Concerns about Quadrupole ICP-MS Lead Isotopic Data and Interpretations in the Environment and Health Fields
Abstract
:1. Introduction
2. Methods
How Can One Check the Data and Evaluation of Sources?
3. Results
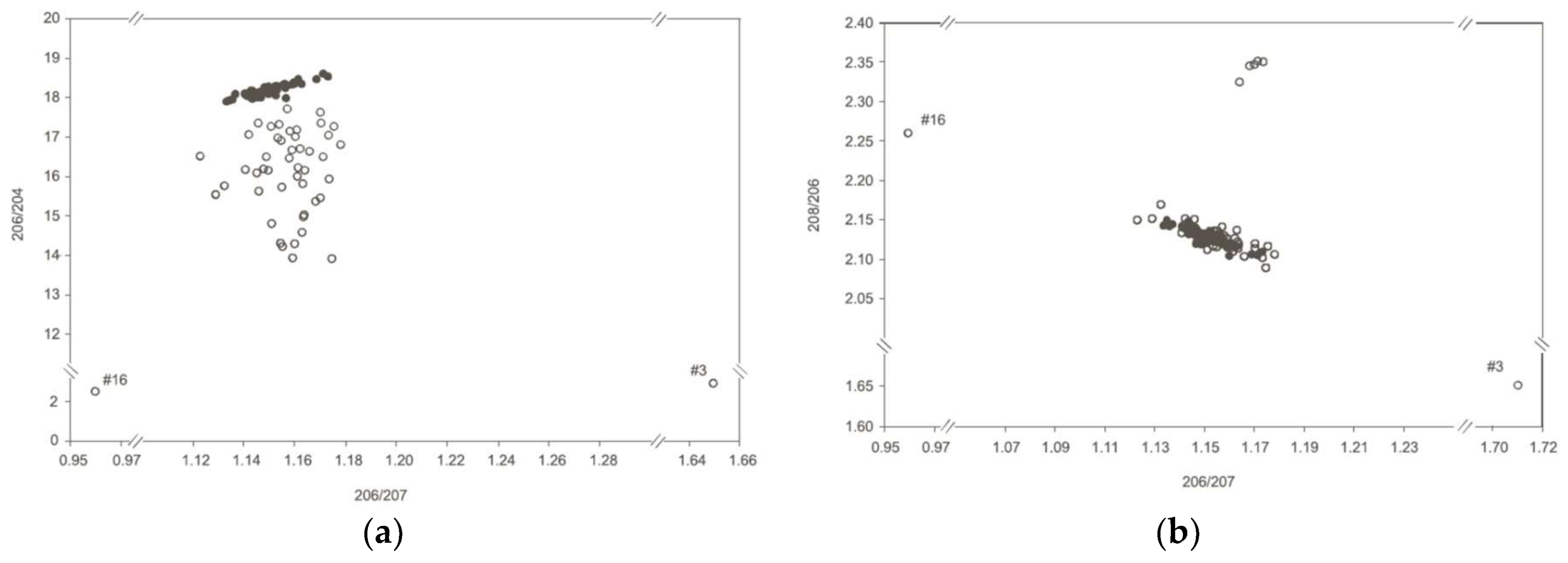
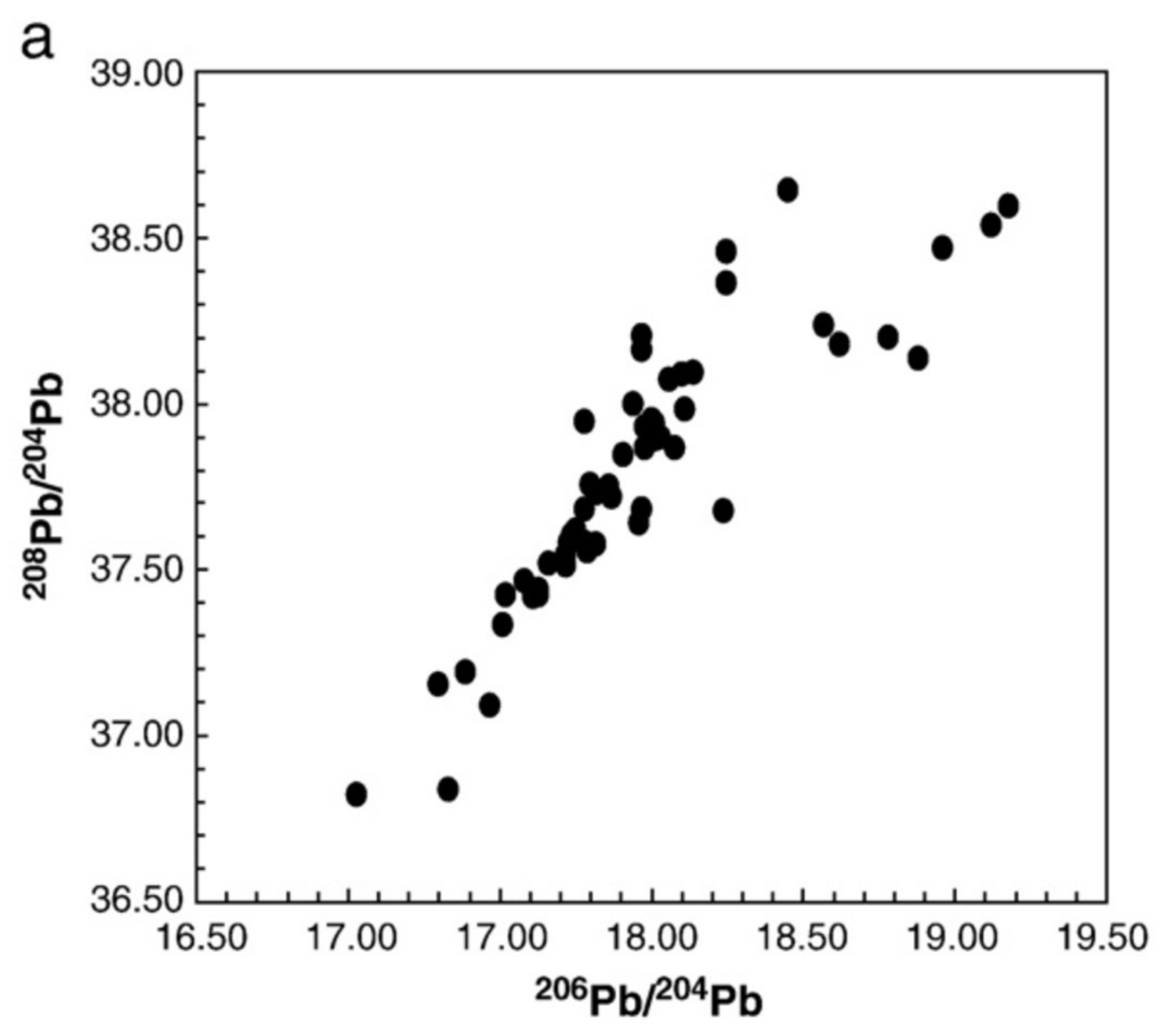
3.1. Examples Using Organic Extractions and Different ICP Instruments
3.2. Other Complications
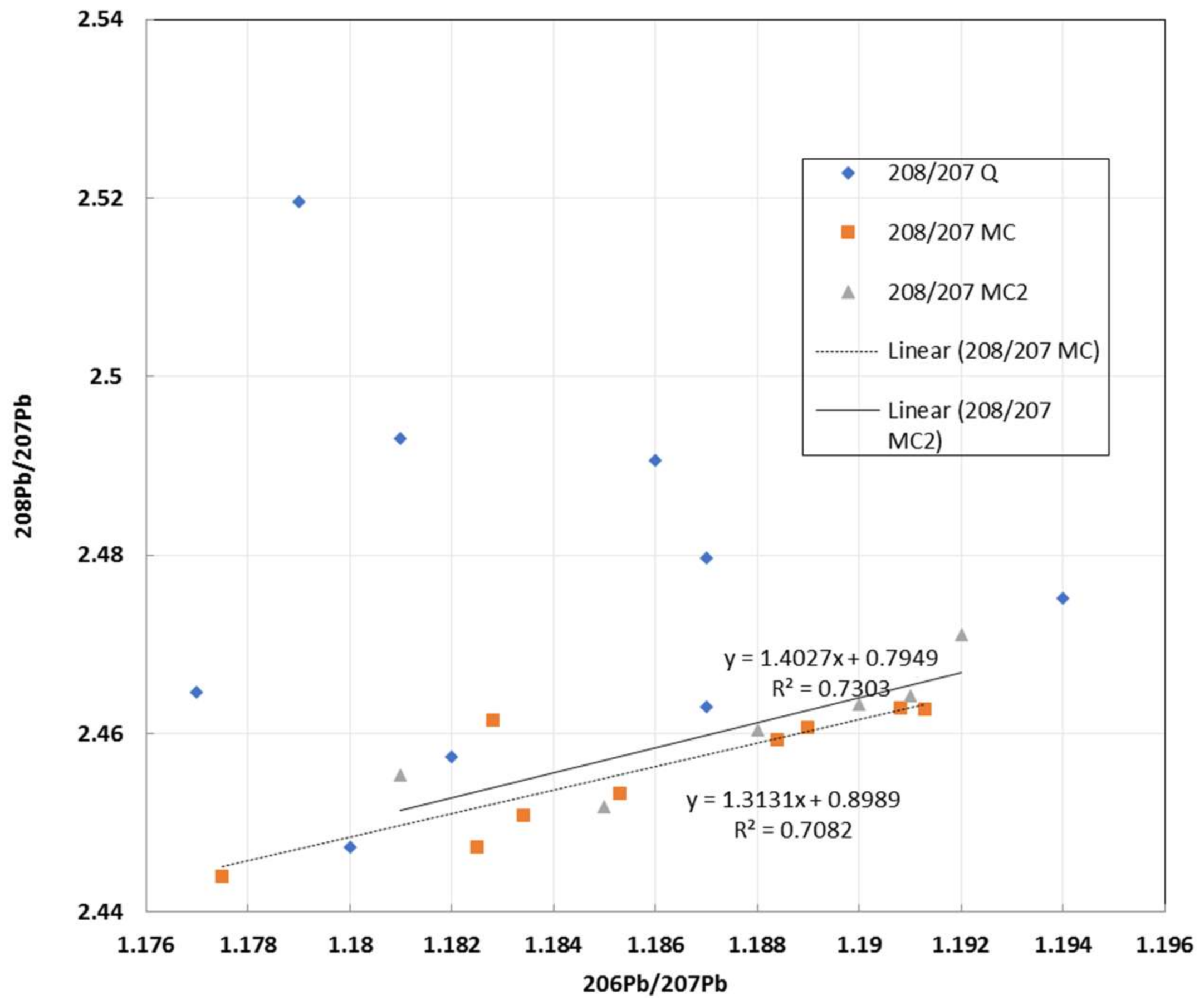
3.3. Comparison of ICP-MS and TIMS/MC-ICP-MS
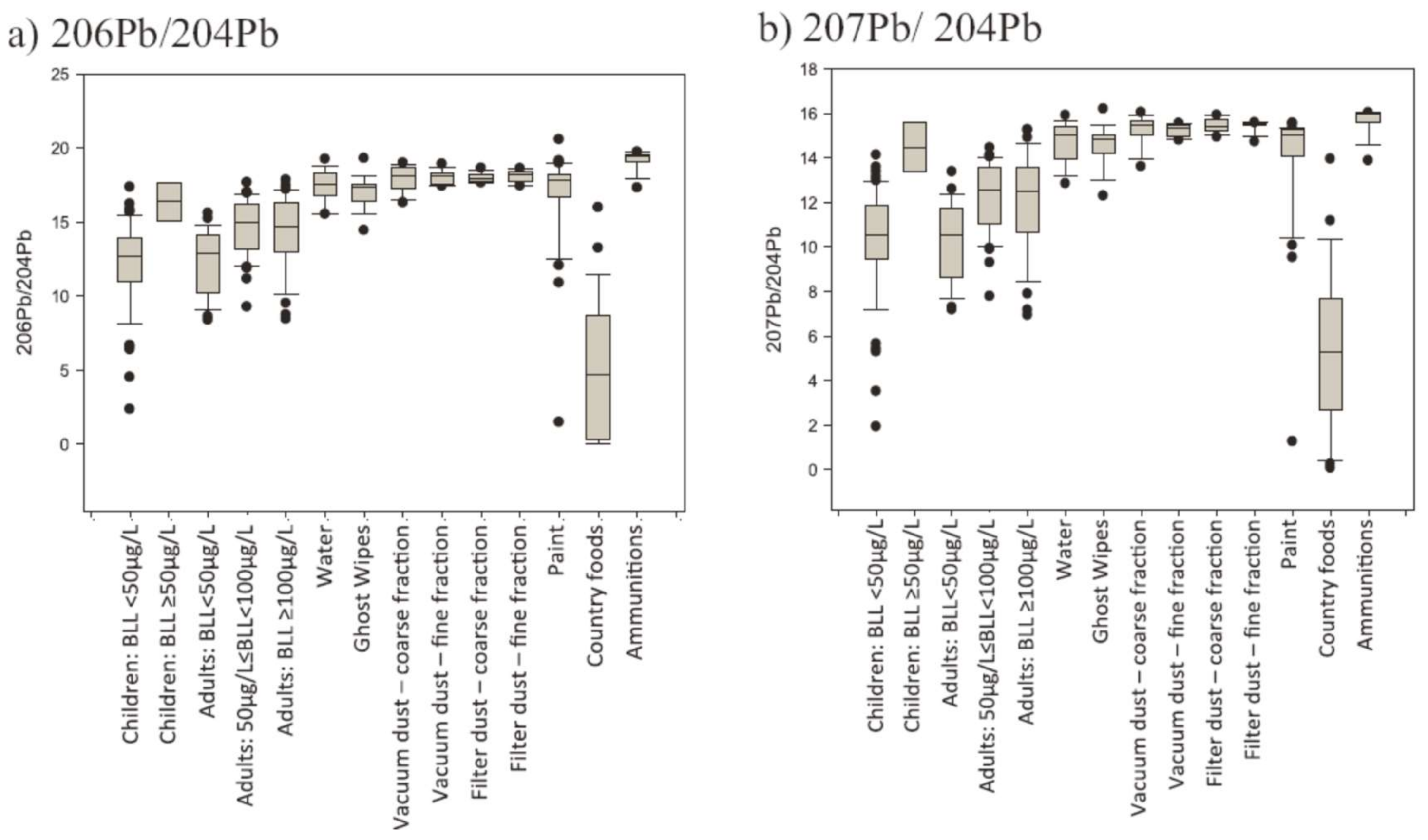
3.4. Examples Where the Major Isotopes Fail
3.4.1. Esperance Western Australia
3.4.2. Ryde Bridge
3.4.3. Atmospheric Aerosols
3.5. Isotopic Fractionation in Biological Samples—Fact or Fiction?
- (1)
- Digestion was with HNO3 and HCLO4 in quartz vessels but such equipment can contain small amounts of Pb and no processing blank data were provided.
- (2)
- It is also unrealistic to quote Pb isotopic data to 5 decimal places, especially from Q-ICP-MS analyses, although the incredible agreement for blood and urine data from both studies shown in Figure 11 would permit such claims. It is also noteworthy that in the Wu et al. [40] paper the isotopic data