Effects of Mining Activities on the Release of Heavy Metals (HMs) in a Typical Mountain Headwater Region, the Qinghai-Tibet Plateau in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.1.1. Geology
2.1.2. Anthropogenic Activity
2.2. Water Sample Collection and Analysis
2.3. Sediments Sample Collection and Analysis
2.4. Factor Analysis
3. Results and Discussion
3.1. Water Quality Assessment
3.2. Distribution Characteristics of Heavy Metals in Sediment and Water
3.3. The Interaction of Surface Water, Groundwater, and Mining Waste Leaching Water
3.4. Variability of Heavy Metals Along the Main Course
3.5. Heavy Metal Provenance Tracing (Factor Analysis)
3.6. Identifying Hydrogeochemical Processes
3.7. Impact of Mining Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Förstner, U.; Wittmann, G. Metal Pollution in the Aquatic Environment; Springer: Berlin, Germany, 1983; ISBN 9783540128564. [Google Scholar]
- Meng, Q.; Zhang, J.; Zhang, Z.; Wu, T. Geochemistry of dissolved trace elements and heavy metals in the Dan River Drainage (China): Distribution, sources, and water quality assessment. Environ. Sci. Pollut. Res. 2016, 23, 8091–8103. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, L.; Wang, J. Multivariate analysis of heavy metal pollution in street dusts of Xianyang city, NW China. Environ. Earth Sci. 2013, 69, 1973–1979. [Google Scholar] [CrossRef]
- Kumar, M.; Ramanatahn, A.L.; Tripathi, R.; Farswan, S.; Kumar, D.; Bhattacharya, P. A study of trace element contamination using multivariate statistical techniques and health risk assessment in groundwater of Chhaprola Industrial Area, Gautam Buddha Nagar, Uttar Pradesh, India. Chemosphere 2017, 166, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-M.; Dang, Z.; Cai, M.-F.; Liu, C.-Q. Soil Heavy Metal Pollution Around the Dabaoshan Mine, Guangdong Province, China. Pedosphere 2007, 17, 588–594. [Google Scholar] [CrossRef]
- Zhao, L.J.; Yin, L.; Xiao, H.L.; Cheng, G.D.; Zhou, M.X.; Yang, Y.G.; Li, C.Z.; Zhou, J. Isotopic evidence for the moisture origin and composition of surface runoff in the headwaters of the Heihe River basin. Chin. Sci. Bull. 2011, 56, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Li, X.; Zhao, W.; Xu, Z.; Feng, Q.; Xiao, S.; Xiao, H. Integrated study of the water-ecosystem-economy in the Heihe River Basin. Natl. Sci. Rev. 2014, 1, 413–428. [Google Scholar] [CrossRef]
- Zhong, Z. The Chinese western development initiative: New opportunities for mineral investment. Resour. Policy 2002, 28, 117–131. [Google Scholar] [CrossRef]
- Xiao, C.; Zou, X.; Jia, Q.; Yang, Z. Present situation of the mineral resource in Qilian metallogenic belt and thinking. Northwest. Geol. 2003, 36, 38–49. [Google Scholar]
- Bu, J.; Sun, Z.; Zhou, A.; Xu, Y.; Ma, R.; Wei, W.; Liu, M. Heavy metals in surface soils in the upper reaches of the Heihe River, northeastern Tibetan Plateau, China. Int. J. Environ. Res. Public Health 2016, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Yan China Punishes Officials Over Environmental Violations in Qilian Mountains. Available online: http://www.xinhuanet.com/english/2017-07/20/c_136459844.htm (accessed on 20 July 2018).
