Induced Mitogenic Activity in AML-12 Mouse Hepatocytes Exposed to Low-dose Ultra-Wideband Electromagnetic Radiation
Abstract
:Introduction
Materials and Methods
Chemicals
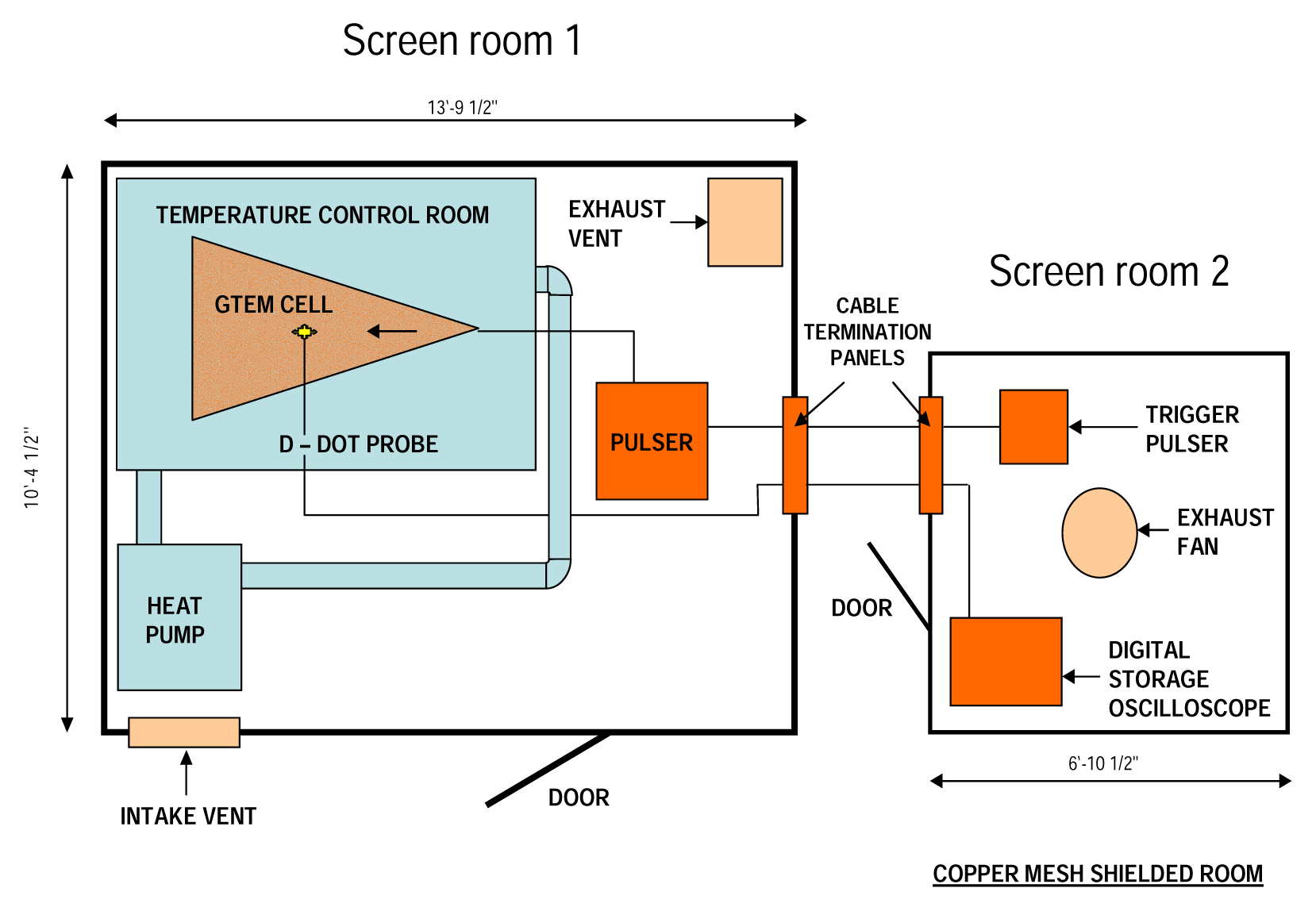
Electromagnetic Field Exposure System
Cell Culture
Exposure of Samples to UWBR
Cell Viability Assay
Sample Collection and Protein Determination
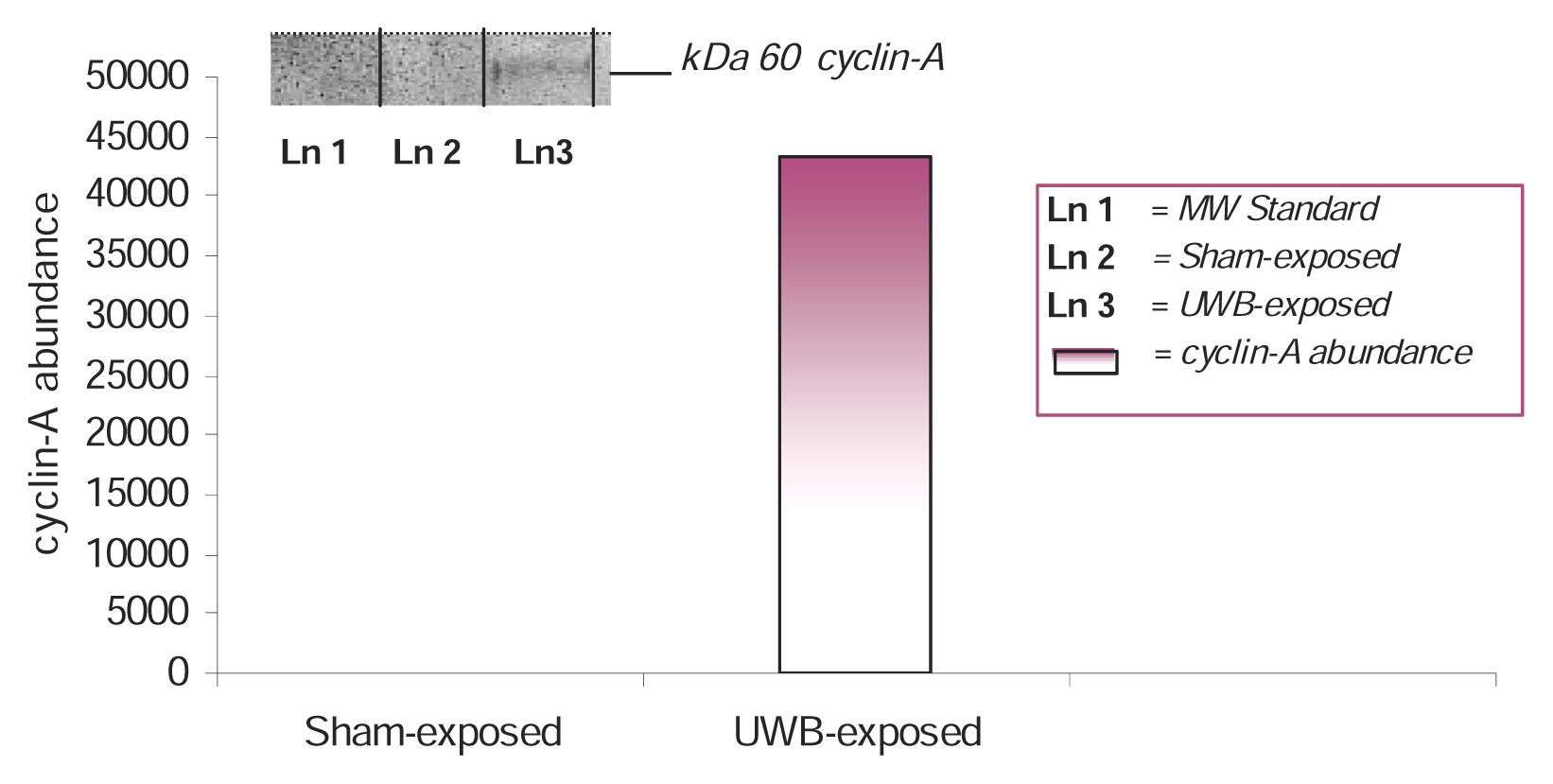
Western Blot and Densitometric Analyses for Cyclin A Expression
Statistical Analysis
Results
Discussion
Conclusions



Acknowledgments
References
- Ross, G. F. A time domain criterion for the design of wideband radiating elements. IEEE Transactions on Antennas and Propagation 1968, 16(3), 355. [Google Scholar]
- Lu, S. T.; Mathur, S. P.; Akyel, Y.; Lee, J.C. Ultra-wideband electromagnetic pulses induced hypotension in rats. Physio Behav 1999, 65(4–5), 753–761. [Google Scholar]
- Seamen, R. L.; Parker, J. E.; Kiel, J. L.; Mathur, S. P.; Grubbs; Prol, H. K. Ultra-wideband pulses increase nitric oxide production by RAW 264.7 macrophages incubated in nitrate. Bioelectromagnetics 2002, 23, 83–87. [Google Scholar]
- Lai, H. Genetic effects of nonionizing electromagnetic fields. Paper presented at the International Workshop on Biological Effects of Ionizing Radiation, Electromagnetic Fields and Chemical Toxic Agents, Sinaia, Romania, October 2–6; 2001. [Google Scholar]
- Maes, A.; Verschaeve, L.; Arroyo, A.; De Wagter, C.; Vercruyssen, L. In vitro cytogenetic effects of 2450 MHz waves on human peripheral blood lymphocytes. Bioelectromagnetics 1993, 14, 495–501. [Google Scholar]
- Simko, M.; Kriehuber, R.; Weiss, D. G.; Luben, R. A. Effects of 50 Hz EMF exposure on micro-nucleus formation and apoptosis in transformed and nontransformed human cell lines. Bioelectromagnetics 1998, 19, 85–91. [Google Scholar]
- Zotti-Martelli, L.; Peccatori, M.; Scarpato, R.; Migliore, L. Induction of micronuclei in human lymphocytes exposed in vitro to microwave radiation. Mutat Res 2000, 472, 51–58. [Google Scholar]
