Implications of Soil Pollution with Diesel Oil and BP Petroleum with ACTIVE Technology for Soil Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Plant
2.3. Petroleum Products
2.4. Experimental Procedure
2.5. Methodology of Microbiological Analyses
2.5.1. Bacterial and Fungal Counts
2.5.2. DNA Extraction and Bioinformatic Analysis of Specific Bacterial Taxa
2.6. Methodology of Biochemical Analyses
2.7. Methodology of Chemical and Physiochemical Analyses of Soil
2.8. Statistical Analysis
3. Results
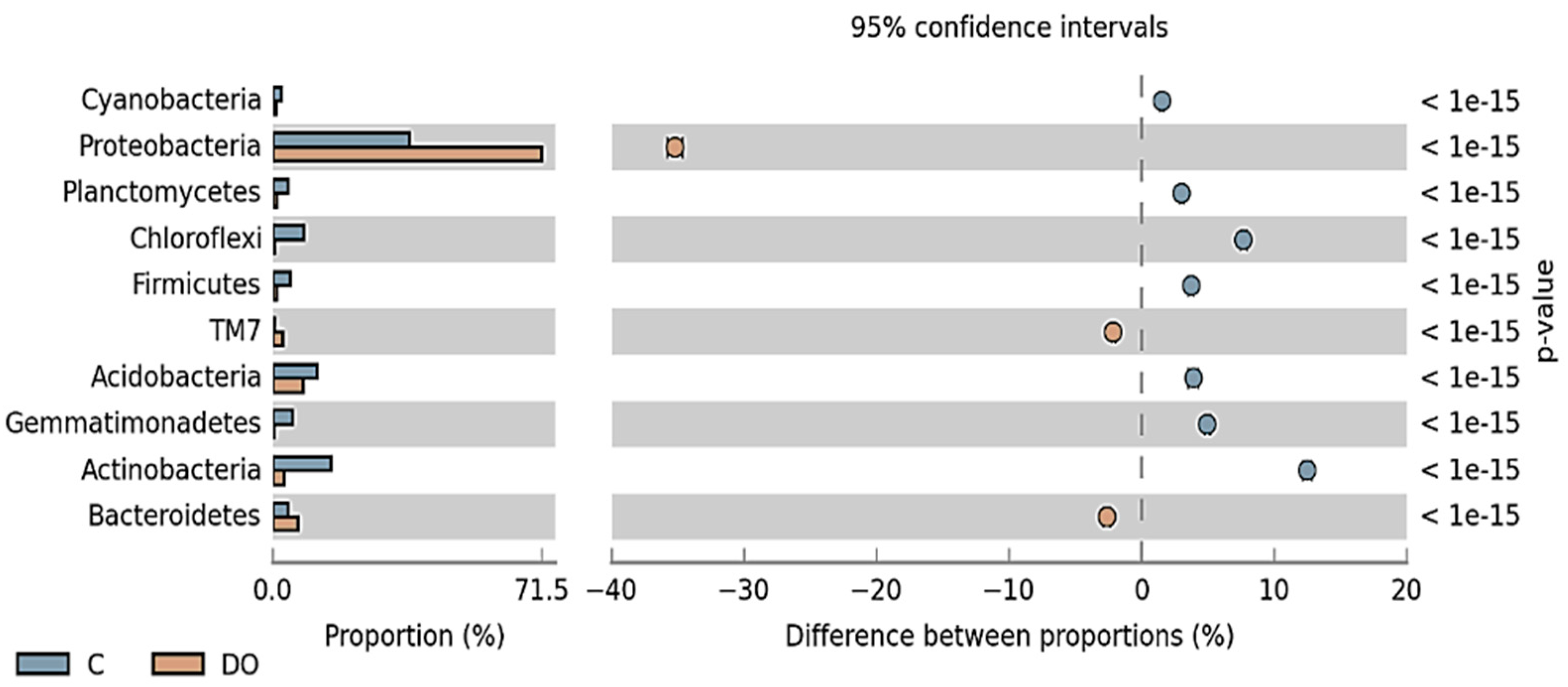
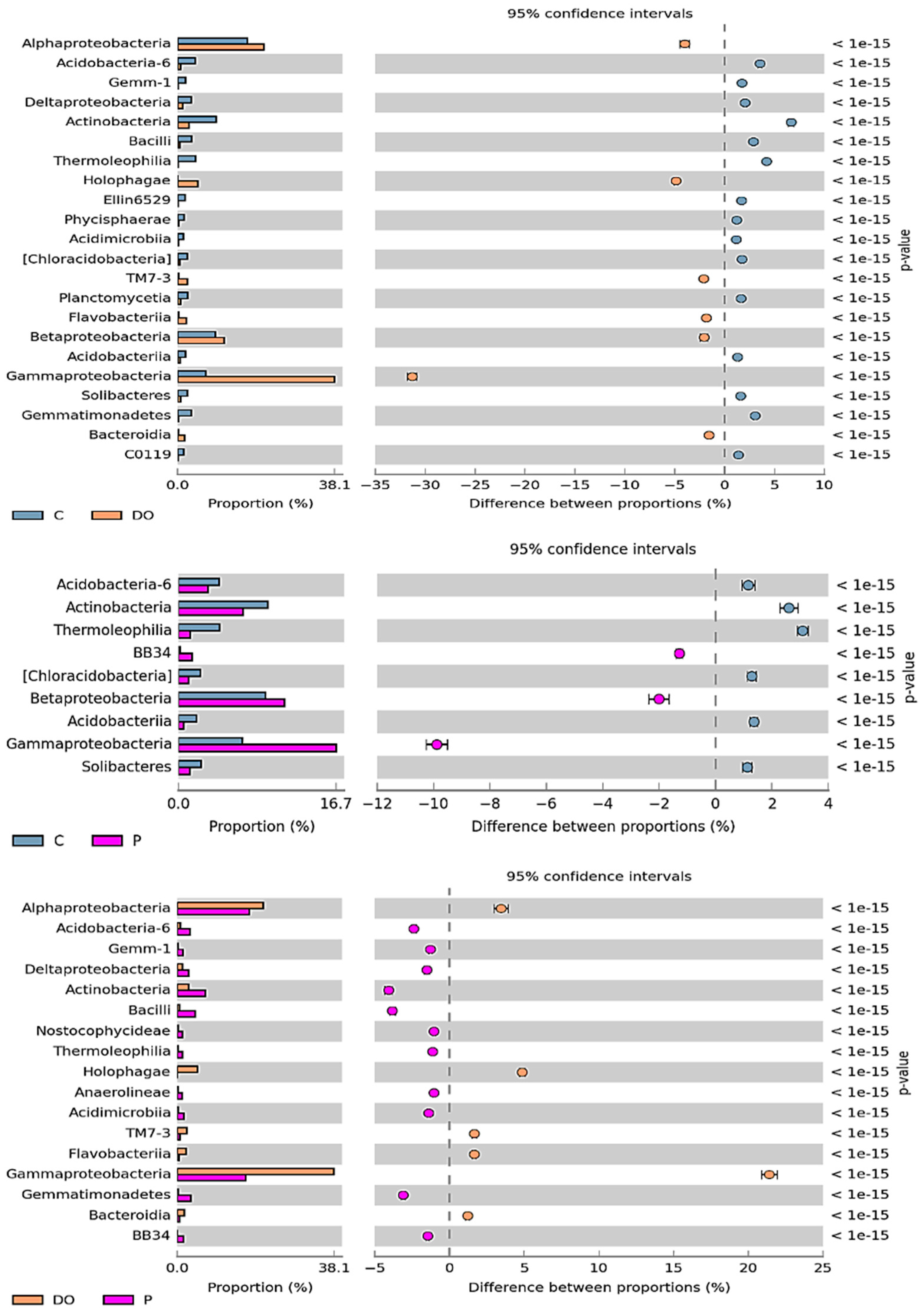
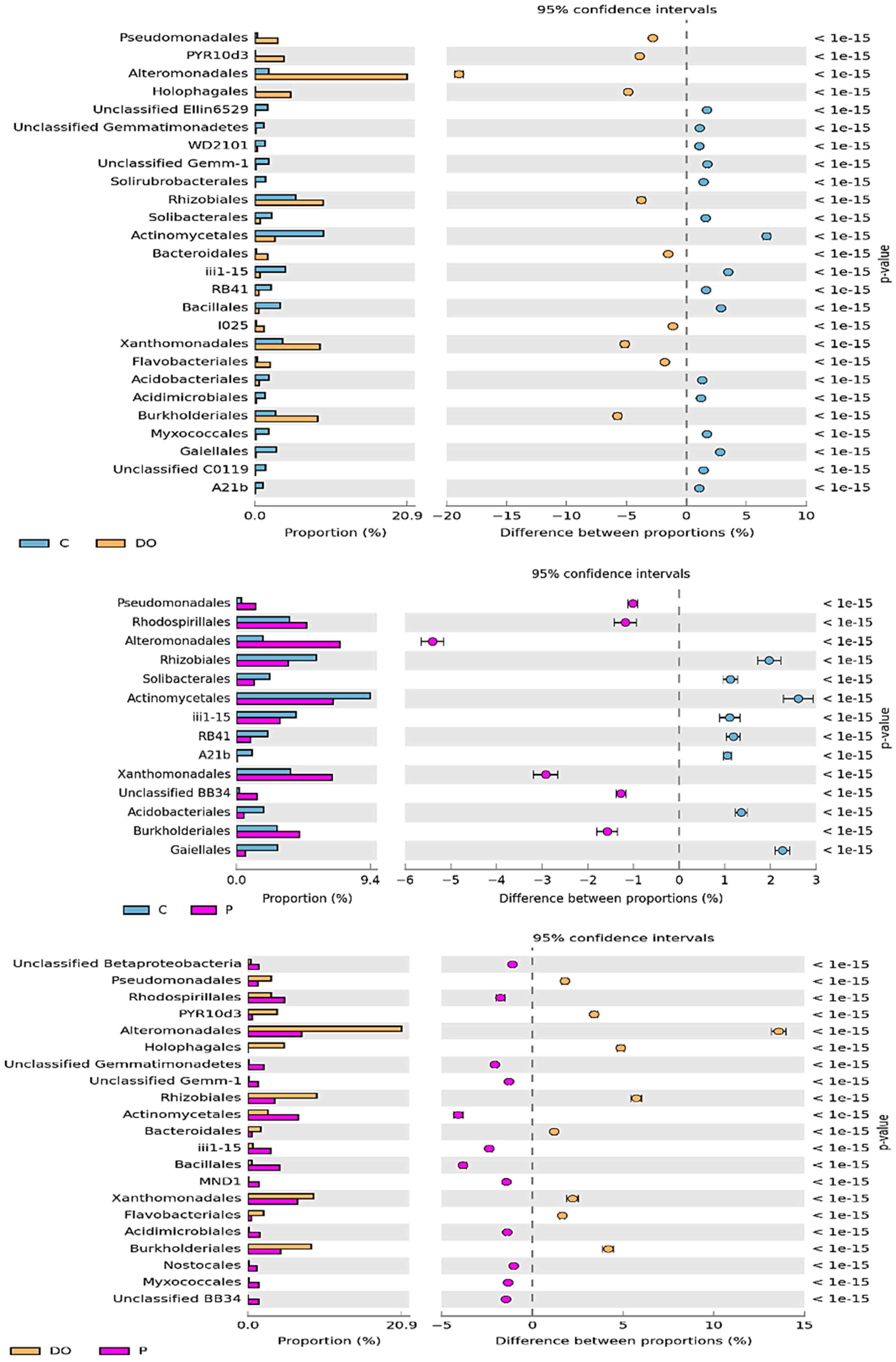