- Gao, B.; Qin, Y.; Wang, Y.; Yang, D.; Zheng, Y. Modeling ecohydrological processes and spatial patterns in the upper Heihe basin in China. Forests 2016, 7, 10. [Google Scholar] [CrossRef]
- Ma, R.; Sun, Z.; Hu, Y.; Chang, Q.; Wang, S.; Xing, W.; Ge, M. Hydrological connectivity from glaciers to rivers in the Qinghai-Tibet Plateau: Roles of suprapermafrost and subpermafrost groundwater. Hydrol. Earth Syst. Sci. 2017, 21, 4803–4823. [Google Scholar] [CrossRef]
- Yang, Q.; Xiao, H.; Zhao, L.; Yang, Y.; Li, C.; Zhao, L.; Yin, L. Hydrological and isotopic characterization of river water, groundwater, and groundwater recharge in the Heihe River basin, northwestern China. Hydrol. Process. 2011, 25, 1271–1283. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, H.; Wei, Y.; Zhao, L.; Zou, S.; Yin, Z.; Yang, Q. Hydrologic processes in the different landscape zones of Mafengou River basin in the alpine cold region during the melting period. J. Hydrol. 2011, 409, 149–156. [Google Scholar] [CrossRef]
- Yang, Y.G.; Xiao, H.L.; Zou, S.B.; Zhao, L.J.; Zhou, M.X.; Hou, L.G.; Wang, F. Hydrochemical and hydrological processes in the different landscape zones of alpine cold region in China. Environ. Earth Sci. 2012, 65, 609–620. [Google Scholar] [CrossRef]
- Li, Z.; Qi, F.; Li, J.; Pan, Y.; Wang, T.; Li, L.; Xiaoyan, G.; Yan, G.; Bing, J.; Rui, G. Environmental significance and hydrochemical processes at a cold alpine basin in the Qilian Mountains. Environ. Earth Sci. 2015, 73, 4043–4052. [Google Scholar] [CrossRef]
- Li, X.; Cheng, G.; Ge, Y.; Li, H.; Han, F.; Hu, X.; Tian, W.; Tian, Y.; Pan, X.; Nian, Y.; et al. Hydrological Cycle in the Heihe River Basin and Its Implication for Water Resource Management in Endorheic Basins. J. Geophys. Res. Atmos. 2018, 123, 890–914. [Google Scholar] [CrossRef]
- Xia, X.H.; Song, S.G. Forming age and tectono-petrogenises of the Jiugequan ophiolite in the North Qilian Mountain, NW China. Chin. Sci. Bull. 2010, 55, 1899–1907. [Google Scholar] [CrossRef]
- Song, S.; Niu, Y.; Su, L.; Xia, X. Tectonics of the North Qilian orogen, NW China. Gondwana Res. 2013, 23, 1378–1401. [Google Scholar] [CrossRef]
- Baranova, A.; Schickhoff, U.; Wang, S.; Jin, M. Mountain pastures of Qilian Shan: Plant communities, grazing impact and degradation status (Gansu province, NW China). Geophys. Res. Abstr. 2016, 21–35. [Google Scholar] [CrossRef]
- Xia, L.; Xia, Z.; Xu, X. Early Palaeozoic Mid-Ocean Rudge-Ocean Island and Back-Arc Basin Volcanism in the North Qilian Mountains. Acta Geol. Sin. 1998, 72, 301–312. [Google Scholar]
- Yi, Y.; Dong, X.; Du, Z.; Yuan, K.; Cao, S. Analysis of metallogenic conditions of metamorphic sedimentary iron ore deposit in the upper stream of the Black River along Qilian mountain range. Miner. Explor. 2015, 6, 397–404. [Google Scholar]
- Bowen, H.J.M. Environmental Chemistry of the Elements; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Xiao, Y.; Niu, Y.; Wang, K.L.; Lee, D.C.; Iizuka, Y. Geochemical behaviours of chemical elements during subduction-zone metamorphism and geodynamic significance. Int. Geol. Rev. 2016, 58, 1253–1277. [Google Scholar] [CrossRef]
- Gran, G.; Dahlenborg, H.; Laurell, S.; Rottenberg, M. Determination of the Equivalent Point in Potentiometric Titrations. Acta Chem. Scand. 1950, 4, 559–577. [Google Scholar] [CrossRef]
- Sun, Z.; Long, X.; Ma, R. Water uptake by saltcedar (Tamarix ramosissima) in a desert riparian forest: Responses to intra-annual water table fluctuation. Hydrol. Process. 2016, 30, 1388–1402. [Google Scholar] [CrossRef]
- Goyer, R.; Mehlman, M. Toxicology of Trace Elements; Hemisphere Publishing Corporation: Dubrovnik, Croatia, 1977. [Google Scholar]
- Sheng, J.; Weather, B.; Office, M.; Wang, X.; Gong, P.; Tian, L. Heavy metals of the Tibetan top soils. Environ. Sci. Pollut. Res. 2012, 19, 3362–3370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Liu, J.; Liu, Q.; Zhou, Q. Multivariate and geostatistical analyses of the sources and spatial distribution of heavy metals in agricultural soil in Gongzhuling, Northeast China. J. Soils Sediments 2016, 16, 634–644. [Google Scholar] [CrossRef]
- Doabi, S.A.; Afyuni, M.; Karami, M. Multivariate statistical analysis of heavy metals contamination in atmospheric dust of Kermanshah province, western Iran, during the spring and summer 2013. J. Geochem. Explor. 2017, 180, 61–70. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Zhou, X.; You, X.; Shi, Y.; Xu, J. Source identification and spatial distribution of heavy metals in tobacco-growing soils in Shandong province of China with multivariate and geostatistical analysis. Environ. Sci. Pollut. Res. 2017, 24, 5964–5975. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, G.; Liu, H.; Lam, P.K.S. Multivariate statistical evaluation of dissolved trace elements and a water quality assessment in the middle reaches of Huaihe River, Anhui, China. Sci. Total Environ. 2017, 583, 421–431. [Google Scholar] [CrossRef] [PubMed]
- State Environmental Protection Administration of China (SEPA); General Administration of Quality Supervision (AQSIQ). Chinese National Standards GB 3838-2002: Environmental Quality Standards for Surface Water; China Water Risk: Hongkong, China, 2002.
- Ministry of Health of the People’s Republic of China (MOH); Standardization Administration of China (SAC). Chinese National Standards GB 3838-2002: Sanitary Standards for Drinking Water Quality; China Water Risk: Hongkong, China, 2006.
