The Effects of Annatto Tocotrienol Supplementation on Cartilage and Subchondral Bone in an Animal Model of Osteoarthritis Induced by Monosodium Iodoacetate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatment
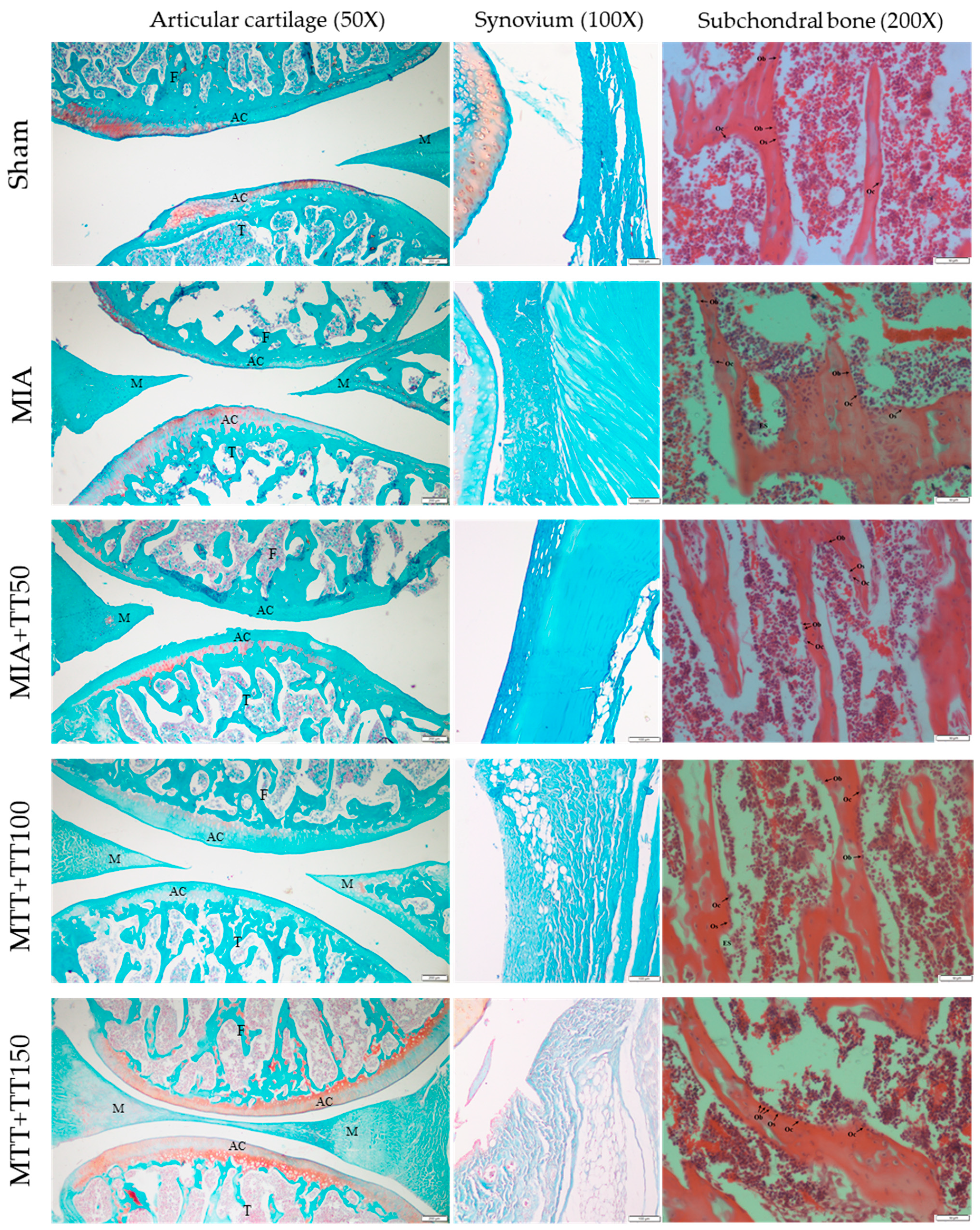
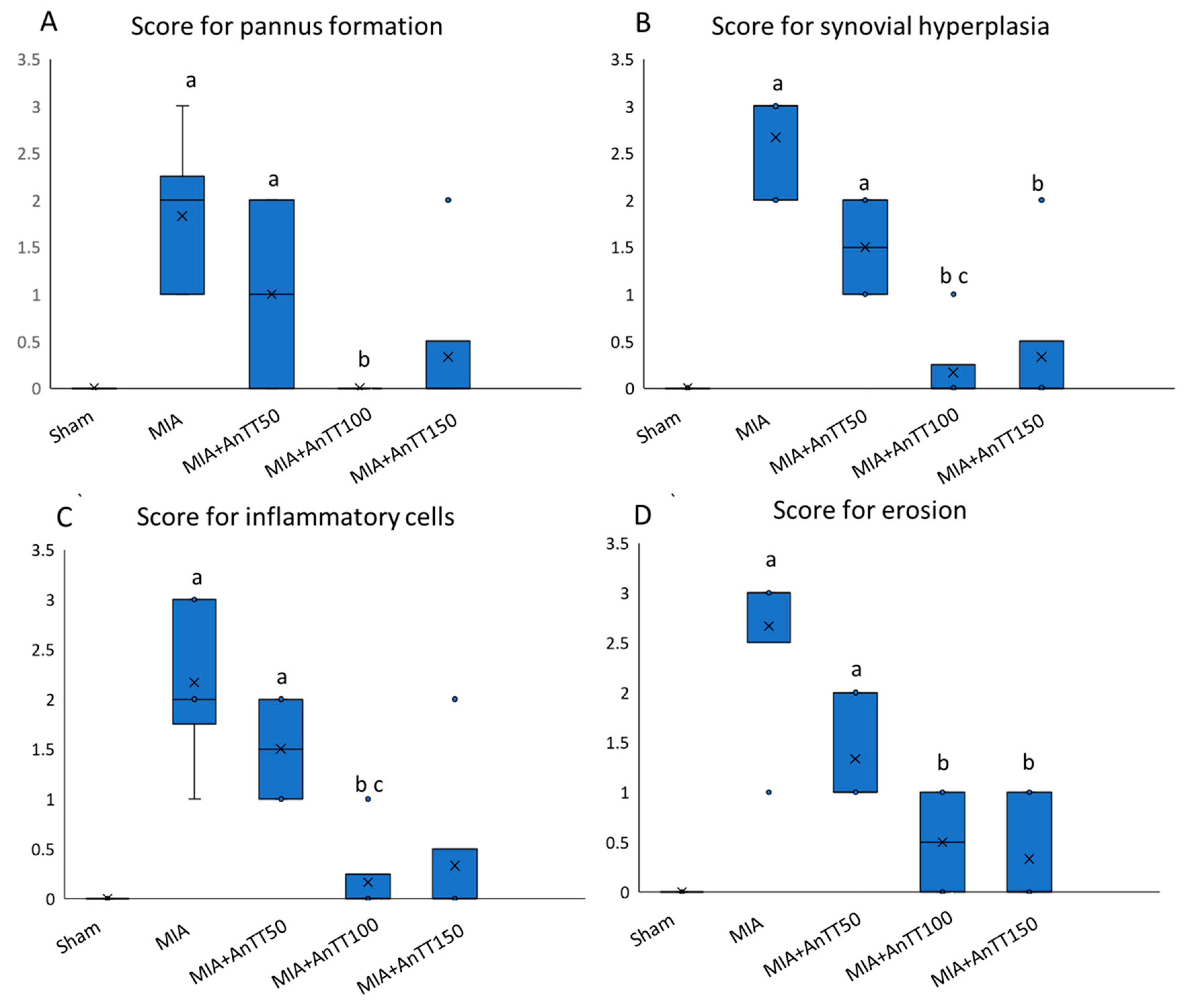
2.3. Histological Analysis
2.4. Biochemical Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (dalys) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Adatia, A.; Rainsford, K.D.; Kean, W.F. Osteoarthritis of the knee and hip. Part i: Aetiology and pathogenesis as a basis for pharmacotherapy. J. Pharm. Pharm. 2012, 64, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Findlay, D.M.; Atkins, G.J. Osteoblast-chondrocyte interactions in osteoarthritis. Curr. Osteoporos. Rep. 2014, 12, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Mohan, G.; Perilli, E.; Parkinson, I.H.; Humphries, J.M.; Fazzalari, N.L.; Kuliwaba, J.S. Pre-emptive, early, and delayed alendronate treatment in a rat model of knee osteoarthritis: Effect on subchondral trabecular bone microarchitecture and cartilage degradation of the tibia, bone/cartilage turnover, and joint discomfort. Osteoarthr. Cartil. 2013, 21, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Siebelt, M.; Waarsing, J.H.; Groen, H.C.; Muller, C.; Koelewijn, S.J.; De Blois, E.; Verhaar, J.A.; De Jong, M.; Weinans, H. Inhibited osteoclastic bone resorption through alendronate treatment in rats reduces severe osteoarthritis progression. Bone 2014, 66, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.W. Approach to osteoarthritis management for the primary care provider. Prim. Care 2018, 45, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Buckner, T.; Fan, R.; Kim, Y.; Kim, J.; Chung, S. Annatto tocotrienol attenuates nlrp3 inflammasome activation in macrophages. Curr. Dev. Nutr. 2017, 1, e000760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Park, N.Y.; Jang, Y.; Ma, A.; Jiang, Q. Vitamin e gamma-tocotrienol inhibits cytokine-stimulated nf-kappab activation by induction of anti-inflammatory a20 via stress adaptive response due to modulation of sphingolipids. J. Immunol. 2015, 195, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Pang, K.L.; Soelaiman, I.N. Tocotrienol and its role in chronic diseases. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2016; Volume 928, pp. 97–130. [Google Scholar]
