Cytotoxicity Assessment of Some Carbon Nanotubes and Related Carbon Nanoparticle Aggregates and the Implications for Anthropogenic Carbon Nanotube Aggregates in the Environment
Abstract
:Introduction
Materials and Methods
Experimental Nanomaterials
Nanomaterials Characterization by TEM
Viability Assays
Detection of Cytokines
Clinical Asthma Patient Data Collection
Collection and Analysis of Airborne Carbon Nanotube Aggregates
Results
Nanomaterials Characterization by TEM
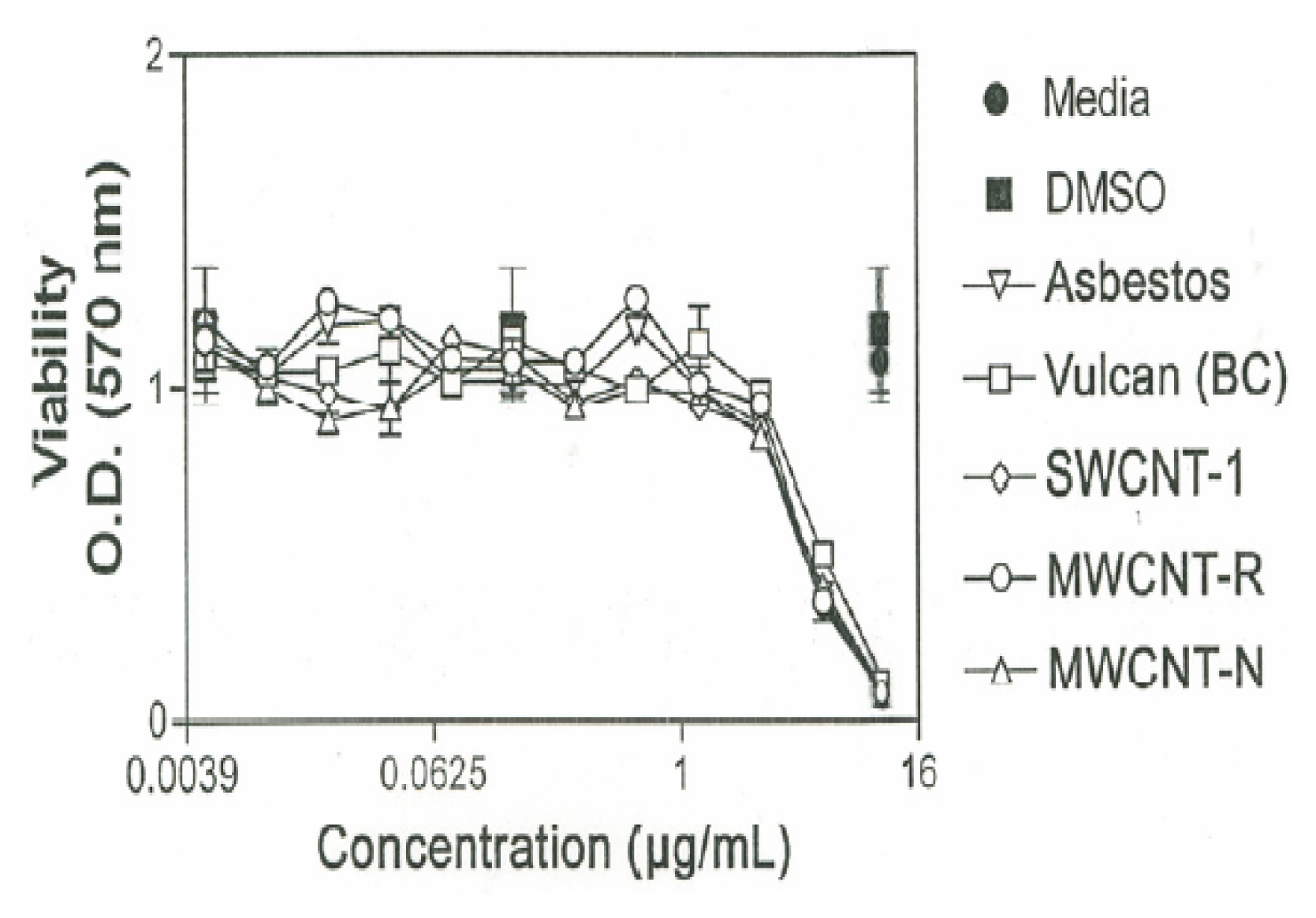
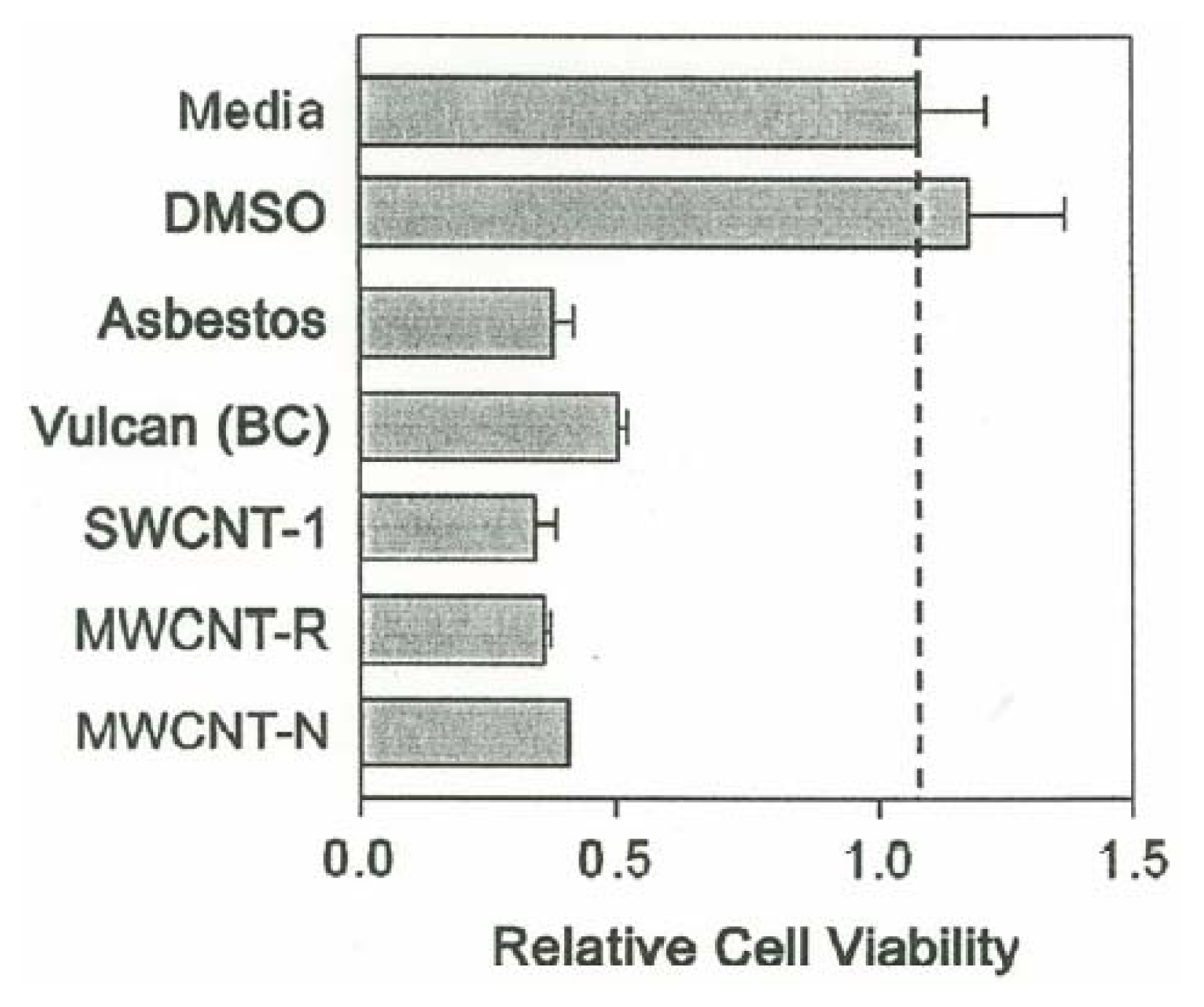
Cytotoxicity Assays
Cytokine Response
Preliminary Clinical Asthma Data
Discussion
Conclusions
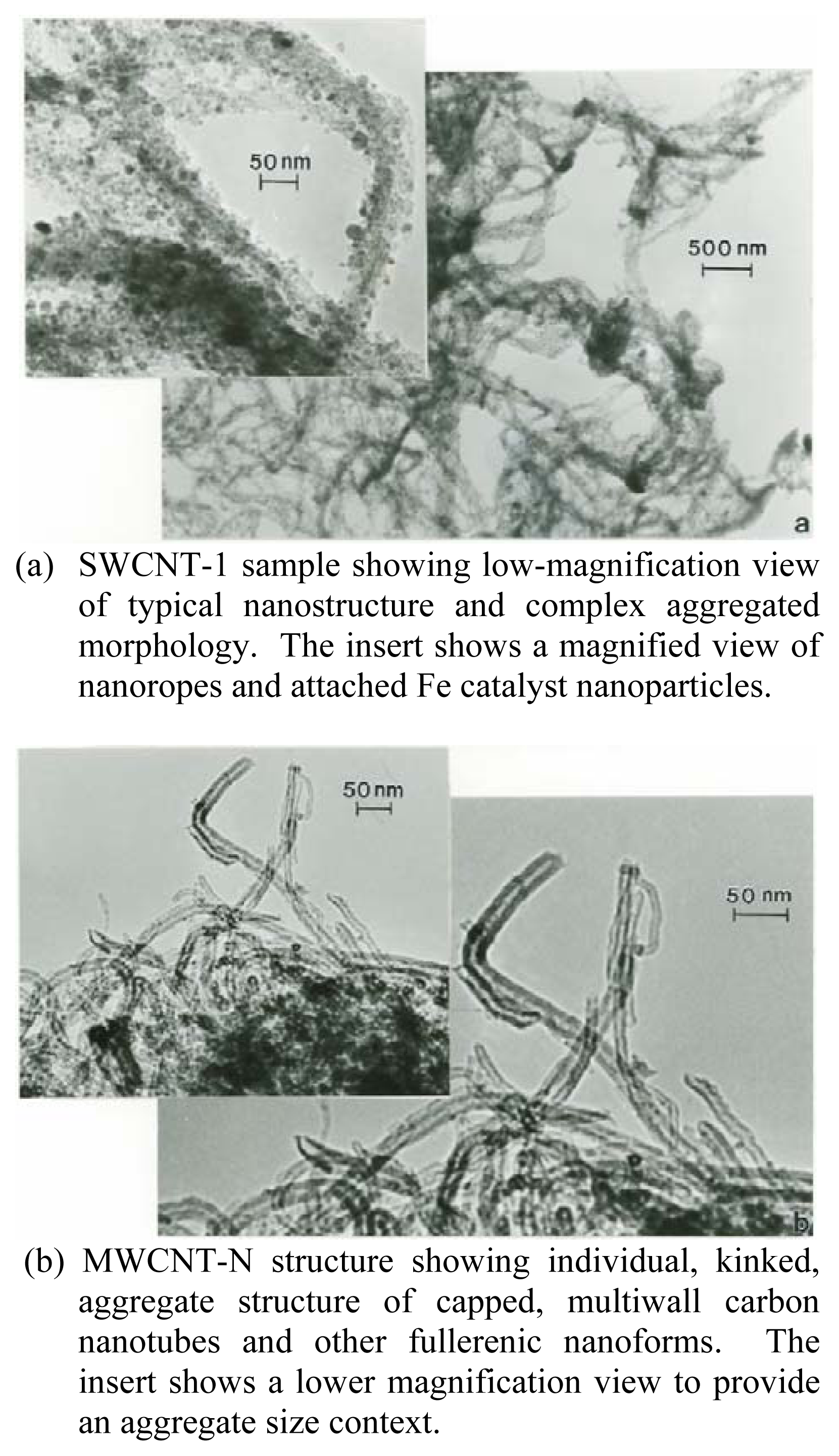
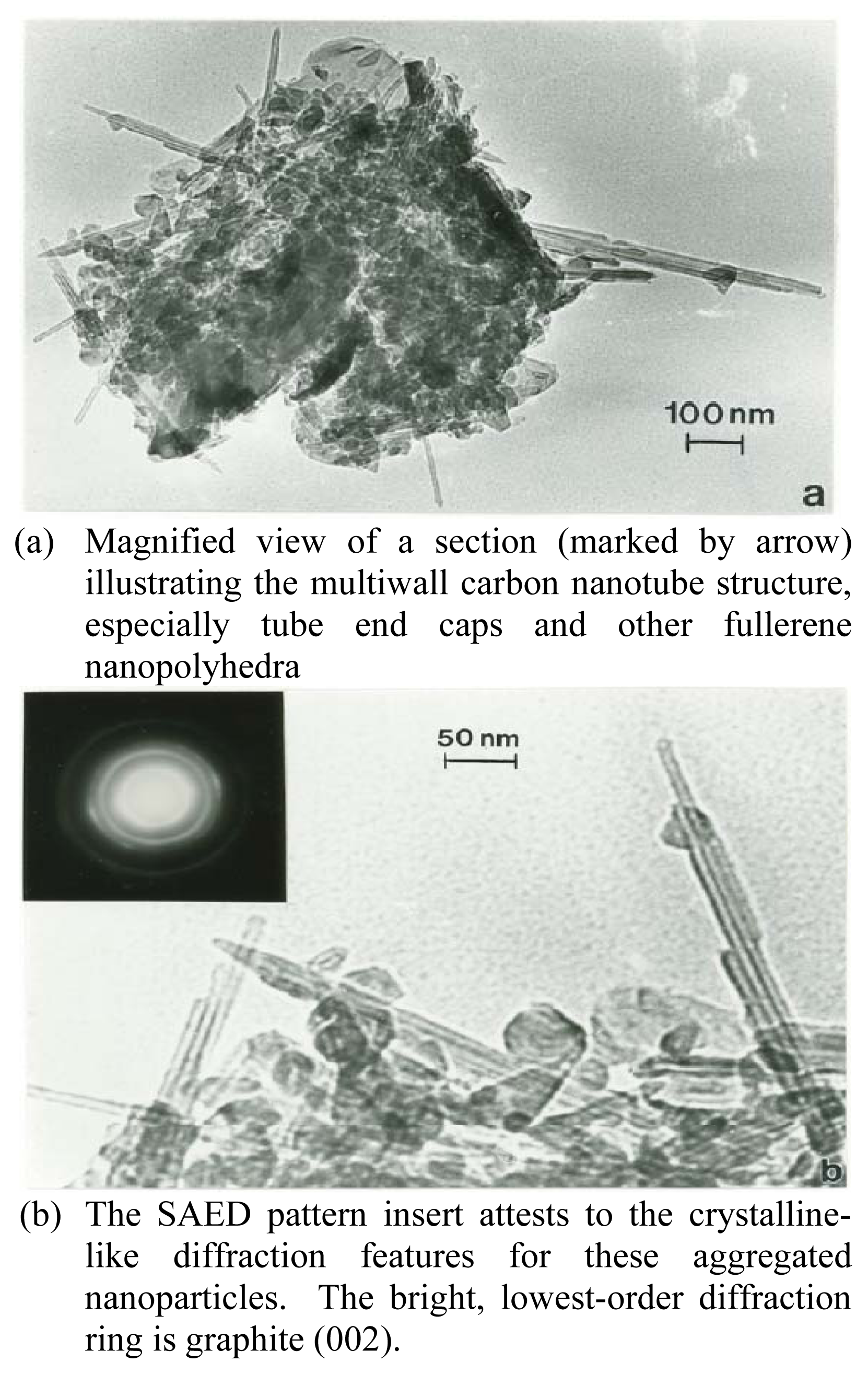
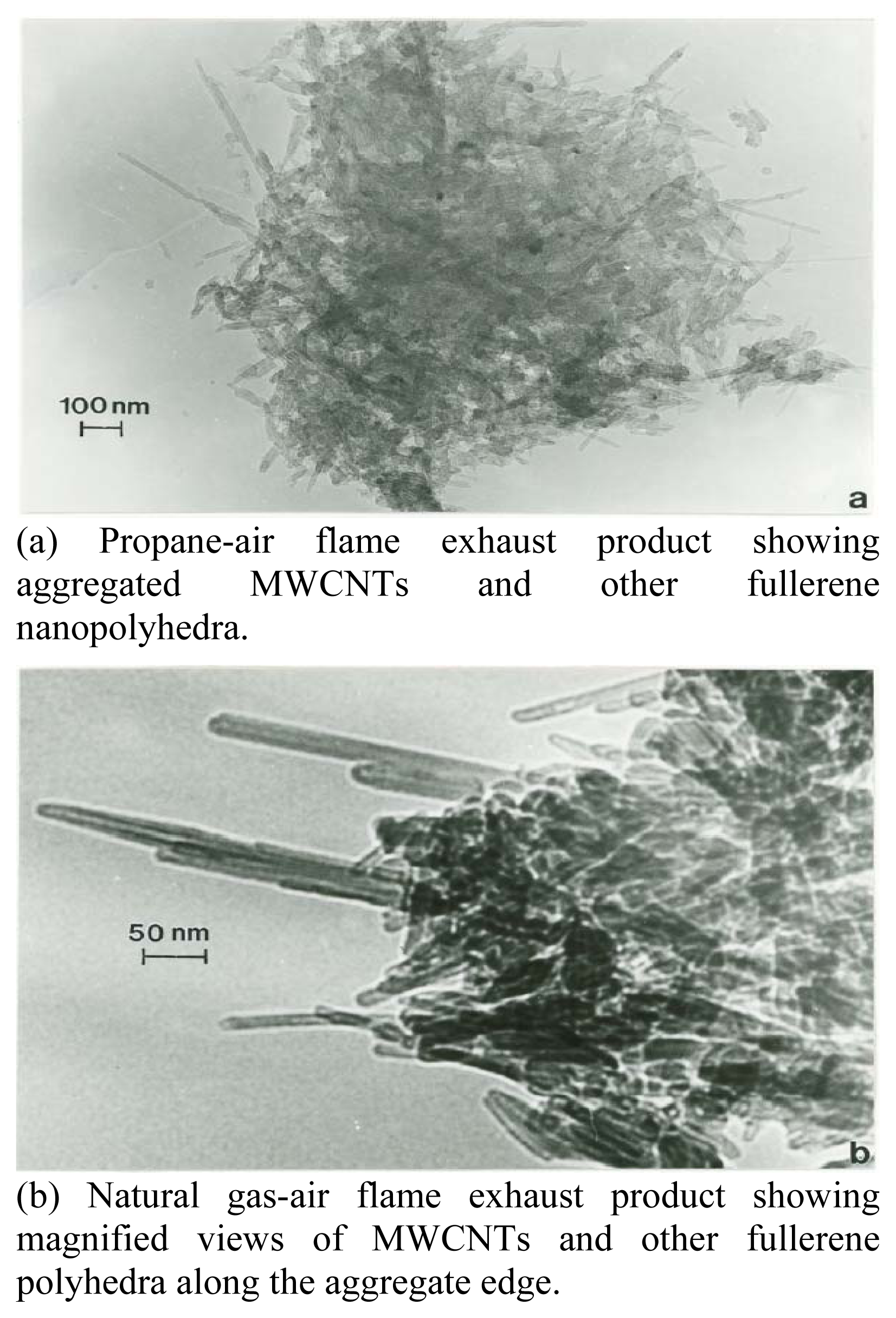

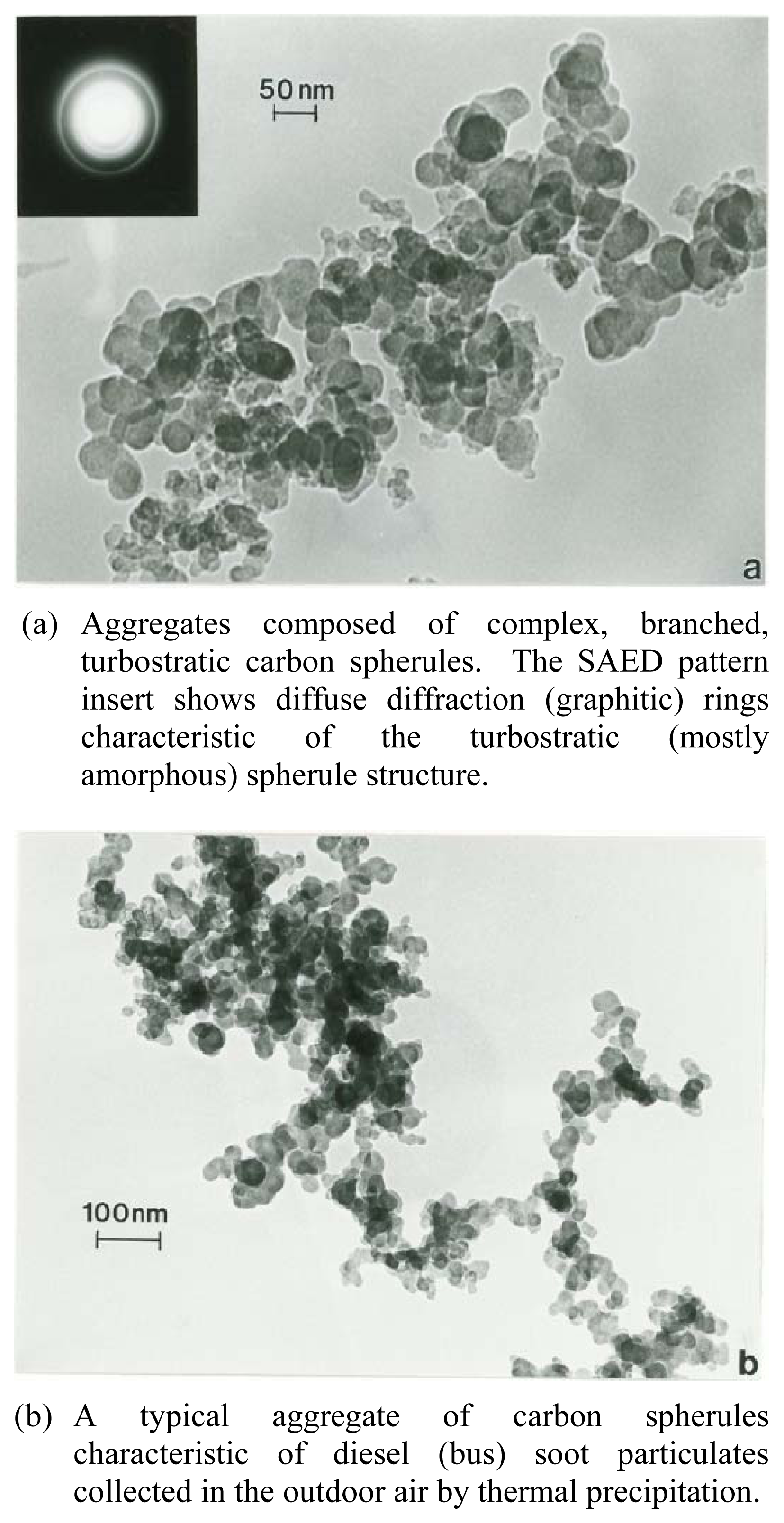