- Pfeifer, H.R.; Derron, M.H.; Rey, D.; Schlegel, C.; Atteia, O.; Piazza, R.D.; Dubois, J.P.; Mandia, Y. Chapter 2 Natural trace element input to the soil-sediment-water-plant system: Examples of background and contaminated situations in Switzerland, Eastern France and Northern Italy. Trace Met. Environ. 2000, 4, 33–86. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. ISBN 978-94-007-4470-7. [Google Scholar]
- Tong, J.; Zhou, M.; Sun, Z.; Chang, Q.; Li, J. Water vapor sources precipitation in the upper reaches of heihe river: Evidence from stable water isotopes and air mass trajectory model. J. Arid Land Resour. Environ. 2013. [Google Scholar] [CrossRef]
- Wang, N.L.; Zhang, S.B.; He, J.Q.; Pu, J.C.; Wu, X.B.; Jiang, X. Tracing the major source area of the mountainous runoff generation of the Heihe River in northwest China using stable isotope technique. Chin. Sci. Bull. 2009, 54, 2751–2757. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, H.; Wei, Y.; Zhao, L.; Zou, S.; Yang, Q.; Yin, Z. Hydrological processes in the different landscape zones of alpine cold regions in the wet season, combining isotopic and hydrochemical tracers. Hydrol. Process. 2012, 26, 1457–1466. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Song, X.F.; Wu, Y.Q. Use of oxygen-18 isotope to quantify flows in the upriver and middle reaches of the Heihe River, northwestern china. Environ. Geol. 2009, 58, 645–653. [Google Scholar] [CrossRef]
- Chang, Q.; Ma, R.; Sun, Z.; Zhou, A.; Hu, Y.; Liu, Y. Using isotopic and geochemical tracers to determine the contribution of glacier-snow meltwater to streamflow in a partly glacierized alpine-gorge catchment in northeastern Qinghai-Tibet Plateau. J. Geophys. Res. Atmos. 2018. [Google Scholar] [CrossRef]
- Förstner, U.; Wittmann, G.T.W. Metal Pollution in the Aquatic Environment; Springer: Berlin/Heidelberg, Germany, 1981; ISBN 978-3-540-12856-4. [Google Scholar]