- de Pomerai, D.; Daniels, C.; David, H.; Allan, J.; Duce, I.; Mutwakil, M.; Thomas, D.; Sewell, P.; Tattersall, J.; Jones, D. Non-thermal heat-shock response to microwaves. Nature 2000, 405, 417–418. [Google Scholar]
- Leszczynski, D.; Joenvaara, S.; Reivinen, J.; Kuokka, R. Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells: molecular mechanism for cancer- and blood-brain barrier-related effects. Differentiation 2002, 70, 120–129. [Google Scholar]
- Mann, K.; Röschke, J. Effects of pulsed high-frequency electromagnetic fields on human sleep. Neuropsychobiology 1996, 33, 41–47. [Google Scholar]
- Lai, H.; Singh, N. P. Acute low-intensity microwave exposure increases DNA single-strand breaks in rat brain cells. Bioelectromagnetics 1995, 16, 207–210. [Google Scholar]
- Garaj-Vrhovac, V. Micronucleus assay and lymphocyte mitotic activity in risk assessment of occupational exposure to microwave radiation. Chemosphere 1999, 39(13), 2301–2312. [Google Scholar]
- McCann, J.; Dietrich, F.; Rafferty, C.; Martin, A. O. A critical review of the genotoxic potential of electric and magnetic fields. Mutat Res 1993, 297, 61–95. [Google Scholar]
- McCann, J.; Dietrich, F.; Rafferty, C. The genotoxic potential of electric and magnetic fields: an update. Mutat Res 1998, 411, 45–86. [Google Scholar]
- Miyakoshi, J.; Koji, Y.; Wakasa, T.; Takebe, H. Long-term exposure to a magnetic field (5 mT at 60 Hz) increases X-ray-induced mutations. J Radiat Res (Tokyo) 1999, 40, 13–21. [Google Scholar]
- Walleczek, J.; Shiu, E. C.; Hahn, G. M. Increase in radiation-induced HPRT gene mutation frequency after nonthermal exposure to nonionizing 60 Hz electromagnetic fields. Radiat Res 1999, 151, 489–497. [Google Scholar]
- Wei, M.; Guizzetti, M.; Yost, M.; Costa, L. G. Exposure to 60-Hz magnetic fields and proliferation of human astrocytoma cells. in vitro. Toxicol Appl Pharmacol 2000, 162(3), 166–76. [Google Scholar]