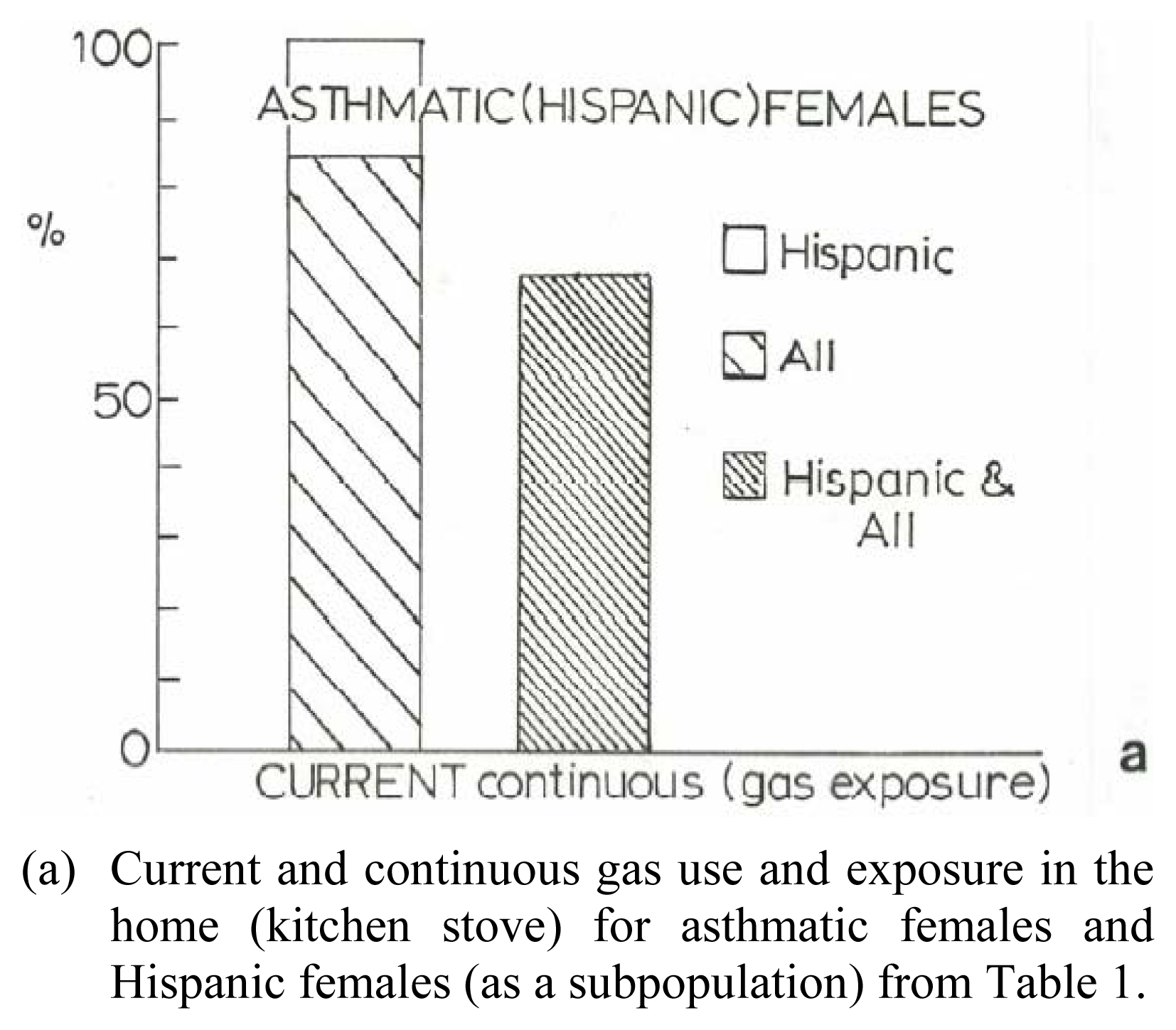
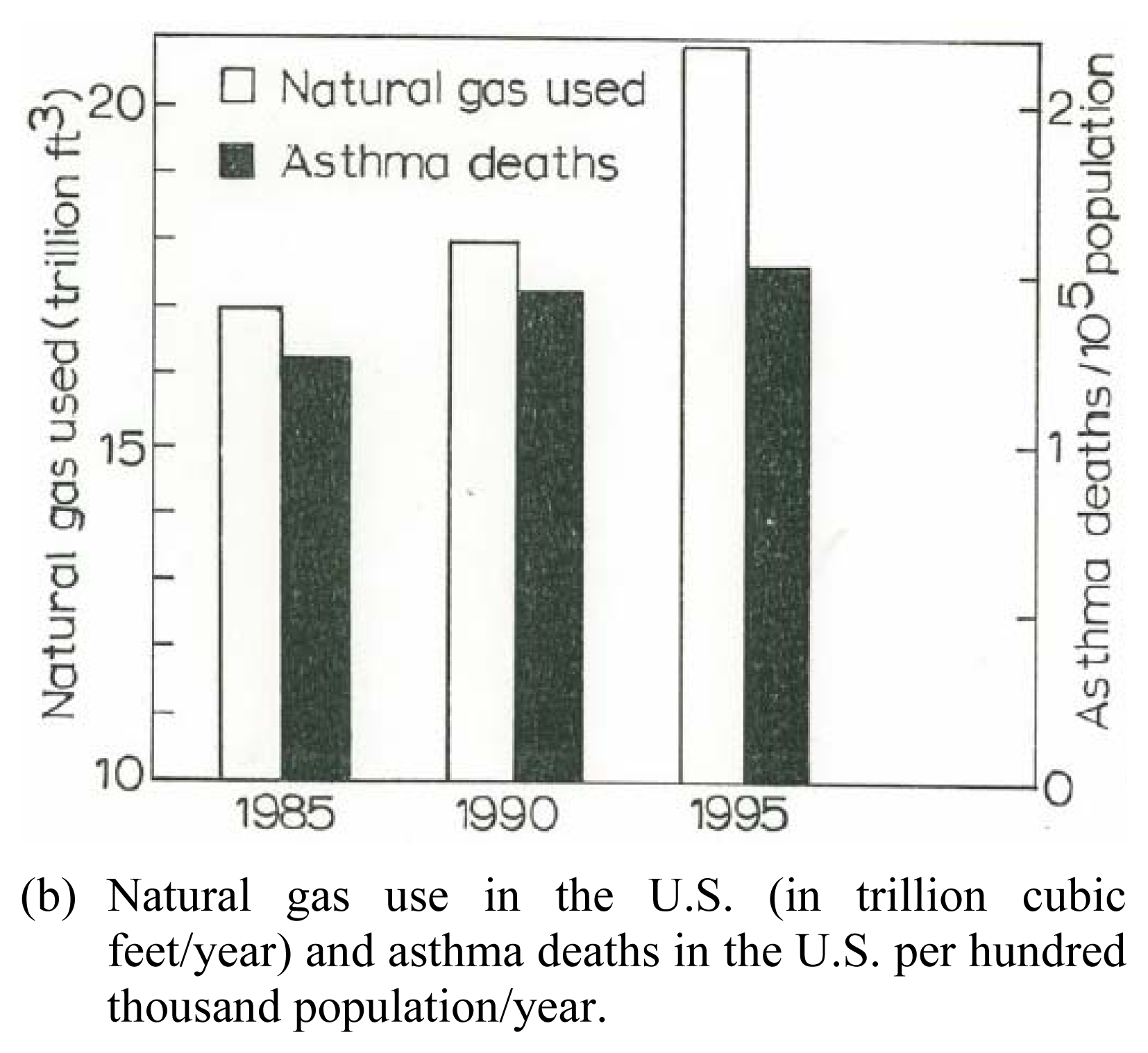
| Patient | Age | Hispanic | Asthma Classification* | Fev1* | ΔFev1* | Current Gas Stove | Prior Gas Stove | No Gas Exposure | Continuous Gas Exposure |
|---|---|---|---|---|---|---|---|---|---|
| Male | 11 | √ | Mild | 107 | 5 | √ | √ | √ | |
| Female | 15 | √ | Mild | 114 | −1 | √ | √ | √ | |
| Male | 16 | √ | Mild | 104 | 8 | √ | √ | √ | |
