Tuberculosis Specific Interferon-Gamma Production in a Current Refugee Cohort in Western Europe
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.1.1. Study Population and Sample Collection
2.1.2. Data Collection
2.2. Analysis
2.3. Study Approval
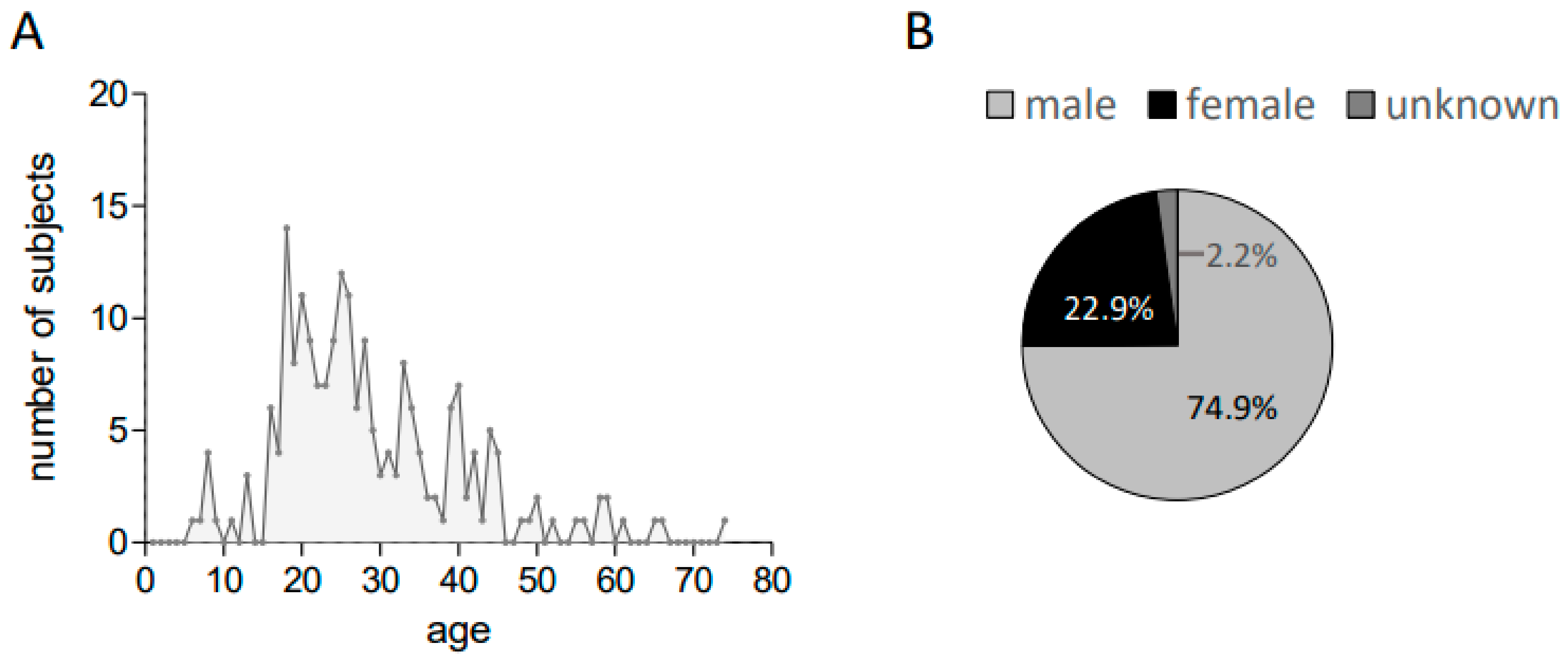
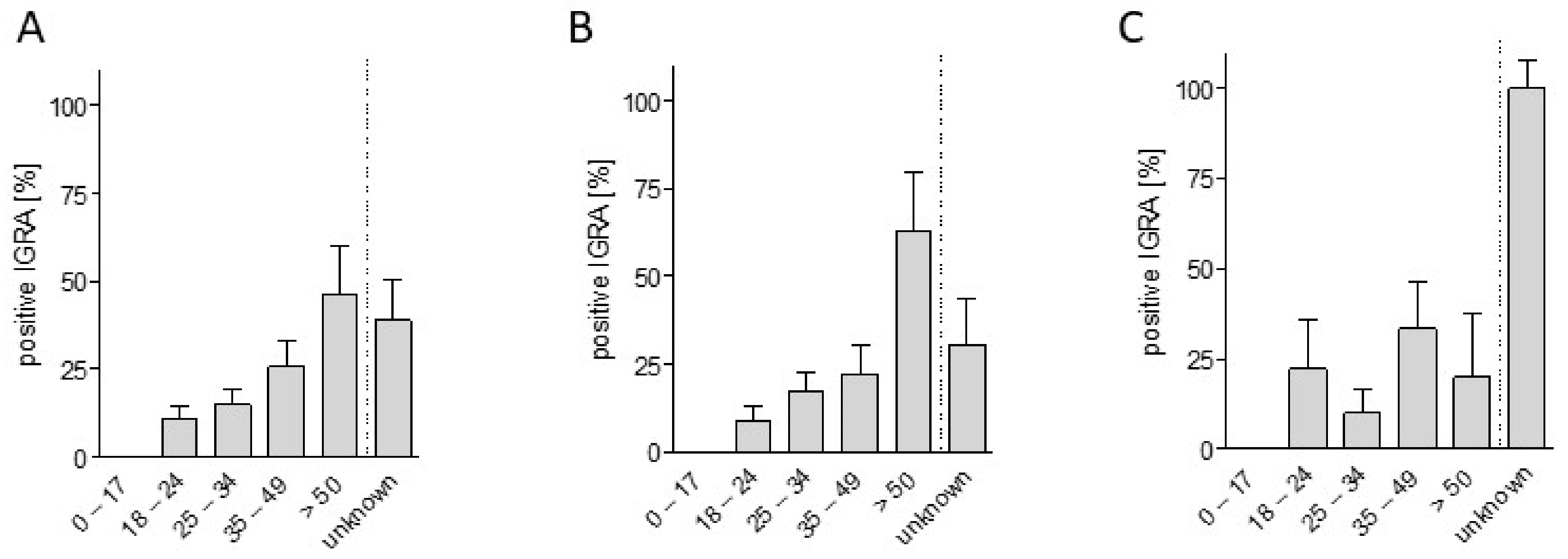
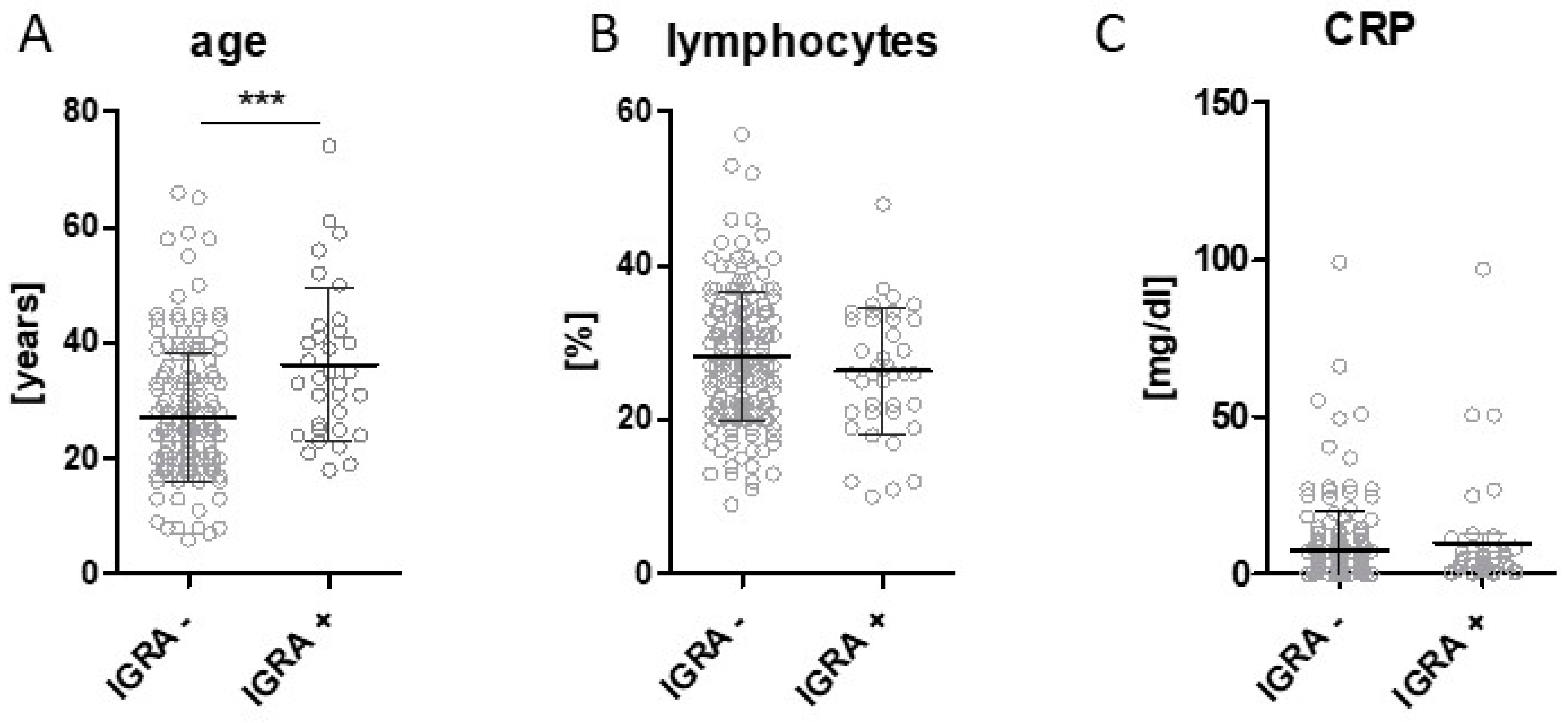
3. Results
4. Discussion
5. Conclusions
Author Contributions
Grants and Funding
Acknowledgments
Conflicts of Interest
References
- Kotsiou, O.S.; Srivastava, D.S.; Kotsios, P.; Exadaktylos, A.K.; Gourgoulianis, K.I. The emergency medical system in Greece: Opening Aeolus’ bag of winds. Int. J. Environ. Res. Public Health 2018, 15, 745. [Google Scholar] [CrossRef] [PubMed]
- Bozorgmehr, K.; Wahedi, K. Reframing solidarity in Europe: Frontex, frontiers, and the fallacy of refugee quota. Lancet Public Health 2017, 2, e10–e11. [Google Scholar] [CrossRef]
- Lam, E.; McCarthy, A.; Brennan, M. Vaccine-preventable diseases in humanitarian emergencies among refugee and internally-displaced populations. Hum. Vaccines Immunother. 2015, 11, 2627–2636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouadio, I.K.; Koffi, A.K.; Attoh-Toure, H.; Kamigaki, T.; Oshitani, H. Outbreak of measles and rubella in refugee transit camps. Epidemiol. Infect. 2009, 137, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, L.; Centis, R.; Dara, M.; Solovic, I.; Sulis, G.; Zumla, A.; Migliori, G.B. European policies in the management of tuberculosis among migrants. Int. J. Infect. Dis. 2017, 56, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, A.; Hauer, B.; Brodhun, B.; Haas, W.; Fiebig, L. Find and treat or find and lose? Tuberculosis treatment outcomes among screened newly arrived asylum seekers in Germany 2002 to 2014. Euro Surveill. 2018, 23, 17-00042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronald, L.A.; Campbell, J.R.; Balshaw, R.F.; Romanowski, K.; Roth, D.Z.; Marra, F.; Cook, V.J.; Johnston, J.C. Demographic predictors of active tuberculosis in people migrating to British Columbia, Canada: A retrospective cohort study. CMAJ 2018, 190, E209–E216. [Google Scholar] [CrossRef] [PubMed]