- Peh, H.Y.; Tan, W.S.; Liao, W.; Wong, W.S. Vitamin e therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, A.; Arita, M.; Sato, Y.; Kiyose, C.; Ueda, T.; Igarashi, O.; Arai, H.; Inoue, K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin e analogs. FEBS Lett. 1997, 409, 105–108. [Google Scholar] [CrossRef]
- Khor, H.T.; Ng, T.T. Effects of administration of alpha-tocopherol and tocotrienols on serum lipids and liver hmg coa reductase activity. Int. J. Food Sci. Nutr. 2000, 51, S3–S11. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Pearce, B.C.; Nor, R.M.; Gapor, A.; Peterson, D.M.; Elson, C.E. Dietary alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme a reductase activity in chickens. J. Nutr. 1996, 126, 389–394. [Google Scholar] [CrossRef]
- Frega, N.; Mozzon, M.; Bocci, F. Identification and estimation of tocotrienols in the annatto lipid fraction by gas chromatography-mass spectrometry. J. Am. Oil Chem. Soc. 1998, 75, 1723–1727. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ima-Nirwana, S. The role of tocotrienol in preventing male osteoporosis—A review of current evidence. Int. J. Mol. Sci. 2019, 20, 1355. [Google Scholar] [CrossRef]
- Kamarudin, T.A.; Othman, F.; Ramli, E.S.; Isa, N.; Das, S. Protective effect of curcumin on experimentally induced arthritic rats: Detailed histopathological study of the joints and white blood cell count. EXCLI J. 2012, 11, 226–236. [Google Scholar]
- Vidal, B.; Pinto, A.; Galvao, M.J.; Santos, A.R.; Rodrigues, A.; Cascao, R.; Abdulghani, S.; Caetano-Lopes, J.; Ferreira, A.; Fonseca, J.E.; et al. Bone histomorphometry revisited. Acta Reum. Port. 2012, 37, 294–300. [Google Scholar]
- Saif, A.; Norazlina, M.; Ima Nirwana, S. Quantification of bone histomorphometric parameters using the weibel technique in animals. Med. Health 2016, 11, 278–288. [Google Scholar]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef]
- Yuan, G.H.; Tanaka, M.; Masuko-Hongo, K.; Shibakawa, A.; Kato, T.; Nishioka, K.; Nakamura, H. Characterization of cells from pannus-like tissue over articular cartilage of advanced osteoarthritis. Osteoarthr. Cartil. 2004, 12, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.M.; Okda, A.A.K.; Dessouky, I.S.; Hewedy, W.A.; Zahran, N.M.; Alamrani, B.A.W. L-carnitine ameliorates knee lesions in mono-iodoacetate induced osteoarthritis in rats. Alex. J. Med. 2017, 53, 61–66. [Google Scholar] [CrossRef]