- Chen, G.; Upham, B.L.; Sun, W.; Chang, C. C.; Rothwell, E.J.; Chen, K.M.; Yamasaki, H.; Trosko, J. E. Effect of electromagnetic field exposure on chemically induced differentiation of Friend erythroleukemia cells. Environmental Health Perspectives 2000, 108(10), 967–972. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Applications to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
- Dorsey, W. C.; Tchounwou, P. B.; Sutton, D. Mitogenic and cytotoxic effects of pentachloro-phenol to AML 12 Mouse Hepatocytes. Int. J. Environ. Res. Public Health 2004, 1(2), 100–105. [Google Scholar]
- Popovic, M.; Hagness, S. C. Finite-difference time-domain analysis of a complete transverse electromagnetic cell loaded with liquid biological media in culture dishes. IEEE Transactions on Biomedical Engineering 1998, 45(8), 1067–1076. [Google Scholar]
- Gilroy, D. W.; Saunders, M. A.; Sansores-Garcia, L.; Matijevic-Aleksic, N.; Wu, K. K. Cell cycle-dependent expression of cyclooxygenase-2 in human fibroblasts. FASEB J 2001, 15, 288–290. [Google Scholar]
- Walleczek, J.; Liburdy, R. P. Nonthermal 60 Hz sinusoidal magnetic-field exposure enhances 45Ca2+ uptake in rat thymocytes: dependence on mitogen activation. FEBS Lett 1990, 271, 157–160. [Google Scholar]
- Pezetti, F.; De Mattei, M.; Caruso, A.; Cadossi, R.; Zucchini, P.; Carinci, F.; Traina, G. C.; Sollazzo, V. Effects of pulsed electromagnetic fields on human chondrocytes: an in vitro study. Calcif Tissue Int 1999, 65, 396–401. [Google Scholar]
- LeBoeuf, R. A.; Laishes, B. A.; Hoeskstra, W. G. Effects of selenium on cell proliferation in rat liver and mammalian cells as indicated by cytokinetic and biochemical analysis. Cancer Research 1985, 45(11), 5496–5504. [Google Scholar]
- Cossarizza, A.; Monti, D.; Bersani, F.; Paganelli, R.; Montagnani, G.; Cadossi, R.; Cantini, M.; Franceschi, C. Extremely low frequency pulsed electromagnetic fields increase in interleukin-2 (IL-2) utilization and IL-2 receptor expression in mitogen-stimulated human lymphocytes from old subjects. FEBS Lett 1989, 248, 141–144. [Google Scholar]
- Chellappan, S. P.; Giordano, A.; Fisher, P. B. Role of cyclin-dependent kinases and their inhibitors in cellular differentiation and development. Curr Top Microbiol Immunol 1998, 227, 57–103. [Google Scholar]