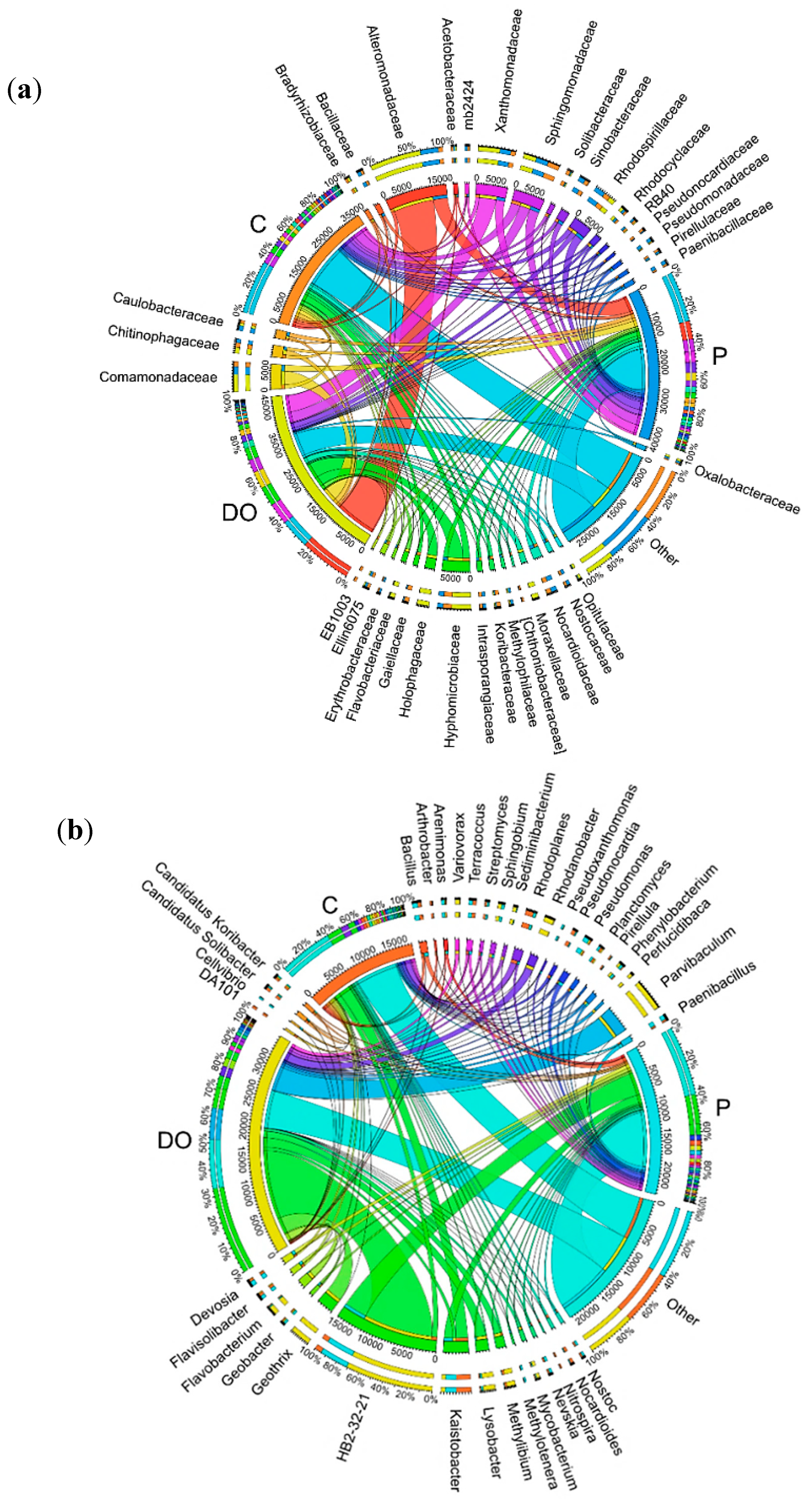
3.1. Counts and Diversity of Microorganisms in the Soil
3.2. Activity of Soil Enzymes
3.3. Physicochemical Properties of Soil
3.4. Degradation of Hydrocarbons
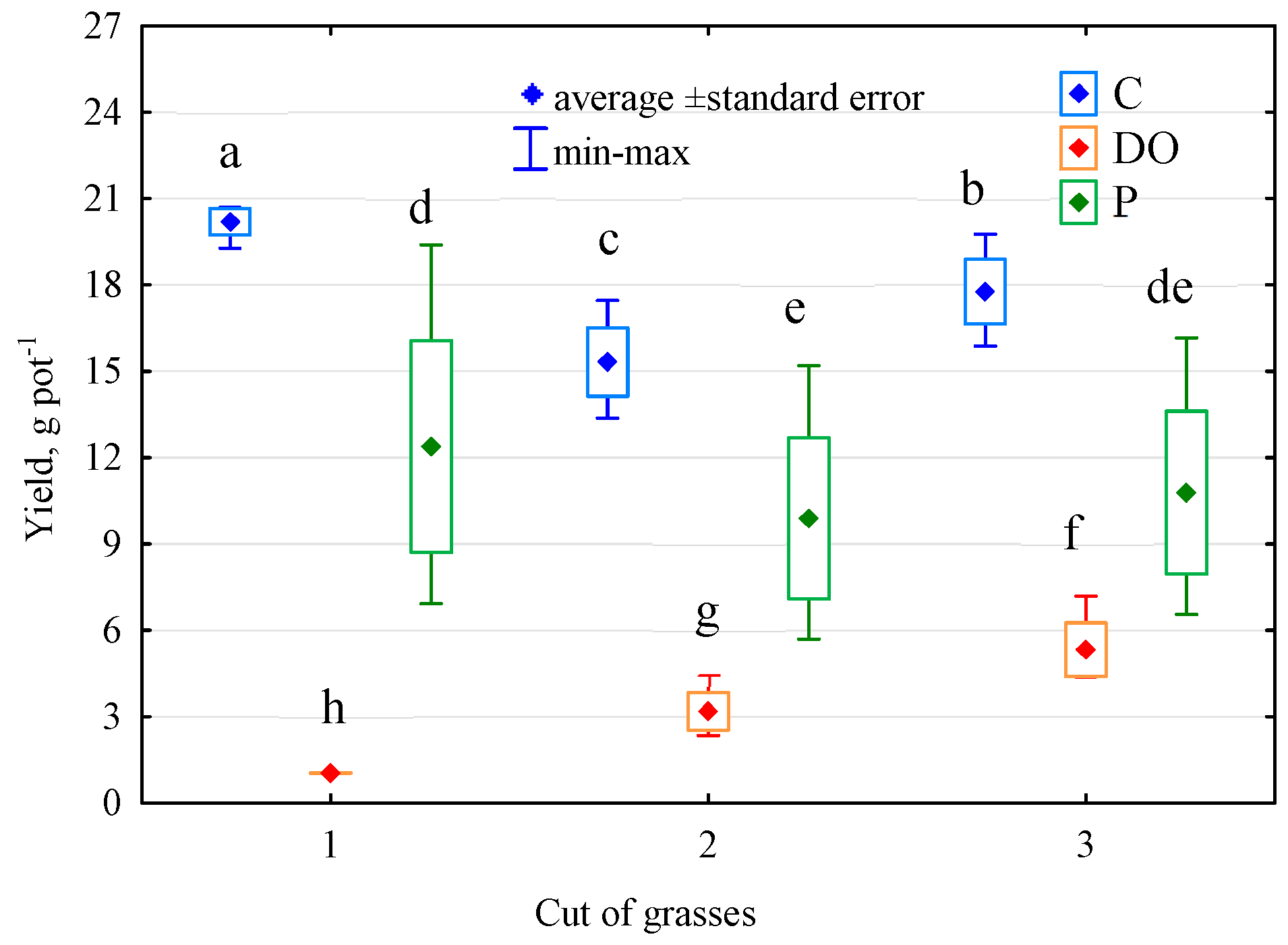
3.5. Response of Elymus Elongatus
4. Discussion
4.1. Counts and Diversity of Microorganisms in the Soil
4.2. Activity of Soil Enzymes
4.3. Degradation of Hydrocarbons
4.4. Physicochemical Properties of Soil
4.5. Plants Response
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency (IEA). Oil Market Report; International Energy Agency: Paris, France, 2019; p. 55.