| Male | 16 | √ | Mild | 102 | 7 | √ | |||
| Female | 19 | Mild | 87 | 10 | √ | ||||
| Female | 20 | √ | Moderate | 75 | 19 | √ | √ | √ | |
| Female | 23 | √ | Moderate | 69 | −28 | √ | |||
| Female | 28 | Mild | 84 | 11 | √ | ||||
| Female | 28 | √ | Moderate | 69 | 23 | √ | √ | √ | |
| Female | 30 | Mild | 87 | 9 | √ | √ | √ | ||
| Female | 30 | Mild | 94 | 4 | √ | √ | √ | ||
| Female | 35 | √ | Moderate | 74 | 5 | √ | √ | √ | |
| Female | 37 | Severe | 35 | 92 | √ | ||||
| Female | 38 | Moderate | 69 | 31 | √ | √ | √ | ||
| Female | 44 | √ | Moderate | 64 | 30 | √ | √ | √ | |
| Female | 45 | Mild | 90 | 5 | √ | ||||
| Female | 46 | Moderate | 71 | −2 | √ | √ | √ | ||
| Female | 47 | √ | Moderate | 67 | 10 | √ | |||
| Female | 51 | √ | Moderate | 97 | 11 | √ | √ | √ | |
| Female | 52 | Severe | 43 | 82 | √ | √ | √ | ||
| Female | 54 | √ | Moderate | 66 | 9 | √ | √ | √ | |
| Male | 61 | √ | Mild | 120 | −3 | √ | √ | √ | |
| Male | 65 | √ | Moderate | 61 | 0 | √ | √ | √ | |
| Male | 89 | √ | Moderate | 69 | 3 | √ | √ | √ |
Acknowledgments
References
- Brumfiel, G. A little knowledge…. Nature 2003, 424, 246–248. [Google Scholar]
- Green Peace Report, Future Technologies, Today’s Choices; Green Peace Environmental Trust: London, July 2003.