- Pavletich, N. P. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol 1999, 287, 821–828. [Google Scholar]
- Shen, W. H.; Yin, Y.; Broussard, S. R.; McCusker, R. H.; Freund, G. G.; Dantzer, R.; Kelly, K. W. Tumor necrosis factor alpha inhibits cyclin A expression and retinoblastoma hyperphosphorylation triggered by insulin-like growth factor-I induction of new E2F-1 synthesis. J. Biol. Chem 2004, 279(9), 7438–7446. [Google Scholar]
- Pagano, M.; Pepperkok, R.; Verde, F.; Ansorge, W.; Draetta, G. Cyclin A is required at two points in the human cell cycle. The EMBO J 1992, 11, 961–971. [Google Scholar]
- Velizaro, S.; Raskmark, P.; Kwee, S. The effects of radiofrequency fields on cell proliferation are non-thermal. Bioelectrochem Bioenerg 1999, 48(1), 177–180. [Google Scholar]
- Fesenko, E. E.; Geletyuk, V. I.; Kazachenko, V. N.; Chemeris, N. K. Preliminary microwave irradiation of water solutions changes their channel-modifying activity. FEBS Lett 1995, 366(1), 49–52. [Google Scholar]
- Goswami, P. C.; Albee, L. D.; Parsian, A. J.; Baty, J. D.; Moros, E. G.; Pickard, W. F.; Roti-Roti, J. L.; Hunt, C. R. Expression of proto-oncogene and activities of multiple transcription factors in RF exposed cells, using C3H10T1/2 mouse embryo fibroblast cells exposed to 835.62 NS 847.74 MHz cellphone radiations. Radiat Res 1999, 151(3), 300–309. [Google Scholar]
- Donnelian, M.; McKenzie, D. R.; French, P. W. Effects of exposure to electromagnetic radiation at 385 MHz pm growth, morphology and secretory characteristics of a mast cell analogue, RBL-2H3. Cell Biol Int 1997, 21, 427–439. [Google Scholar]
- Brocklehurst, B.; McLauchlan, K. A. Free radical mechanism for the effects of environmental electro- magnetic fields on biological systems. Int. H. Radiat Biol 1996, 69, 3–24. [Google Scholar]
- Mohtat, N.; Cozens, F. L.; Hancock-Chen, T.; Scaiano, J. C.; McLean, J.; Kim, J. Magnetic field effects on the behavior of radicals in protein and DNA environments. Photochem Photobiol 1998, 67, 111–118. [Google Scholar]