- Hawrot-Paw, M.; Nowak, A. An attempt at mathematical modelling of the process of microbiological biodegradation of diesel oil. Environ. Prot. Eng. 2012, 38, 23–29. [Google Scholar]
- Hunt, L.J.; Duca, D.; Dan, T.; Knopper, L.D. Petroleum hydrocarbon (PHC) uptake in plants: A literature review. Environ. Pollut. 2019, 245, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.M.; Sangal, P.; Shukla, S. Microbiological analysis for hydrocarbon exploration. Environ. Risk Assess. Remediat. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2010, 2011, 941810. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J. Response of Avena sativa L. and the soil microbiota to the contamination of soil with shell diesel oil. Plant Soil Environ. 2018, 64, 102–107. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Bioaugmentation of soil contaminated with diesel oil. J. Elementol. 2018, 23, 1161–1178. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Roh, H.; Yang, J.K. Improving the clean-up efficiency of field soil contaminated with diesel oil by the application of stabilizers. Environ. Technol. 2013, 34, 1481–1487. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2017, 68, 12–26. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of aerobic microorganisms and soil enzyme response to soil contamination with Ekodiesel Ultra fuel. Environ. Sci. Pollut. Res. Int. 2017, 24, 24346–24363. [Google Scholar] [CrossRef] [Green Version]
- Naylor, D.; Coleman-Derr, D. Drought Stress and Root-Associated Bacterial Communities. Front. Plant Sci. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G.; Valori, F. Effects of root exudates in microbial diversity and activity in rhizosphere soil. In Soil Biology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 15, pp. 339–365. [Google Scholar]
- Haney, C.H.; Samuel, B.S.; Bush, J.; Ausubel, F.M. Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nat. Plants 2015, 1, 15051. [Google Scholar] [CrossRef] [PubMed]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Ann. Agric. Sci. 2018, 63, 123–127. [Google Scholar] [CrossRef]
- Anyasi, R.; Atagana, H.I. Assessment of plants at petroleum contaminated site for phytoremediation. In Proceedings of the International Conference of Recent Trends in Environmental Science and Engineering (RTESE’17), Toronto, ON, Canada, 23–25 August 2017. [Google Scholar]
- Shen, Y.; Ji, Y.; Li, C.; Luo, P.; Wang, W.; Zhang, Y.; Nover, D. Effects of phytoremediation treatment on bacterial community structure and diversity in different petroleum-contaminated soils. Int. J. Environ. Res. Public Health 2018, 15, 2168. [Google Scholar] [CrossRef] [PubMed]
- Peuke, A.D.; Rennenberg, H. Phytoremediation. EMBO Rep. 2005, 6, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Cho, K.S.; Kim, J. Comparative study of rhizobacterial community structure of plant species in oil-contaminated soil. J. Microbiol. Biotechnol. 2010, 20, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Jiang, H.; Cai, H.; Zhou, Y.; Krumholz, L.R. Complex interactions between the macrophyte acorus calamus and microbial fuel cells during pyrene and benzo[a]pyrene degradation in sediments. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J. Remediation of soil contaminated with diesel oil. J. Elementol. 2018, 23, 767–788. [Google Scholar] [CrossRef]
- Sipilä, T.P.; Keskinen, A.K.; Åkerman, M.L.; Fortelius, C.; Haahtela, K.; Yrjälä, K. High aromatic ring-cleavage diversity in birch rhizosphere: PAH treatment-specific changes of I.E.3 group extradiol dioxygenases and 16S rRNA bacterial communities in soil. ISME J. 2008, 2, 968–981. [Google Scholar] [CrossRef] [Green Version]
- Bell, T.H.; El-Din Hassan, S.; Lauron-Moreau, A.; Al-Otaibi, F.; Hijri, M.; Yergeau, E.; St-Arnaud, M. Linkage between bacterial and fungal rhizosphere communities in hydrocarbon-contaminated soils is related to plant phylogeny. ISME J. 2014, 8, 331–343. [Google Scholar] [CrossRef]
- Meagher, R.B. Phytoremediation of toxic elemental and organic pollutants. Curr. Opin. Plant Biol. 2000, 3, 153–162. [Google Scholar] [CrossRef]
- Lu, B.R. Meiotic studies of Elymus nutans and E. jacquemontii (Poaceae, Triticeae) and their hybrids with Pseudoroegneria spicata and seventeen Elymus species. Plant Syst. Evol. 1993, 186, 193–211. [Google Scholar] [CrossRef]
- Ma, X.; Chen, S.; Zhang, X.; Bai, S.; Zhang, C. Assessment of worldwide genetic diversity of Siberian wild rye (Elymus sibiricus L.) germplasm based on gliadin analysis. Molecules 2012, 17, 4424–4434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xie, W.; Zhang, J.; Zhao, X.; Zhao, Y.; Wang, Y. Phenotype-and SSR-based estimates of genetic variation between and within two important Elymus species in western and northern china. Genes 2018, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Guo, Y.; Zhou, H.; Wang, K. Isozyme variability among Elymus species indigenous to the Tibetan and Inner Mongolian Plateaus. Grassl. Sci. 2007, 53, 91–96. [Google Scholar] [CrossRef]
- Martyniak, D.; Żurek, G.; Prokopiuk, K. Biomass yield and quality of wild populations of tall wheatgrass [Elymus elongatus (Host.) Runemark]. Biomass Bioenergy 2017, 101, 21–29. [Google Scholar] [CrossRef]
- Paliwa BP. Available online: https://www.bp.com/pl_pl/poland/home/produkty_uslugi/paliwa.html (accessed on 3 June 2019).