- Choudhary, D.; Kothari, P.; Tripathi, A.K.; Singh, S.; Adhikary, S.; Ahmad, N.; Kumar, S.; Dev, K.; Mishra, V.K.; Shukla, S.; et al. Spinacia oleracea extract attenuates disease progression and sub-chondral bone changes in monosodium iodoacetate-induced osteoarthritis in rats. BMC Complement. Altern. Med. 2018, 18, 69. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ima-Nirwana, S. The role of vitamin e in preventing and treating osteoarthritis—A review of the current evidence. Front. Pharm. 2018, 9, 946. [Google Scholar] [CrossRef]
- Suantawee, T.; Tantavisut, S.; Adisakwattana, S.; Tanavalee, A.; Yuktanandana, P.; Anomasiri, W.; Deepaisarnsakul, B.; Honsawek, S. Oxidative stress, vitamin e, and antioxidant capacity in knee osteoarthritis. J. Clin. Diagn. Res. 2013, 7, 1855–1859. [Google Scholar]
- Surapaneni, K.M.; Venkataramana, G. Status of lipid peroxidation, glutathione, ascorbic acid, vitamin e and antioxidant enzymes in patients with osteoarthritis. Indian J. Med. Sci. 2007, 61, 9–14. [Google Scholar] [CrossRef]
- Sutipornpalangkul, W.; Morales, N.P.; Charoencholvanich, K.; Harnroongroj, T. Lipid peroxidation, glutathione, vitamin e, and antioxidant enzymes in synovial fluid from patients with osteoarthritis. Int. J. Rheum. Dis. 2009, 12, 324–328. [Google Scholar] [CrossRef]
- Heidar, E.H.; Al Faya, F.F.; Hassan, W.N.; Eid, R.A.; Haidara, M.A. The impact of antioxidants on inflammation and oxidative stress markers in osteoarthritis rat model: Scanning electron microscope insights. Am. J. Pharmacol. Toxicol. 2014, 9, 157–167. [Google Scholar] [CrossRef]
- Rhouma, M.; de Oliveira El Warrak, A.; Troncy, E.; Beaudry, F.; Chorfi, Y. Anti-inflammatory response of dietary vitamin e and its effects on pain and joint structures during early stages of surgically induced osteoarthritis in dogs. Can. J. Vet. Res. 2013, 77, 191–198. [Google Scholar]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef]
- Yuan, X.L.; Meng, H.Y.; Wang, Y.C.; Peng, J.; Guo, Q.Y.; Wang, A.Y.; Lu, S.B. Bone-cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef]
- Berry, P.A.; Maciewicz, R.A.; Cicuttini, F.M.; Jones, M.D.; Hellawell, C.J.; Wluka, A.E. Markers of bone formation and resorption identify subgroups of patients with clinical knee osteoarthritis who have reduced rates of cartilage loss. J. Rheumatol. 2010, 37, 1252–1259. [Google Scholar] [CrossRef]