- Buber-Ennser, I.; Kohlenberger, J.; Rengs, B.; Al Zalak, Z.; Goujon, A.; Striessnig, E.; Potancokova, M.; Gisser, R.; Testa, M.R.; Lutz, W. Human capital, values, and attitudes of persons seeking refuge in Austria in 2015. PLoS ONE 2016, 11, e0163481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonka, A.; Happle, C.; Grote, U.; Schleenvoigt, B.T.; Hampel, A.; Dopfer, C.; Hansen, G.; Schmidt, R.E.; Behrens, G.M. Measles, mumps, rubella, and varicella seroprevalence in refugees in Germany in 2015. Infection 2016, 44, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Jablonka, A.; Behrens, G.M.; Stange, M.; Dopfer, C.; Grote, U.; Hansen, G.; Schmidt, R.E.; Happle, C. Tetanus and diphtheria immunity in refugees in Europe in 2015. Infection 2017, 45, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Mulder, C.; van Deutekom, H.; Huisman, E.M.; Toumanian, S.; Koster, B.F.; Meijer-Veldman, W.; van Loenhout-Rooyackers, J.H.; Appel, M.; Arend, S.M.; Borgdorff, M.W.; et al. Role of the QuantiFERON®-TB gold in-tube assay in screening new immigrants for tuberculosis infection. Eur. Respir. J. 2012, 40, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Castelli, F.; Tomasoni, L.R.; El Hamad, I. Migration and chronic noncommunicable diseases: Is the paradigm shifting? J. Cardiovasc. Med. 2014, 15, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Castelli, F.; Sulis, G. Migration and infectious diseases. Clin. Microbiol. Infect. 2017, 23, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Henjum, S.; Barikmo, I.; Strand, T.A.; Oshaug, A.; Torheim, L.E. Iodine-induced goitre and high prevalence of anaemia among Saharawi refugee women. Public Health Nutr. 2012, 15, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Blanck, H.M.; Bowman, B.A.; Serdula, M.K.; Khan, L.K.; Kohn, W.; Woodruff, B.A. Angular stomatitis and riboflavin status among adolescent Bhutanese refugees living in southeastern Nepal. Am. J. Clin. Nutr. 2002, 76, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Jakab, Z. Population Movement Is a Challenge for Refugees and Migrants as well as for the Receiving Population. 2015. Available online: http://www.euro.who.int/en/health-topics/health-determinants/migration-and-health/news/news/2015/09/population-movement-is-a-challenge-for-refugees-and-migrants-as-well-as-for-the-receiving-population (accessed on 21 April 2018).