- De Leij, F.A.A.M.; Whipps, J.M.; Lynch, J.M. The use of colony development for the characterization of bacterial communities in soil and on roots. Microb. Ecol. 1993, 27, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Öhlinger, R. Dehydrogenase activity with the substrate TTC. In Methods in Soil Biology; Schinner, F., Ohlinger, R., Kandler, E., Margesin, R., Eds.; Springer: Berlin, Germany, 1996; pp. 241–243. [Google Scholar]
- Johnson, J.I.; Temple, K.L. Some variables affecting the measurement of catalase activity in soil. Soil Sci. Soc. Am. Pro. 1964, 28, 207–216. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic: London, UK, 1998; pp. 316–365. [Google Scholar]
- ISO 10390. Soil Quality—Determination of pH; International Organization for Standardization: Geneva, Switzerland, 2005. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. (Eds.) Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 1224. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Method of Soil Analysis: Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- ISO 11261. Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Egner, H.; Riehm, H.; Domingo, W.R. Untersuchun-gen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extractionsmethoden zur Phospor-und Kaliumbestimmung. Ann. R. Agric. Coll. Swed. 1960, 26, 199–215. [Google Scholar]
- Schlichting, E.; Blume, H.P.; Stahr, K. Bodenkundliches Praktikum. Pareys Studientexte; Blackwell Wissenschafts: Berlin, Germany, 1995; p. 81. [Google Scholar]
- ISO 11260 Preview. Soil Quality—Determination of Effective Cation Exchange Capacity and Base Saturation Level Using Barium Chloride Solution; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- ISO 18287. Soil Quality—Determination of Polycyclic Aromatic Hydrocarbons (PAH)—Gas Chromatographic Method with Mass Spectrometric Detection (GC-MS); International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- EN ISO 16703. Soil Quality—Determination of Content of Hydrocarbon in the Range C10 to C40 by Gas Chromatography; International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- EN ISO 22155. Soil Quality—Gas Chromatographic Determination of Volatile Aromatic and Halogenated Hydrocarbons and Selected Ethers—Static Headspace Method; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- Dell Inc. Dell Statistica (Data Analysis Software System), version 13.1; Dell Inc.: Tulsa, OK, USA, 2016. [Google Scholar]
- Orwin, K.H.; Wardle, D.A. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biol. Biochem. 2004, 36, 1907–1912. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.I.; Schein, J.E.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, E.; Tanee, F. A laboratory trial of bioaugmentation for removal of total petroleum hydrocarbon (TPH) in Niger Delta soil using Oscillatoria bornettia. J. Microbiol. Biotechnol. 2011, 1, 147–168. [Google Scholar]
- Gałązka, A.; Grządziel, J.; Gałązka, R.; Ukalska-Jaruga, A.; Strzelecka, J.; Smreczak, B. Genetic and functional diversity of bacterial microbiome in soils with long term impacts of petroleum hydrocarbons. Front. Microbiol. 2018, 9, 1923. [Google Scholar] [CrossRef] [PubMed]
- Shabir, G.; Arslan, M.; Fatima, K.; Amin, I.; Khan, Q.M.; Afzal, M. Effects of inoculum density on plant growth and hydrocarbon degradation. Pedosphere 2016, 26, 774–778. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Oszust, K. Changes in the functional diversity of bacterial communities in soil contaminated with diesel oil. J. Elementol. 2018, 23, 1099–1117. [Google Scholar] [CrossRef]
- Kucharski, J.; Tomkiel, M.; Boros, E.; Wyszkowska, J. The effect of soil contamination with diesel oil and petrol on the nitrification process. J. Elementol. 2010, 15, 111–118. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, J. Response of Avena sativa, microorganisms and enzymes to contamination of soil with diesel oil. Plant Soil Environ. 2015, 61, 483–488. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Kucharski, J. Correlation between number of microbes and degree of soil contamination with petrol. Pol. J. Environ. Stud. 2001, 10, 175–181. [Google Scholar]
- Adam, I.K.U.; Duarte, M.; Pathmanathan, J.; Miltner, A.; Brüls, T.; Kästner, M. Microbial communities in pyrene amended soil-compost mixture and fertilized soil. AMB Express 2017, 7, 7. [Google Scholar] [CrossRef]
- Niepceron, M.; Martin-Laurent, F.; Crampon, M.; Portet-Koltalo, F.; Akpa-Vinceslas, M.; Legras, M.; Bru, D.; Bureau, F.; Bodilis, J. GammaProteobacteria as a potential bioindicator of a multiple contamination by polycyclic aromatic hydrocarbons (PAHs) in agricultural soils. Environ. Pollut. 2013, 180, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, J.; Jastrzębska, E. Effects of heating oil on the count of microorganisms and physico-chemical properties of soil. Pol. J. Environ. Stud. 2005, 14, 195–204. [Google Scholar]