- Pesesse, L.; Sanchez, C.; Walsh, D.A.; Delcour, J.P.; Baudouin, C.; Msika, P.; Henrotin, Y. Bone sialoprotein as a potential key factor implicated in the pathophysiology of osteoarthritis. Osteoarthr. Cartil. 2014, 22, 547–556. [Google Scholar] [CrossRef] [Green Version]
- Attur, M.; Krasnokutsky-Samuels, S.; Samuels, J.; Abramson, S.B. Prognostic biomarkers in osteoarthritis. Curr. Opin. Rheumatol. 2013, 25, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Legrand, C.B.; Lambert, C.J.; Comblain, F.V.; Sanchez, C.; Henrotin, Y.E. Review of soluble biomarkers of osteoarthritis: Lessons from animal models. Cartilage 2017, 8, 211–233. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. Vitamin e as an antiosteoporotic agent via receptor activator of nuclear factor kappa-b ligand signaling disruption: Current evidence and other potential research areas. Evid. Based Complement. Altern. Med. 2012, 2012, 747020. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Dev. Ther. 2015, 9, 2049–2061. [Google Scholar] [CrossRef]
- Chin, K.Y.; Mo, H.; Soelaiman, I.N. A review of the possible mechanisms of action of tocotrienol—A potential antiosteoporotic agent. Curr. Drug Targets 2013, 14, 1533–1541. [Google Scholar] [CrossRef]
- Wan Hasan, W.N.; Abd Ghafar, N.; Chin, K.Y.; Ima-Nirwana, S. Annatto-derived tocotrienol stimulates osteogenic activity in preosteoblastic mc3t3-e1 cells: A temporal sequential study. Drug Des. Dev. Ther. 2018, 12, 1715–1726. [Google Scholar] [CrossRef]
- Marchev, A.S.; Dimitrova, P.A.; Burns, A.J.; Kostov, R.V.; Dinkova-Kostova, A.T.; Georgiev, M.I. Oxidative stress and chronic inflammation in osteoarthritis: Can nrf2 counteract these partners in crime? Ann. N. Y. Acad. Sci. 2017, 1401, 114–135. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Khalid, B.A.K.; Luke, D.A.; Ima Nirwana, S. Tocotrienol offers better protection than tocopherol from free radical-induced damage of rat bone. Clin. Exp. Pharm. Physiol. 2005, 32, 761–770. [Google Scholar] [CrossRef]
- Maniam, S.; Mohamed, N.; Shuid, A.N.; Soelaiman, I.N. Palm tocotrienol exerted better antioxidant activities in bone than α-tocopherol. Basic Clin. Pharmacol. Toxicol. 2008, 103, 55–60. [Google Scholar] [CrossRef]