- Zhang, X.P.; Deng, W.; Yang, X.M. The background concentrations of 13 soil trace elements and their relationships to parent materials and vegetation in Xizang (Tibet), China. J. Asian Earth Sci. 2002, 21, 167–174. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Nanogeochemistry: Nanostructures, emergent properties and their control on geochemical reactions and mass transfers. Chem. Geol. 2014, 378–379, 1–23. [Google Scholar] [CrossRef]
- Jin, Z.; You, C.F.; Yu, T.L.; Wang, B.S. Sources and flux of trace elements in river water collected from the Lake Qinghai catchment, NE Tibetan Plateau. Appl. Geochem. 2010, 25, 1536–1546. [Google Scholar] [CrossRef]
- Chessa, G.; Calaresu, G.; Ledda, G.; Testa, M.C.; Orrù, A. Chapter 18 Lead, zinc and cadmium in biological tissues of sheep bred in a polluted area. In Trace Metals in the Environment; Elsevier: Amsterdam, The Netherlands, 2000; Volume 4, pp. 479–483. ISBN 0927-5215. [Google Scholar]
- Sarmiento, A.M.; Nieto, J.M.; Olías, M.; Cánovas, C.R. Hydrochemical characteristics and seasonal influence on the pollution by acid mine drainage in the Odiel river Basin (SW Spain). Appl. Geochem. 2009, 24, 697–714. [Google Scholar] [CrossRef]
- Waseem, A.; Arshad, J.; Iqbal, F.; Sajjad, A.; Mehmood, Z.; Murtaza, G. Pollution Status of Pakistan: A Retrospective Review on Heavy Metal Contamination of Water, Soil, and Vegetables. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Banks, D.; Younger, P.L.; Arnesen, R.T.; Iversen, E.R.; Banks, S.B. Mine-water chemistry: The good, the bad and the ugly. Environ. Geol. 1997, 32, 157–174. [Google Scholar] [CrossRef]
- Lee, P.K.; Baillif, P.; Touray, J.C. Geochemical behaviour and relative mobility of metals (Mn, Cd, Zn and Pb) in recent sediments of a retention pond along the A-71 motorway in Sologne, France. Environ. Geol. 1997, 32, 142–152. [Google Scholar] [CrossRef]
- Khan, R.; Israili, S.H.; Ahmad, H.; Mohan, A. Heavy Metal Pollution Assessment in Surface Water Bodies and its Suitability for Irrigation around the Neyevli Lignite Mines and Associated Industrial Complex, Tamil Nadu, India. Mine Water Environ. 2005, 24, 155–161. [Google Scholar] [CrossRef]