- Houben, R.M.; Dodd, P.J. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Global Tuberculosis Control 2015; Document WHO/HTM/BT/2015.22; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Getahun, H.; Matteelli, A.; Abubakar, I.; Aziz, M.A.; Baddeley, A.; Barreira, D.; Den Boon, S.; Borroto Gutierrez, S.M.; Bruchfeld, J.; Burhan, E.; et al. Management of latent mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur. Respir. J. 2015, 46, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Lonnroth, K.; Migliori, G.B.; Abubakar, I.; D’Ambrosio, L.; de Vries, G.; Diel, R.; Douglas, P.; Falzon, D.; Gaudreau, M.A.; Goletti, D.; et al. Towards tuberculosis elimination: An action framework for low-incidence countries. Eur. Respir. J. 2015, 45, 928–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korthals Altes, H.; Kloet, S.; Cobelens, F.; Bootsma, M. Latent tuberculosis infection in foreign-born communities: Import versus Transmission in The Netherlands derived through mathematical modelling. PLoS ONE 2018, 13, e0192282. [Google Scholar]
- Alami, N.N.; Yuen, C.M.; Miramontes, R.; Pratt, R.; Price, S.F.; Navin, T.R. Trends in tuberculosis—United States, 2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 229–233. [Google Scholar] [PubMed]
- Van Leth, F.; Kalisvaart, N.A.; Erkens, C.G.; Borgdoff, M.W. Projection of the number of patients with tuberculosis in The Netherlands in 2030. Eur. J. Public Health 2009, 19, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.M.; Painter, J.; Posey, D.L.; Zhou, W.; Shetty, S. Latent tuberculosis infection among immigrant and refugee children arriving in the United States: 2010. J. Immigr. Minor. Health 2016, 18, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Dara, M.; Solovic, I.; Sotgiu, G.; D’Ambrosio, L.; Centis, R.; Tran, R.; Goletti, D.; Duarte, R.; Aliberti, S.; de Benedictis, F.M.; et al. Tuberculosis care among refugees arriving in Europe: A ERS/WHO Europe region survey