- Tantavisut, S.; Tanavalee, A.; Honsawek, S.; Suantawee, T.; Ngarmukos, S.; Adisakwatana, S.; Callaghan, J.J. Effect of vitamin e on oxidative stress level in blood, synovial fluid, and synovial tissue in severe knee osteoarthritis: A randomized controlled study. BMC Musculoskelet. Disord. 2017, 18, 281. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Saxena, R.; Gupta, V. Efficacy of vitamin e in knee osteoarthritis management of north indian geriatric population. Ther. Adv. Musculoskelet. Dis. 2012, 4, 11–19. [Google Scholar] [CrossRef]
- Sasaki, E.; Tsuda, E.; Yamamoto, Y.; Maeda, S.; Inoue, R.; Chiba, D.; Fujita, H.; Takahashi, I.; Umeda, T.; Nakaji, S.; et al. Serum hyaluronic acid concentration predicts the progression of joint space narrowing in normal knees and established knee osteoarthritis—A five-year prospective cohort study. Arthritis Res. Ther. 2015, 17, 283. [Google Scholar] [CrossRef]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef] [Green Version]
- Sniekers, Y.H.; Weinans, H.; Bierma-Zeinstra, S.M.; Van Leeuwen, J.P.; Van Osch, G.J. Animal models for osteoarthritis: The effect of ovariectomy and estrogen treatment—A systematic approach. Osteoarthr. Cartil. 2008, 16, 533–541. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, K.-Y.; Wong, S.K.; Japar Sidik, F.Z.; Abdul Hamid, J.; Abas, N.H.; Mohd Ramli, E.S.; Afian Mokhtar, S.; Rajalingham, S.; Ima Nirwana, S. The Effects of Annatto Tocotrienol Supplementation on Cartilage and Subchondral Bone in an Animal Model of Osteoarthritis Induced by Monosodium Iodoacetate. Int. J. Environ. Res. Public Health 2019, 16, 2897. https://doi.org/10.3390/ijerph16162897
Chin K-Y, Wong SK, Japar Sidik FZ, Abdul Hamid J, Abas NH, Mohd Ramli ES, Afian Mokhtar S, Rajalingham S, Ima Nirwana S. The Effects of Annatto Tocotrienol Supplementation on Cartilage and Subchondral Bone in an Animal Model of Osteoarthritis Induced by Monosodium Iodoacetate. International Journal of Environmental Research and Public Health. 2019; 16(16):2897. https://doi.org/10.3390/ijerph16162897
Chicago/Turabian StyleChin, Kok-Yong, Sok Kuan Wong, Fadhlullah Zuhair Japar Sidik, Juliana Abdul Hamid, Nurul Hafizah Abas, Elvy Suhana Mohd Ramli, Sabarul Afian Mokhtar, Sakthiswary Rajalingham, and Soelaiman Ima Nirwana. 2019. "The Effects of Annatto Tocotrienol Supplementation on Cartilage and Subchondral Bone in an Animal Model of Osteoarthritis Induced by Monosodium Iodoacetate" International Journal of Environmental Research and Public Health 16, no. 16: 2897. https://doi.org/10.3390/ijerph16162897