- Huang, X.; Sillanpää, M.; Gjessing, E.T.; Peräniemi, S.; Vogt, R.D. Environmental impact of mining activities on the surface water quality in Tibet: Gyama valley. Sci. Total Environ. 2010, 408, 4177–4184. [Google Scholar] [CrossRef] [PubMed]
- Wiggering, H. Sulfide oxidation—An environmental problem within colliery spoil dumps. Environ. Geol. 1993, 22, 99–105. [Google Scholar] [CrossRef]
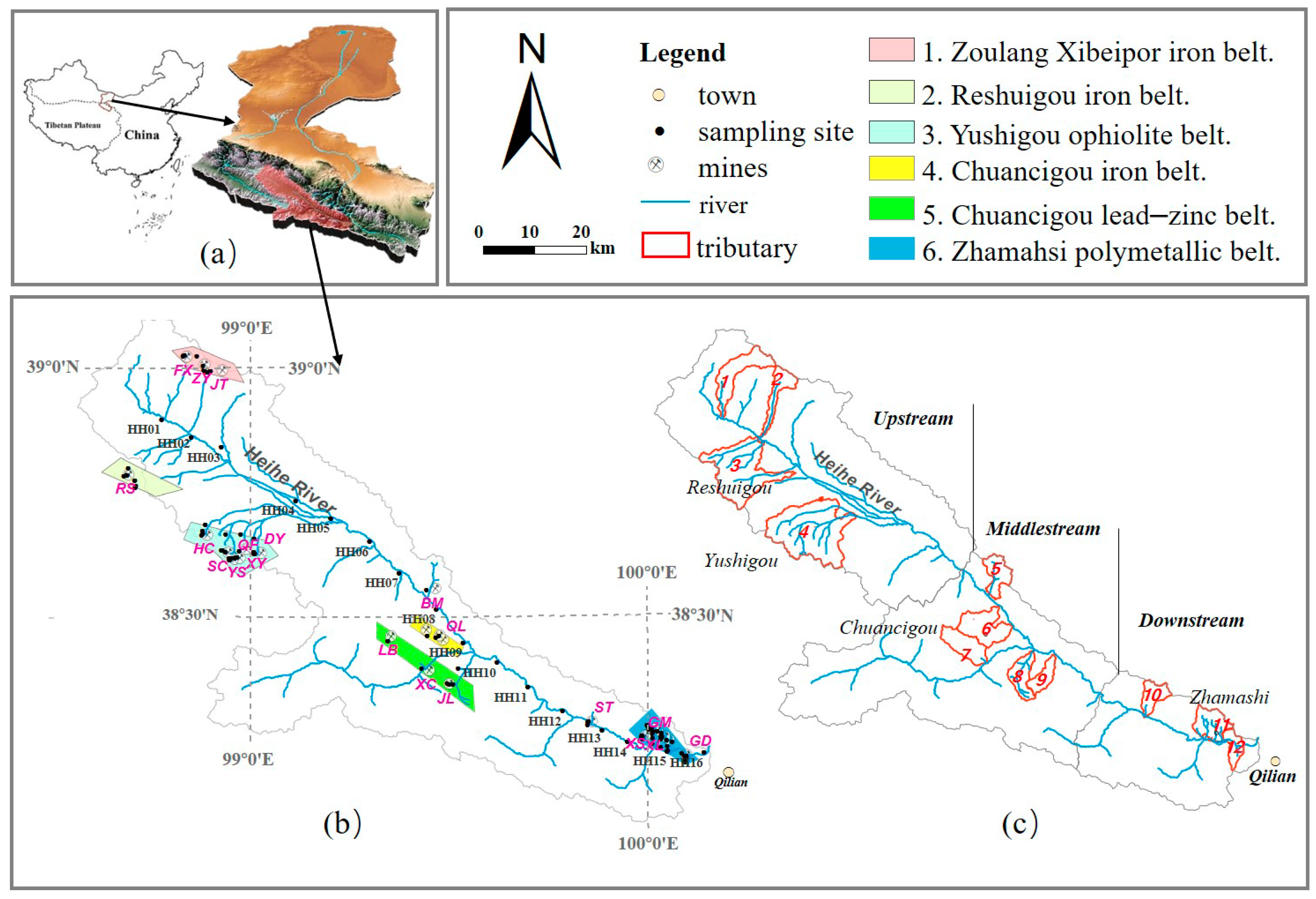

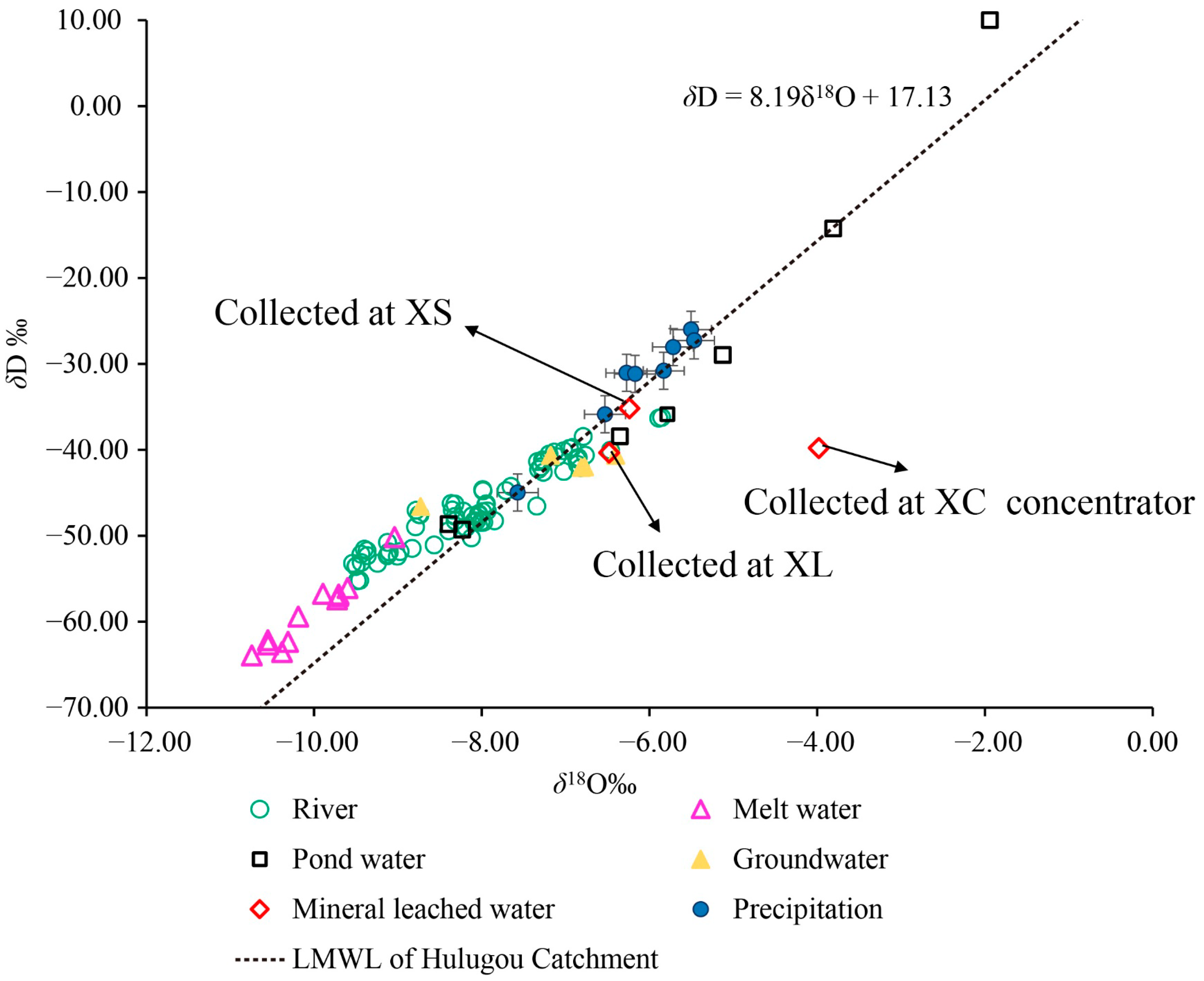
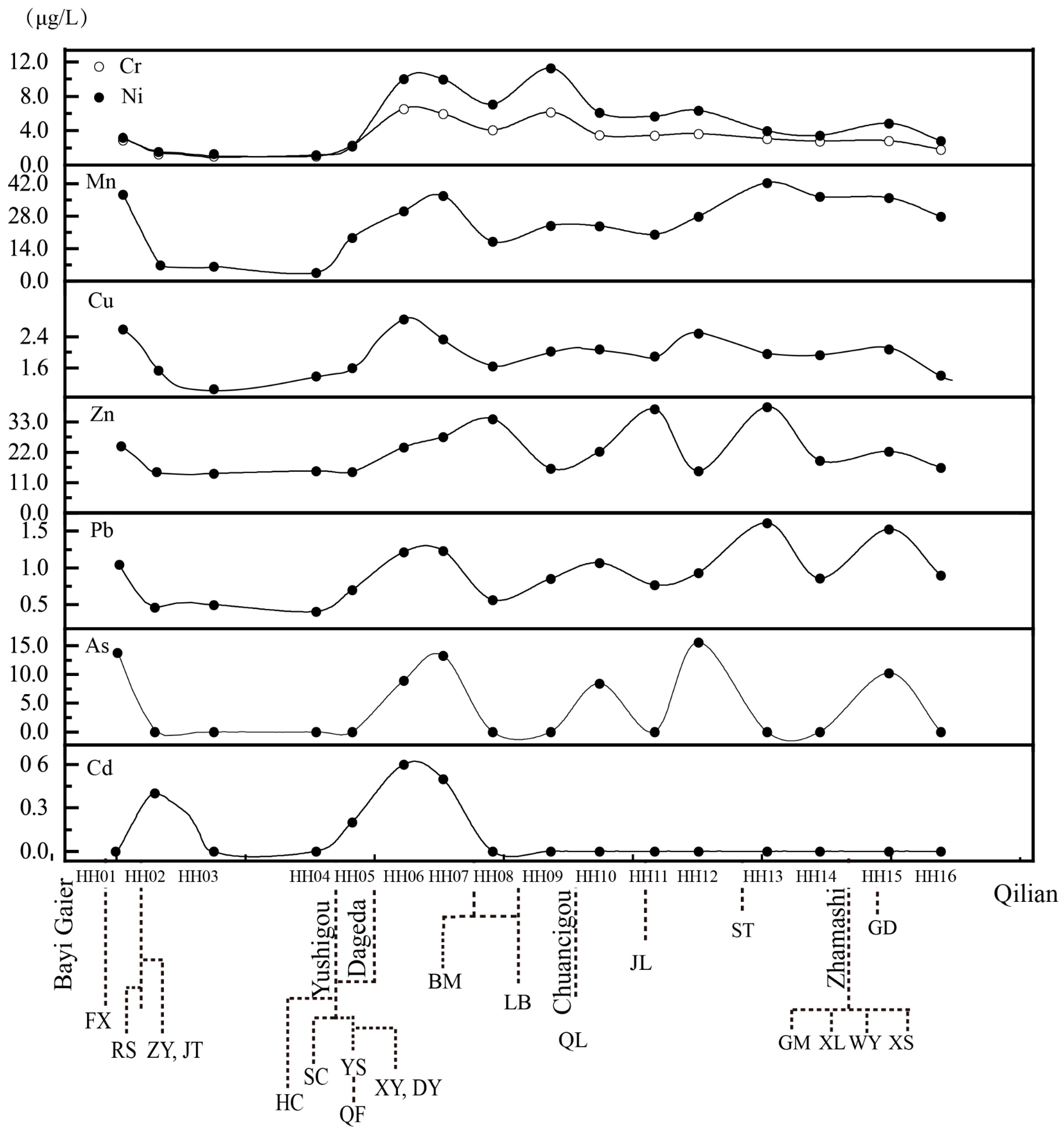

| Reaches | ID a | Mine b | Date | Mine Area (km2) | Ore Type | Elevation (m) | Mining Method (OP/UG) c | Water Sample Collected from Several Mines | Sediment | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meltwater | River Water | Groundwater | Pond Water | Mineral Leached Water | Total | |||||||||
| Upstream | 1 | FX | 20/August | 0.087 | Marble | 4280–4582 | OP | 1 | 1 | 3 | 5 | 5 | ||
| 2 | JT | 19/August | 1.115 | Polymetallic | 3920–4100 | UG | 2 | 1 | 3 | 2 | ||||
| ZY | 19/August | 0.498 | Iron ore | 4300–4600 | OP | 4 | 1 | 5 | 3 | |||||
| 3 | RS | 18/August | 0.570 | Iron ore | 4190–4290 | OP | 4 | 5 | 9 | 3 | ||||
| 4 | HC | 21/August | 0.105 | Serpentine | 4238–4308 | OP | 3 | 1 | 4 | 3 | ||||
| XY | 21/August | 0.070 | Serpentine | 4200–4330 | OP | 2 | 2 | 1 | ||||||
| YS | 22/August | 0.056 | Chromite | 4360–4539 | OP | 5 | 1 | 6 | 1 | |||||
| SC | 23/August | 1.299 | Asbestos | 4362–4630 | OP | 3 | 2 | 5 | 6 | |||||
| QF | 23/August | 0.313 | Chromite | 4280–4560 | OP | 2 | 1 | 3 | 2 | |||||
| DY | 24/August | 0.034 | Serpentine | 4220–4290 | OP | 3 | 1 | 4 | 3 | |||||
| Middle-stream | 5 | BM | 25/August | 0.107 | Manganese | 3620–3650 | UG | 1 | 1 | 5 | ||||
| 6 | LB | 26/August | 0.523 | Lead–Zinc | 4020-4460 | OP/UG | 2 | 2 | 1 | |||||
| 7 | QL | 25/August | 0.520 | Iron ore | 3520–3959 | UG | 3 | 3 | 7 | |||||
| 8 | JL | 15/August | 0.121 | Polymetallic | 3920–4100 | UG | 3 | 1 | 1 | 5 | 4 | |||
| 9 | XC | 26/August | Ore dressing | 1 | 1 | 3 | ||||||||
| Down-stream | 10 | ST | 14/August | 0.160 | Manganese | 3018–3130 | UG | 4 | 1 | 5 | 3 | |||