of current practices. Eur. Respir. J. 2016, 48, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, J.; Manabe, T.; Morino, E.; Muto, Y.; Hashimoto, M.; Iikura, M.; Izumi, S.; Sugiyama, H.; Kudo, K. Sensitivity and specificity of QuantiFERON-TB Gold Plus compared with QuantiFERON-TB gold In-Tube and T-SPOT.TB on active tuberculosis in Japan. J. Infect. Chemother. 2018, 24, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.; Park, K.U.; Song, E.Y.; Kim, S.J.; Lee, Y.J.; Park, J.S.; Cho, Y.J.; Yoon, H.I.; Yim, J.J.; Lee, C.T.; et al. Comparison of the sensitivity of Quantiferon-TB gold In-Tube and T-SPOT.TB according to patient age. PLoS ONE 2016, 11, e0156917. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, M.W.; Sebek, M.; Geskus, R.B.; Kremer, K.; Kalisvaart, N.; van Soolingen, D. The incubation period distribution of tuberculosis estimated with a molecular epidemiological approach. Int. J. Epidemiol. 2011, 40, 964–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlopoulou, I.D.; Tanaka, M.; Dikalioti, S.; Samoli, E.; Nisianakis, P.; Boleti, O.D.; Tsoumakas, K. Clinical and laboratory evaluation of new immigrant and refugee children arriving in Greece. BMC Pediatr. 2017, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Bennet, R.; Eriksson, M. Tuberculosis infection and disease in the 2015 cohort of unaccompanied minors seeking asylum in Northern Stockholm, Sweden. Infect. Dis. 2017, 49, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Piergallini, T.J.; Turner, J. Tuberculosis in the elderly: Why inflammation matters. Exp. Gerontol. 2018, 105, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Mirsaeidi, M.; Sadikot, R.T. Patients at high risk of tuberculosis recurrence. Int. J. Mycobacteriol. 2018, 7, 1–6. [Google Scholar] [PubMed]
- Cohn, D.L.; O’Brien, R.J.; Geiter, L.J.; Rockville, M.D.; Gordin, F.M.; Hershfield, E.; Horsburgh, C.R., Jr.; Jereb, J.A.; Jordan, T.J.; Kaplan, J.E.; et al. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 2000, 161, S221–S247, (Joint statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC) 1999). [Google Scholar]