- Robertson, D. H.; Brenner, D. W.; Mintmire, J. S. Energetics of nanoscale graphitic tubules. Phys. Rev 1992, B45(21), 12592–12598. [Google Scholar]
- Krewski, D.; Burnett, R.; Goldberg, M. S.; Hoover, K.; Siemiatycki, J.; Jerrett, M.; Abramawicz, M.; White, W.; et al. Reanalysis of the Harvard six cities study and the American Cancer Society study of particulate air pollution and mortality: Investigators Report, Park I: Replication and Validation; Part II: Sensitivity Analysis; Health Effects Institute: Boston, MA, 2000. [Google Scholar]
- Churg, R.; Brauer, M.; Vedal, S.; Stevens, B. Ambient mineral particles in the small airways of the normal human lung. J. Environm. Med 1999, 1(1), 39–47. [Google Scholar]
- Renwick, L. C.; Donaldson, K.; Clouter, A. Impairment of alveolar macrophage phagocytosies by ultrafine particles. Toxicol. Appl. Pharmacol 2001, 172(2), 119–127. [Google Scholar]
- Oberdörster, G.; Finkelstein, J. N.; Johnston, C.; Gelein, R.; Cox, C.; Baggs, R.; Elder, A. C. Acute pulmonary effects of ultrafine particles in rats and mice. In Res. Report 96; Health Effects Institute: Cambridge, MA, 2000. [Google Scholar]
- Momarca, S.; Creberlli, R.; Feretti, D.; Zanardini, A.; Fuselli, J.; Fillini, L. Mutagens and carcinogenesis in size-classified air particulates of a northern Italian town. Sci Total Environ 1997, 205(2–3), 137–144. [Google Scholar]
- Murr, L. E.; Bang, J. J. Electron microscope comparisons of fine and ultra-fine carbonaceous and non-carbonaceous, airborne particulates. Atmos. Environ 2003, 37, 4795–4806. [Google Scholar]
- Murr, L. E.; Esquivel, E. V.; Bang, J. J. Characterization of nanostructure phenomena in airborne particulate aggregates and their potential for respiratory health effects. J. Mater. Sci.: Mater. In Medicine 2004, 15, 237–247. [Google Scholar]
- Murr, L. E.; Soto, K. F.; Esquivel, E. V.; Bang, J. J.; Guerrero, P. A.; Lopez, D. A.; Ramirez, D. A. Carbon nanotubes and other fullerene-related nanocrystals in the environment: A TEM Study. JOM 2004, 56(6), 28–31. [Google Scholar]
- Warheit, D. B. Nanoparticles: Health risks? Mater Today; February 2004, 32–35. [Google Scholar]
- Heinrich, U.; Peters, L.; Creutzenberg, O.; Dasenbrock, C.; Hopmann, H. G. Inhalation exposure of rats to tar/pitch condensation aerosol or carbon black alone or in combination with irritant gas. Toxic. Carcinog. Eff. Solid Par. Respir. Tract; 1994, 433–439. [Google Scholar]
- Mauderly, J. L. Diesel emissions: Is more health research still needed? Toxicol. Sci 2001, 62(1), 6–15. [Google Scholar]
- Lam, C. W.; James, J. T.; McCloskey, R.; Hunter, R. L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci 2004, 77(1), 126–134. [Google Scholar]
- Warheit, D. B.; Lawrence, B. R.; Reed, K. L.; Roach, D. H.; Reynolds, G. A. M.; Webb, T. R. Comparative pulmonary toxicity assessment of single wall carbon nanotubes in rats. Toxicol. Sci 2004, 77(1), 117–125. [Google Scholar]
- Murr, L. E.; Bang, J. J.; Esquivel, E. V.; Guerrero, P. A.; Lopez, D. A. Carbon nanotubes and nanocrystal forms, and complex nanoparticle aggregates in common fuel-gas combustion sources and the ambient air. J. Nanoparticle Res 2004, 6, 241–251. [Google Scholar]
- Esquivel, E. V.; Murr, L. E. TEM analysis of nanoparticulates in a Polar ice core. Mater. Character 2004, 52(1), 15–25. [Google Scholar]
- Lippmann, M. Asbestos exposure indices. Environ. Res 1988, 46, 106–118. [Google Scholar]
- Lippmann, M. Nature of exposure to chrysotile. Ann. Occup. Hyg 1994, 38(4), 459–467. [Google Scholar]
- Manning, C. B.; Vallyathan, V.; Mossmann, B. T. Diseases caused by asbestos: mechanisms of injury and disease development. Int. Immunopharma 2002, 2, 191–200. [Google Scholar]
- Murr, L. E.; Soto, K. F. TEM comparison of chrystotile (asbestos) nanotube and carbon nanotubes. J. Mater. Sci. Lett 2004, 39, 4941–4947. [Google Scholar]
- Service, R. F. Nanotubes: The next asbestos? Science 1998, 281(5379), 941. [Google Scholar]
- Murr, L. E. Electron and Ion Microscopy and Microanalysis: Principles and Applications, 2nd Edition ed; Marcel Dekker, Inc.: New York, 1991. [Google Scholar]
- Mahler, D. A. (Ed.) Pulmonary Function Testing. Clin. Chest Med 1989, 10(2), 145–160.
- Bang, J. J.; Murr, L. E.; Esquivel, E. V. Collection and characterization of airborne nanoparticulates. Mater. Character 2004, 52, 1–14. [Google Scholar]
- Bang, J. J.; Trillo, E. A.; Murr, L. E. Utilization of selected area electron diffraction (SAED) patterns for characterization of air submicron particulate matter collected by a thermophoretic precipitator. J. Air & Waste Managmt. Assoc 2003, 53, 227–236. [Google Scholar]
- Bang, J. J.; Murr, L. E. Collection and TEM characterization of atmospheric nanoparticles. JOM 2002, 54(12), 28–30. [Google Scholar]
- Schwarz, J. A.; Contescu, C. I.; Putyera, K. (Eds.) Dekker Encyclopedia of Nanoscience and Nanotechnology; Marcel Dekker, Inc.: New York, 2004; Volume 1–5.