- Lai, H.; Singh, N. P. Melatonin and N-tert-butyl-α-phenylnitrone blocked 60-Hz magnetic field-induced DNA single and double strand breaks in rat brain cells. J Pineal Res 1997, 22, 152–162. [Google Scholar]
- Mashevich, M.; Folkman, D.; Kesar, A.; Barbul, A.; Korenstein, R.; Jerby, E.; Avivi, L. Exposure of human peripheral blood lymphocytes to electromagnetic fields associated with cellular phones leads to chromosomal instability. Bioelectromagnetics 2003, 24(2), 82–90. [Google Scholar]
- Phillips, J. L.; Haggren, W.; Thomas, W.J.; Ishida, T. T.; Adey, W. R. Magnetic field-induced changes in specific gene transcription. Biochim Biophys Acta 1992, 1132, 140–144. [Google Scholar]
- Fanelli, C.; Coppola, S.; Barone, R.; Colussi, C.; Gualandi, G.; Volpe, P.; Ghibelli, L. Magnetic fields increase cell survival by inhibiting apoptosis via modulation of Ca2+ influx. FASEB J 1999, 13, 95–102. [Google Scholar]
- Cho, M. R.; Thatte, H. S.; Silvia, M. T.; Golan, D. E. Transmembrane calcium influx induced by ac electric fields. FASEB J 1999, 13, 677–683. [Google Scholar]
© 2005 MDPI. All rights reserved.
Share and Cite
Dorsey, W.C.; Ford, B.D.; Roane, L.; Haynie, D.T.; Tchounwou, P.B. Induced Mitogenic Activity in AML-12 Mouse Hepatocytes Exposed to Low-dose Ultra-Wideband Electromagnetic Radiation. Int. J. Environ. Res. Public Health 2005, 2, 24-30. https://doi.org/10.3390/ijerph2005010024
Dorsey WC, Ford BD, Roane L, Haynie DT, Tchounwou PB. Induced Mitogenic Activity in AML-12 Mouse Hepatocytes Exposed to Low-dose Ultra-Wideband Electromagnetic Radiation. International Journal of Environmental Research and Public Health. 2005; 2(1):24-30. https://doi.org/10.3390/ijerph2005010024
Chicago/Turabian StyleDorsey, W. C., B. D. Ford, L. Roane, D. T. Haynie, and P. B. Tchounwou. 2005. "Induced Mitogenic Activity in AML-12 Mouse Hepatocytes Exposed to Low-dose Ultra-Wideband Electromagnetic Radiation" International Journal of Environmental Research and Public Health 2, no. 1: 24-30. https://doi.org/10.3390/ijerph2005010024