- Bennett, R.J.; Brodine, S.; Waalen, J.; Moser, K.; Rodwell, T.C. Prevalence and treatment of latent tuberculosis infection among newly arrived refugees in San Diego County, January 2010–October 2012. Am. J. Public Health 2014, 104, e95–e102. [Google Scholar] [CrossRef] [PubMed]
- Cain, K.P.; Haley, C.A.; Armstrong, L.R.; Garman, K.N.; Wells, C.D.; Iademarco, M.F.; Castro, K.G.; Laserson, K.F. Tuberculosis among foreign-born persons in the United States: Achieving tuberculosis elimination. Am. J. Respir. Crit. Care Med. 2007, 175, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Rennert-May, E.; Hansen, E.; Zadeh, T.; Krinke, V.; Houston, S.; Cooper, R. A step toward tuberculosis elimination in a low-incidence country: Successful diagnosis and treatment of latent tuberculosis infection in a refugee clinic. Can. Respir. J. 2016, 2016, 7980869. [Google Scholar] [CrossRef] [PubMed]
| Age | Total n | % Positive IGRA |
|---|---|---|
| 0–17 years | 21 | 0 |
| 18–24 years | 65 | 10.8 |
| 25–34 years | 67 | 14.9 |
| 35–49 years | 39 | 25.6 |
| ≥50 years | 13 | 46.2 |
| unknown | 18 | 38.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jablonka, A.; Dopfer, C.; Happle, C.; Sogkas, G.; Ernst, D.; Atschekzei, F.; Hirsch, S.; Schäll, A.; Jirmo, A.; Solbach, P.; et al. Tuberculosis Specific Interferon-Gamma Production in a Current Refugee Cohort in Western Europe. Int. J. Environ. Res. Public Health 2018, 15, 1263. https://doi.org/10.3390/ijerph15061263
Jablonka A, Dopfer C, Happle C, Sogkas G, Ernst D, Atschekzei F, Hirsch S, Schäll A, Jirmo A, Solbach P, et al. Tuberculosis Specific Interferon-Gamma Production in a Current Refugee Cohort in Western Europe. International Journal of Environmental Research and Public Health. 2018; 15(6):1263. https://doi.org/10.3390/ijerph15061263
Chicago/Turabian StyleJablonka, Alexandra, Christian Dopfer, Christine Happle, Georgios Sogkas, Diana Ernst, Faranaz Atschekzei, Stefanie Hirsch, Annabelle Schäll, Adan Jirmo, Philipp Solbach, and et al. 2018. "Tuberculosis Specific Interferon-Gamma Production in a Current Refugee Cohort in Western Europe" International Journal of Environmental Research and Public Health 15, no. 6: 1263. https://doi.org/10.3390/ijerph15061263