- Grieco, W. J.; Howard, J. B.; Rainey, L. C.; Vander Sande, J. B. Fullerenic carbon in combustion-generated soot. Carbon 2000, 38, 597–614. [Google Scholar]
- Vander Wal, R. L.; Tomasek, A. J. Soot nanostructure: dependence upon synthesis conditions. Combustion and Flame 2004, 136, 129–140. [Google Scholar]
- Peters, A.; Wichmann, H. E.; Tuch, T.; Heinrich, J.; Heyder, J. Respiratory effects are associated with the number of ultrafine particles. Amer. J. Respiratory and Crit. Care Med 1997, 155, 1376–1383. [Google Scholar]
- Mossman, B. T.; Churg, A. Mechanisms in the pathogenesis of asbestos and silicosis. Am. J. Respir. Crit. Care Med 1998, 157, 1666–1680. [Google Scholar]
- Hammond, E. C.; Selikoff, I. J.; Seidman, H. Asbestos exposure, cigarette smoking and death rates. Ann. N.Y. Acad. Sci 1979, 330, 473–490. [Google Scholar]
- Mossman, B. T.; Gee, J. B. L. Medical; progress; Asbestos-related diseases. In N. Engl. J. Med; 1989; Volume 320, pp. 1721–1738. [Google Scholar]
- Chorg, A.; Green, F. H. Y. Pathology of Occupational Lung Disease; Williams and Wilkins: Baltimore, MD, 1999. [Google Scholar]
- Alleman, J. E.; Mossman, B. T. Asbestos revisited. Sci. Amer 1997, 70–75. [Google Scholar]
- Mossman, B. T.; Bignon, J.; Corn, M.; Seaton, A.; Gee, J. B. Asbestos: scientific developments and implications for public policy. Science 1990, 14, 466–480. [Google Scholar]
- Gibbs, A. R. WHO/IARC Sci. Pub. No. 90; 1989; pp. 219–228. [Google Scholar]
- IARC Monograph on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, vol. 68: Silica some silicates, coal dust and para-aramid fibrils; IARC Press: Lyon, France, 1997.
- Heinrich, J.; Hoelscher, B.; Wichman, H. E. Decline of ambient air pollution and respiratory symptoms in children. Am. J. Respir. Crit. Care Med 2000, 161(6), 1930–1936. [Google Scholar]
- Jedrychowski, W.; Flak, E.; Mroz, E. The adverse effect of low levels of ambient air pollutants on lung function growth in preadolescent children. Environ. Health Prspect 1999, 107(8), 669–674. [Google Scholar]
- Nikula, K. J.; Vallyathan, V.; Green, F. H. Y.; Hahn, F. Influence of exposure concentration or dose on the distribution of particulate material in rat and human lungs. Environ. Health Perspectives 2001, 109, 311–319. [Google Scholar]
- National Research Council. Committee on Geosciences, Environment, and Resources, Research. In Priorities for Airborne Particulate Matter II; Washington, D.C.: National Academy Press, 1999.
- Spengler, J. D.; Treitman, R. D.; Tosteson, T. D.; Mage, D. T.; Soczek, M. L. Personal exposures to respirable particulates and implications for air pollution epidemiology. Environ. Sci Technol 1985, 19, 700–707. [Google Scholar]
- Ott, W. R. Human exposure assessment: The birth of a new science. JEAEE 1995, 5, 449–472. [Google Scholar]
- Rodes, C. E.; Lawless, P. A.; Evans, G. F.; Sheldon, L. S.; Williams, R. W.; Vette, A. F.; Creason, J. P.; Walsh, D. The relationship between personal PM exposures for elderly populations and indoor and outdoor concentrations for three retirement center scenarios; JEAEE 2001, 11, 103–105. [Google Scholar]
- Rodes, C. E.; Wiener, R. W. Indoor aerosols and exposure assessment in Chap. 29 Aerosol Measurement: Principles, Techniques and Applications, Second Edition; Baron, P. A., Willeke, K., Eds.; Wiley-Interscience, Inc.: New York, 2001; pp. 859–88. [Google Scholar]
- Kim, S.; Jaques, P. A.; Chang, M.; Friones, J. R.; Sioutas, C. Versatile aerosol concentration enrichment system (VACES) for simultaneous in vivo and in vitro evaluation of toxic effects of ultrafine, fine, and coarse ambient particles. Part I: Development and laboratory characterization. J. Aerosol Sci 2001, 32. [Google Scholar]
- Kim, S.; Jaques, P. A.; Chang, M.; Barone, T.; Xiong, C.; Friedlander, S. K.; Sioutas, C. Versatile aerosol concentration enrichment system (VACES) for simultaneous in vivo and in vitro evaluation of toxic effects of ultrafine, fine, and coarse ambient particles. Part II: Field evaluation. J. Aerosol Sci 2001, 32, 1299–1314. [Google Scholar]
- Misra, C.; Kim, S.; Shea, S.; Sioutas, C. Design and evaluation of a high-flow rate, very low pressure drop impactor for separation and collection of fine from ultrafine particles. J. Aerosol Sci 2002, 33(5), 736–752. [Google Scholar]
© 2005 MDPI. All rights reserved.
Share and Cite
Murr, L.E.; Garza, K.M.; Soto, K.F.; Carrasco, A.; Powell, T.G.; Ramirez, D.A.; Guerrero, P.A.; Lopez, D.A.; Venzor, J., III. Cytotoxicity Assessment of Some Carbon Nanotubes and Related Carbon Nanoparticle Aggregates and the Implications for Anthropogenic Carbon Nanotube Aggregates in the Environment. Int. J. Environ. Res. Public Health 2005, 2, 31-42. https://doi.org/10.3390/ijerph2005010031
Murr LE, Garza KM, Soto KF, Carrasco A, Powell TG, Ramirez DA, Guerrero PA, Lopez DA, Venzor J III. Cytotoxicity Assessment of Some Carbon Nanotubes and Related Carbon Nanoparticle Aggregates and the Implications for Anthropogenic Carbon Nanotube Aggregates in the Environment. International Journal of Environmental Research and Public Health. 2005; 2(1):31-42. https://doi.org/10.3390/ijerph2005010031
Chicago/Turabian StyleMurr, L. E., K. M. Garza, K. F. Soto, A. Carrasco, T. G. Powell, D. A. Ramirez, P. A. Guerrero, D. A. Lopez, and J. Venzor, III. 2005. "Cytotoxicity Assessment of Some Carbon Nanotubes and Related Carbon Nanoparticle Aggregates and the Implications for Anthropogenic Carbon Nanotube Aggregates in the Environment" International Journal of Environmental Research and Public Health 2, no. 1: 31-42. https://doi.org/10.3390/ijerph2005010031