| 11 | WY | 8/August | 0.189 | Polymetallic | 2790–3150 | UG | 6 | 1 | 1 | 1 | 9 | 5 | ||
| XL | 9/August | 0.117 | Polymetallic | 3084–3460 | UG | 2 | 1 | 1 | 3 | 2 | ||||
| XS | 10/August | 0.540 | Polymetallic | 3150–3680 | OP/UG | 6 | 1 | 3 | 10 | 10 | ||||
| GM | 11/August | 0.134 | Polymetallic | 3000–3530 | UG | 1 | 3 | 4 | 8 | 4 | ||||
| XG | 12/August | 0.195 | Polymetallic | 2730–3159 | UG | 4 | 4 | 8 | ||||||
| 12 | GD | 13/August | 0.550 | Polymetallic | 3760–4030 | UG | 2 | 1 | 3 | 6 | 5 | |||
| HH | 27, 28/August | 16 | 16 | 18 | ||||||||||
| Total | 14 | 104 | 10 | 74 | 7 | 6 | 22 | 119 | 104 | |||||
| Water Standard | As | Mn | Cr | Ni | Cu | Cd | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| Grade I a | 10 | – | 10 | – | 10 | 1 | 10 | 50 |
| Drinking b | 10 | 100 | 10 | 20 | 1000 | 5 | 10 | 1000 |
| Tributary ID | Mines a | Samples b | As | Mn | Cr | Ni | Cu | Cd | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | FX | 2 | 0.00 | 40.02 | 2.18 | 3.03 | 2.56 | 0.10 | 2.92 | 25.70 |
| 2 | ZY, JL | 7 | 1.51 | 11.68 | 1.83 | 2.15 | 1.20 | 0.19 | 1.63 | 38.31 |
| 3 | RS | 9 | 4.12 | 58.06 | 12.86 | 26.26 | 4.26 | 0.38 | 13.61 | 28.23 |
| 4 | HC, SC, YS, QF, XY, DY | 20 | 6.90 | 64.71 | 42.92 | 163.86 | 11.79 | 0.48 | 1.32 | 26.13 |
| 5 | BM | 1 | 0.00 | 2.65 | 1.02 | 1.69 | 3.56 | 0.50 | 28.15 | 2.74 |
| 6 | LB | 2 | 6.10 | 167.65 | 1.07 | 7.82 | 7.07 | 0.50 | 1.01 | 29.95 |
| 7 | QL | 3 | 2.13 | 66.61 | 7.19 | 8.54 | 3.72 | 0.83 | 1.57 | 23.28 |
| 8 | JL | 3 | 0.00 | 24.64 | 1.22 | 3.85 | 4.95 | 1.07 | 5.10 | 55.20 |
| 10 | ST | 4 | 3.38 | 16.94 | 0.95 | 4.45 | 7.76 | 0.75 | 2.89 | 33.17 |
| 11 | WY, XL, XS, GM, XG | 21 | 1.09 | 46.73 | 1.82 | 3.13 | 19.05 | 2.49 | 12.82 | 489.98 |
| 12 | GD | 3 | 2.53 | 36.72 | 2.51 | 14.67 | 4.42 | 0.43 | 1.69 | 25.40 |
| HH | 16 | 4.39 | 24.62 | 3.27 | 5.00 | 1.92 | 0.11 | 0.91 | 22.26 |
| Metal | This Study | Background a | |||
|---|---|---|---|---|---|
| Min | Max | Median | Mean | Mean | |
| As | 1.12 | 728.03 | 19.73 | 42.57 | 21.60 |
