Commeau, Y. Two-liquid-phase slurry bioreactors to enhance the degradation of high-molecular-weight polycyclic aromatic hydrocarbons in soil. Biotech. Prog 2000, 16, 966–972. [Google Scholar]
- Guerin, T. Bioremediation of phenols and polycyclic aromatic hydrocarbons in creosote contaminated soil using ex-situ land treatment. J Haz Mat 1999, B65, 305–315. [Google Scholar]
- Dagher, F.; Deziel, E.; Lirette, P.; Paquette, G.; Bisaillon, J. G.; Villemur, R. Comparative study of five polycyclic aromatic hydrocarbon degrading bacterial strains isolated from contaminated soils. Can J. Micro 1997, 43, 368–377. [Google Scholar]
- Dong, S.; Hwang, H-M.; Shi, X.; Holloway, L.; Yu, H. UVA induced single strand cleavage by 1-hydroxypyrene and formation of covalent adducts between DNA and 1-hydroxypyrene. Chem. Res. Toxicol 2000, 13, 585–593. [Google Scholar]
- Schirmer, K.; Xhan, A. G. J.; Greenberg, B. M.; Dixon, D. G.; Bols, N. C. Ability of 16 priority PAHs to be phototoxic to a cell line from rainbow trout gill. Toxicol 1998, 127, 143–155. [Google Scholar]
- Boukamp, P.; Petrussevska, R. T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol 1988, 106(3), 761–771. [Google Scholar]
- Quiec, D.; Maziere, C.; Auclair, M.; Santus, R.; Gardette, J.; Redziniak, G.; Franchi, J.; Dubertret, L.; Maziere, J. C. Lovastatin enhances the photocytotoxicity of UVA radiation towards cultured N.C.T.C.2544 human keratinocytes: prevention by cholesterol supplementation and by a cathepsin inhibitor. Biochem J 1995, 310, 305–309. [Google Scholar]
- Niggli, H.; Applegate, L. Glutathione response after UVA irradiation in mitotic and postmitotic human skin fibroblasts and keratinocytes. Photochem Photobiol 1997, 65(4), 680–684. [Google Scholar]
- Heudorf, U.; Angerer, J. Internal exposure to PAHs of children and adults living in homes with parquet flooring containing high levels of PAHs in the parquet glue. Internat Arch. Occup Environ Health 2001, 74, 91–101. [Google Scholar]
- Shyong, E.; Lu, Y.; Goldstein, A.; Lebwohl, M.; Wei, H. Synergistic enhancement of H2O2 production in human epidermoid carcinoma cells by benxo[a]pyrene and ultraviolet A radiation. Toxicol Appl Pharmacol 2003, 188, 104–109. [Google Scholar]
- Da Silva, J.; Da Freitas, T.; Marinho, J.; Speit, G.; Erdtmann, B. An alkaline single-cell gel electrophoresis (comet) assay for environmental bio-monitoring with native rodents. Genet. Mol. Biol 2000, 23(1), 241–245. [Google Scholar]
- Daroui, P.; Desai, S. D.; Li, T. K.; Liu, A. A.; Liu, L. F. Hydrogen peroxide induces topoisomerase I-mediated DNA damage and cell death. J. Biol. Chem 2004, 279(15), 14587–14594. [Google Scholar]
- Slupphaug, G.; Kavli, B.; Krokan, H. E. The interacting pathways for prevention and repair of oxidative DNA damage. Mut. Res 2003, 531(1–2), 231–251. [Google Scholar]
© 2005 MDPI. All rights reserved.
Share and Cite
Ekunwe, S.I.N.; Hunter, R.D.; Hwang, H.-M. Ultraviolet Radiation Increases the Toxicity of Pyrene, 1-Aminopyrene and 1-Hydroxypyrene to Human Keratinocytes. Int. J. Environ. Res. Public Health 2005, 2, 58-62. https://doi.org/10.3390/ijerph2005010058
Ekunwe SIN, Hunter RD, Hwang H-M. Ultraviolet Radiation Increases the Toxicity of Pyrene, 1-Aminopyrene and 1-Hydroxypyrene to Human Keratinocytes. International Journal of Environmental Research and Public Health. 2005; 2(1):58-62. https://doi.org/10.3390/ijerph2005010058
Chicago/Turabian StyleEkunwe, Stephen I. N., Rochelle D. Hunter, and Huey-Min Hwang. 2005. "Ultraviolet Radiation Increases the Toxicity of Pyrene, 1-Aminopyrene and 1-Hydroxypyrene to Human Keratinocytes" International Journal of Environmental Research and Public Health 2, no. 1: 58-62. https://doi.org/10.3390/ijerph2005010058