- Nwaichi, E.O.; Frac, M.; Nwoha, P.A.; Eragbor, P. Enhanced phytoremediation of crude oil-polluted soil by four plant species: Effect of inorganic and organic bioaugumentation. Int. J. Phytoremediation 2015, 17, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.C.; Vessoni-Penna, T.C.; de Souza Oliveira, R.P. Biosurfactant-enhanced hydrocarbon bioremediation: An overview. Int. Biodeterior. Biodegradation 2014, 89, 88–94. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Kucharski, J. Correlation between the number of microorganisms and soil contamination with diesel oil. Pol. J. Environ. Stud. 2005, 14, 359–368. [Google Scholar]
- Hou, J.; Liu, W.; Wang, B.; Wang, Q.; Luo, Y.; Franks, A.E. PGPR enhanced phytoremediation of petroleum contaminated soil and rhizosphere microbial community response. Chemosphere 2015, 138, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Philippot, L.; Park, W. Metagenomic and functional analyses of the consequences of reduction of bacterial diversity on soil functions and bioremediation in diesel-contaminated microcosms. Sci. Rep. 2016, 6, 23012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, N.X.; Yu, J.; Zhao, H.M.; Cheng, Y.T.; Mo, C.H.; Cai, Q.Y.; Li, Y.W.; Li, H.; Wong, M.H. Efficient phytoremediation of organic contaminants in soils using plant–endophyte partnerships. Sci. Total Environ. 2017, 583, 352–368. [Google Scholar] [CrossRef]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic bacteria: Prospects and applicationsfor the phytoremediation of organic pollutants. Chemosphere 2014, 117, 232–242. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chettri, B.; Langpoklakpam, J.S.; Basak, P.; Prasad, A.; Mukherjee, A.K.; Bhattacharyya, M.; Singh, A.K.; Chattopadhyay, D. Bioinformatic approaches including predictive metagenomic profiling reveal characteristics of bacterial response to petroleum hydrocarbon contamination in diverse environments. Sci. Rep. 2017, 7, 1108. [Google Scholar] [CrossRef]
- Czarny, J.; Staninska-Pieta, J.; Powierska-Czarny, J.; Nowak, J.; Wolko, L.; Piotrowska-Cyplik, A. Metagenomic analysis of soil bacterial community and level of genes responsible for biodegradation of aromatic hydrocarbons. Pol. J. Microbiol. 2017, 66, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Walder, G.; Schinner, F. The impact of hydrocarbon remediation (diesel oil and polycyclic aromatic hydrocarbons) on enzyme activities and microbial properties of soil. Acta Biotechnol. 2000, 20, 313–333. [Google Scholar] [CrossRef]
- Murinova, S.; Dercova, K. Response mechanisms of bacterial degraders to environmental contaminants on the level of cell walls and cytoplasmic membrane. Int. J. Microbiol. 2014, 2014, 873081. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Anand, S.; Moulick, A.; Baraniya, D.; Imran Pathan, S.; Rejsek, K.; Vranová, V.; Sharma, M.; Sharma, D.; Kelkar, A.; et al. How enzymes are adsorbed on soil solid phase and factors limiting its activity: A Review. Int. Agrophys. 2017, 31, 287–302. [Google Scholar] [CrossRef]
- Nannipieri, P.; Carmen, T.C.; Richard, P.D. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Telesinski, A.; Krzysko-Łupicka, T.; Cybulska, K.; Wrobel, J. Response of soil phosphatase activities to contamination with two types of tar oil. Environ. Sci. Pollut. Res. Int. 2018, 25, 28642–28653. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowski, M.; Ziółkowska, A. The importance of relieving substances in restricting the effect of soil contamination with oil derivatives on plants. Fresen. Environ. Bull. 2011, 20, 711–719. [Google Scholar]
- Liu, R.; Jadeja, R.N.; Zhou, Q.; Liu, Z. Treatment and remediation of petroleum-contaminated soils using selective ornamental plants. Environ. Eng. Sci. 2012, 29, 494–501. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Košnář, Z.; Mercl, F.; Aranda, E.; Tlustoš, P. A comparative study to evaluate natural attenuation, mycoaugmentation, phytoremediation, and microbial-assisted phytoremediation strategies for the bioremediation of an aged PAH-polluted soil. Ecotoxicol. Environ. Safe 2018, 147, 165–174. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, M. Bioremediation of crude oil-contaminated soil: Comparison of different biostimulation and bioaugmentation treatments. J. Hazard. Mater. 2010, 183, 395–401. [Google Scholar] [CrossRef]