| Mn | 293.21 | 2106.08 | 722.64 | 770.65 | 818.84 |
| Cr | 2.43 | 1148.47 | 45.46 | 88.39 | 57.29 |
| Ni | 8.96 | 1615.34 | 43.31 | 110.45 | 70.22 |
| Cu | 6.66 | 2497.69 | 43.98 | 103.28 | 56.38 |
| Cd | 0.2 | 2349.91 | 2.97 | 27.39 | 2.93 |
| Pb | 0 | 26,538.82 | 23.22 | 470.22 | 37.35 |
| Zn | 31.87 | 210,880.62 | 128.31 | 2460.93 | 178.68 |
| Variables | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| Cd | 0.974 | 0.033 | 0.064 | 0.131 | 0.021 |
| Cr | 0.071 | 0.978 | 0.034 | −0.072 | −0.005 |
| Cu | 0.607 | −0.063 | 0.693 | 0.097 | −0.009 |
| Mn | −0.060 | 0.023 | −0.117 | −0.064 | 0.901 |
| Ni | −0.042 | 0.984 | 0.048 | −0.014 | −0.018 |
| Pb | 0.887 | −0.013 | 0.425 | 0.112 | −0.023 |
| Zn | 0.975 | 0.030 | 0.085 | 0.129 | 0.016 |
| As | 0.160 | 0.123 | 0.881 | 0.012 | −0.063 |
| pH | −0.035 | 0.034 | −0.279 | −0.879 | −0.096 |
| TOC (g/kg) | 0.276 | −0.042 | −0.299 | 0.735 | −0.151 |
| EC (μs/cm) | 0.264 | −0.128 | 0.279 | 0.326 | 0.400 |
| Eigenvalues | 3.239 | 1.966 | 1.710 | 1.486 | 1.010 |
| % of Variance | 29.447 | 17.877 | 15.544 | 13.509 | 9.181 |
| Cumulative % | 29.447 | 47.323 | 62.867 | 76.376 | 85.558 |
| Variables | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| As | 0.99 | 0.02 | 0.01 | −0.01 | 0.07 | 0.11 |
| Cd | 0.40 | 0.05 | 0.01 | 0.01 | 0.89 | 0.02 |
| Li | 0.21 | 0.82 | 0.11 | 0.16 | 0.11 | 0.07 |
| Be | 0.97 | 0.06 | 0.01 | 0.07 | 0.13 | 0.13 |
| Cr | 0.22 | -0.03 | −0.02 | −0.02 | 0.03 | 0.94 |
| Mn | 0.40 | 0.08 | 0.01 | 0.87 | 0.22 | 0.05 |
| Co | 0.96 | 0.08 | 0.01 | 0.06 | 0.15 | 0.21 |
| Ni | 0.26 | 0.01 | −0.07 | 0.01 | 0.10 | 0.91 |
| Cu | 0.31 | 0.08 | 0.01 | 0.08 | 0.89 | 0.04 |
| Zn | −0.04 | 0.12 | 0.00 | 0.04 | 0.96 | −0.03 |
| Rb | −0.07 | 0.23 | 0.76 | 0.46 | 0.06 | 0.03 |
| Mo | 0.17 | 0.56 | −0.08 | −0.14 | 0.09 | −0.07 |
| Sn | 0.99 | 0.03 | 0.02 | −0.01 | 0.08 | 0.11 |
| Cs | 0.00 | 0.07 | 0.14 | 0.94 | −0.04 | 0.07 |
| Ba | −0.11 | 0.09 | −0.21 | −0.07 | 0.14 | −0.37 |
| Pb | 0.07 | 0.20 | −0.07 | −0.09 | 0.16 | 0.02 |
| Bi | 0.99 | 0.02 | 0.01 | −0.01 | 0.07 | 0.11 |
| Th | 0.99 | 0.02 | 0.01 | 0.02 | 0.07 | 0.11 |
| U | 0.91 | 0.27 | −0.01 | 0.04 | 0.04 | 0.06 |
| EC | 0.67 | 0.26 | 0.62 | 0.22 | 0.17 | 0.04 |
| pH | −0.70 | -0.42 | 0.13 | −0.16 | −0.33 | −0.03 |
| CO32− | −0.05 | −0.16 | 0.75 | −0.15 | −0.12 | −0.06 |
| HCO3− | −0.12 | 0.58 | −0.10 | −0.13 | −0.24 | −0.32 |
| F− | 0.61 | 0.50 | −0.01 | 0.27 | 0.26 | 0.01 |
| Cl− | −0.02 | −0.01 | 0.96 | 0.03 | −0.01 | 0.07 |
| NO3− | 0.00 | 0.05 | 0.02 | 0.95 | −0.03 | −0.02 |
| SO42− | 0.96 | 0.13 | 0.04 | 0.10 | 0.16 | 0.08 |
| Ca | 0.38 | 0.40 | 0.09 | 0.33 | 0.30 | −0.16 |
| Fe | 0.99 | 0.03 | 0.01 | −0.01 | 0.07 | 0.11 |
| K | 0.46 | 0.23 | 0.17 | 0.17 | 0.08 | −0.06 |
| Mg | 0.11 | 0.13 | 0.94 | 0.09 | 0.06 | 0.06 |
| Na | 0.06 | 0.81 | 0.24 | 0.36 | −0.04 | 0.03 |
| Si | 0.98 | 0.07 | −0.01 | 0.02 | 0.08 | 0.15 |
| Sr2+ | 0.07 | 0.86 | 0.09 | 0.15 | 0.10 | 0.00 |
| Eigenvalues | 11.77 | 3.70 | 3.55 | 3.32 | 3.14 | 2.18 |
| % of Variance | 34.60 | 10.88 | 10.43 | 9.77 | 9.24 | 6.41 |
| Cumulative % | 34.60 | 45.48 | 55.91 | 65.68 | 74.92 | 81.33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Ma, R.; Sun, Z.; Zhou, A.; Bu, J.; Long, X.; Liu, Y. Effects of Mining Activities on the Release of Heavy Metals (HMs) in a Typical Mountain Headwater Region, the Qinghai-Tibet Plateau in China. Int. J. Environ. Res. Public Health 2018, 15, 1987. https://doi.org/10.3390/ijerph15091987
Wei W, Ma R, Sun Z, Zhou A, Bu J, Long X, Liu Y. Effects of Mining Activities on the Release of Heavy Metals (HMs) in a Typical Mountain Headwater Region, the Qinghai-Tibet Plateau in China. International Journal of Environmental Research and Public Health. 2018; 15(9):1987. https://doi.org/10.3390/ijerph15091987
Chicago/Turabian StyleWei, Wenhao, Rui Ma, Ziyong Sun, Aiguo Zhou, Jianwei Bu, Xiang Long, and Yunde Liu. 2018. "Effects of Mining Activities on the Release of Heavy Metals (HMs) in a Typical Mountain Headwater Region, the Qinghai-Tibet Plateau in China" International Journal of Environmental Research and Public Health 15, no. 9: 1987. https://doi.org/10.3390/ijerph15091987