- Nanekar, S.; Dhote, M.; Kashyap, S.; Singh, S.K.; Juwarkar, A.A. Microbe assisted phytoremediation of oil sludge and role of amendments: A mesocosm study. Int. J. Environ. Sci. Technol. 2013, 12, 193–202. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Bakina, L.G.; Chugunova, M.V.; Mayachkina, N.V.; Gerasimov, A.O.; Bure, V.M. Effect of remediation strategies on biological activity of oil-contaminated soil—A field study. Int. Biodeter. Biodegr. 2018, 126, 57–68. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Ziółkowska, A. Compost, bentonite and calcium oxide used for alleviation of the impact of petroleum products on some soil properties. Pol. J. Nat. Sci. 2013, 28, 327–337. [Google Scholar]
- Wyszkowski, M.; Sivitskaya, V. Changes in the content of organic carbon and available forms of macronutrients in soil under the influence of soil contamination with fuel oil and application of different substances. J. Elementol. 2012, 17, 139–148. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Sivitskaya, V. Effect of different substances on some properties of soil contaminated with heating oil. J. Ecol. Eng. 2015, 16, 62–66. [Google Scholar] [CrossRef]
- Khalilova, H.K. The impact of oil contamination on soil ecosystem. Biol. Chem. Res. 2015, 2015, 133–139. [Google Scholar]
- Wang, Y.; Feng, J.; Lin, Q.; Lyu, X.; Wang, X.; Wang, G. Effects of crude oil contamination on soil physical and chemical properties in Momoge wetland of China. Chin. Geogr. Sci. 2013, 23, 708–715. [Google Scholar] [CrossRef]
- Rasheed, Z.N.; Ahmed, F.R.; Jassim, H.M. Effect of crude oil products on the geotechnical properties of soil, WIT Trans. Ecol. Environ. 2014, 186, 353–362. [Google Scholar] [CrossRef]
- Prasanna, G.; Manoharan, S. A review on effect of crude oil on the geotechnical properties of soil. Int. J. Res. Eng. Technol. 2016, 3, 1234–1236. [Google Scholar]
- Kucharski, J.; Jastrzębska, E. Effect of heating oil on the activity of soil enzymes and the yield of yellow lupine. Plant Soil Environ. 2006, 52, 220–226. [Google Scholar] [CrossRef]
- Fatima, K.; Imran, A.; Amin, I.; Khan, Q.M.; Afzal, M. Successful phytoremediation of crude-oil contaminated soil at an oil exploration and production company by plants-bacterial synergism. Int. J. Phytoremediation 2018, 20, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, A.; Zhang, M.; Li, H.; Du, S.; Bai, L.; Chen, S.; Zhong, M. Compared the physiological response of two petroleum tolerant-contrasting plants to petroleum stress. Int. J. Phytoremediation 2018, 20, 1043–1048. [Google Scholar] [CrossRef] [PubMed]




| Object | Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|---|
| Shannon Index | ||||||
| C | 2.13 a | 3.34 a | 3.87 a | 4.21 a | 4.13 a | 2.75 b |
| DO | 1.23 c | 2.26 c | 3.06 c | 3.04 c | 2.82 b | 1.68 c |
| P | 2.00 b | 3.16 b | 3.75 b | 3.99 b | 4.06 a | 2.87 a |
| Simpson Index | ||||||
| C | 0.81 a | 0.94 a | 0.96 a | 0.98 a | 0.97 a | 0.90 a |
| DO | 0.48 c | 0.79 b | 0.91 a | 0.90 b | 0.84 b | 0.61 b |
| P | 0.74 b | 0.92 a | 0.96 b | 0.97 a | 0.95 a | 0.92 a |
| Object | Corg | EBC | HAC | CEC | BS | pHKCl |
|---|---|---|---|---|---|---|
| g kg−1 | mmol(+) kg−1 | % | ||||
| C | 26.9 b | 69.3 a | 11.3 b | 80.6 a | 86.0 a | 6.7 c |
| DO | 28.3 a | 62.0 b | 11.3 b | 70.3 b | 84.6 b | 7.0 a |
| P | 26.3 b | 58.7 c | 12.0 a | 70.7 b | 83.0 c | 6.9 a,b |
| Object | C6–C12 | C12–C35 | Ben | EtB | Tol | Xyl | Sty | ∑ BTEX | Nap | Ant |
| DO | 89.1 b | 63.0 b | 75.0 b | 99.4 a | 98.6 b | 99.6 a | 0.0 b | 99.3 a | 99.2 b | 87.6 b |
| P | 99.9 a | 81.3 a | 98.1 a | 99.9 a | 100.0 a | 99.9 a | 96.7 a | 99.9 a | 99.8 a | 66.6 a |
| Object | Chr | BaA | DahA | BaP | BbF | BkF | BghiP | IP | 9PAHs | 10PAHs |
| DO | 47.8 b | 37.5 b | 0.0 b | 44.4 b | 25.0 b | 20.0 b | 0.0 b | 25.0 b | 95.3 b | 95.8 a |
| P | 82.4 a | 77.6 a | 50.0 a | 76.5 a | 77.6 a | 80.6 a | 97.3 a | 97.9 a | 99.0 a | 95.6 a |
| Object | RS | RL | ||
|---|---|---|---|---|
| Cut 1 | Cut 2 | Cut 3 | ||
| DO | 0.026 c | 0.116 b,c | 0.176 b | 0.213 a |
| P | 0.442 a | 0.476 a | 0.436 a | 0.056 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. Implications of Soil Pollution with Diesel Oil and BP Petroleum with ACTIVE Technology for Soil Health. Int. J. Environ. Res. Public Health 2019, 16, 2474. https://doi.org/10.3390/ijerph16142474
Borowik A, Wyszkowska J, Kucharski M, Kucharski J. Implications of Soil Pollution with Diesel Oil and BP Petroleum with ACTIVE Technology for Soil Health. International Journal of Environmental Research and Public Health. 2019; 16(14):2474. https://doi.org/10.3390/ijerph16142474
Chicago/Turabian StyleBorowik, Agata, Jadwiga Wyszkowska, Mirosław Kucharski, and Jan Kucharski. 2019. "Implications of Soil Pollution with Diesel Oil and BP Petroleum with ACTIVE Technology for Soil Health" International Journal of Environmental Research and Public Health 16, no. 14: 2474. https://doi.org/10.3390/ijerph16142474