are given as ratios but in the Liu et al. [41] the data are presented as % abundances of each isotope, although they do claim, to the contrary, that the % abundances are a better indicator of fractionation.
- (3)
- There was no indication if the blood, urine and feces in Liu et al. [41] were the same data from the Wu et al. [40] paper although, as shown in Figure 10, the almost identical data for blood and urine from both studies and off-set of the urine data would perhaps indicate this. Such reproducibility for Q-ICP-MS is highly improbable.
- (4)
- There are no Pb concentrations provided for the urine or any other organs.
- (5)
- They state that Smith et al. [45] reported that there were large differences in the lead isotopic composition between paired blood and bone samples from each human subject. But this ignores the fact that the 5 subjects were from different countries, the long-term storage of Pb in bones and that 40–70% of Pb in blood under equilibrium conditions is derived from the skeleton [45,46,47,48,49,50].
- (6)
- The authors appear to have ignored isotope systematics. Instead of fractionation, the linearity of the data for each dosing and control samples are examples of two-component mixing. Any tissue receiving inputs from blood and the “test” or diet will have isotopic data, expressed as ratios, that fall along a mixing line defined by the end-members and the linear distance along that mixing line will represent the relative contributions of each end-member to that tissue. Wu et al. [40] described such mixing but concluded that the isotopic differences in tissues were due to isotopic fractionation. Also, if we take the diet 208Pb/206Pb of about 2.10 and the blood and 208Pb/206Pb (highest close to 2.15) then 2.10/2.15 will be 2.4% fractionation. Such extraordinary fractionation is not even observed in light stable isotopes where fractionation is commonly found. Any variations in the isotopic makeup of the dietary inputs—which were not—as they slowly moved through the body were the drivers of the observed data, rather than fractionation.
- (7)
- The rats were 6 weeks old so the control data should lie on same mixing line as with dietary data but, except for urine, all dosed data are offset from the Pb acetate “test” as in Figure 11.
- (8)
- The bone data are inconsistent with the dosing regime (Supplementary Figure S1). For example, the medium bone data have lower ratios than the test data and the high dose bone data have ratios similar to the control and low dose samples.
- (9)
- Data for blood/kidneys/liver/lung (Supplementary Figure S2) lie on linear trends between the diet and “test” but there are lower ratios in the low dose and medium dose livers that are inconsistent with the dosing regime. The lung data for all samples are identical to within experimental error which would be consistent with the intratracheal dose administration. Likewise, the kidney data for the dosed animals are almost identical to the lung data. For the high dose regime, the data for blood and other organs are the same to within experimental error which is what would be expected from biokinetics. (The higher uptake of Pb from diet or “test” in the liver for all animals is unexpected).
- (10)
- The authors suggest that the 204Pb/206Pb ratio is a more sensitive biomarker in tracing Pb from inhalation but the errors for this ratio are much larger than for 207Pb/206Pb and 208Pb/206Pb ratios (Supplementary Figure S3).
3.6. Comparison with Other Rat Studies
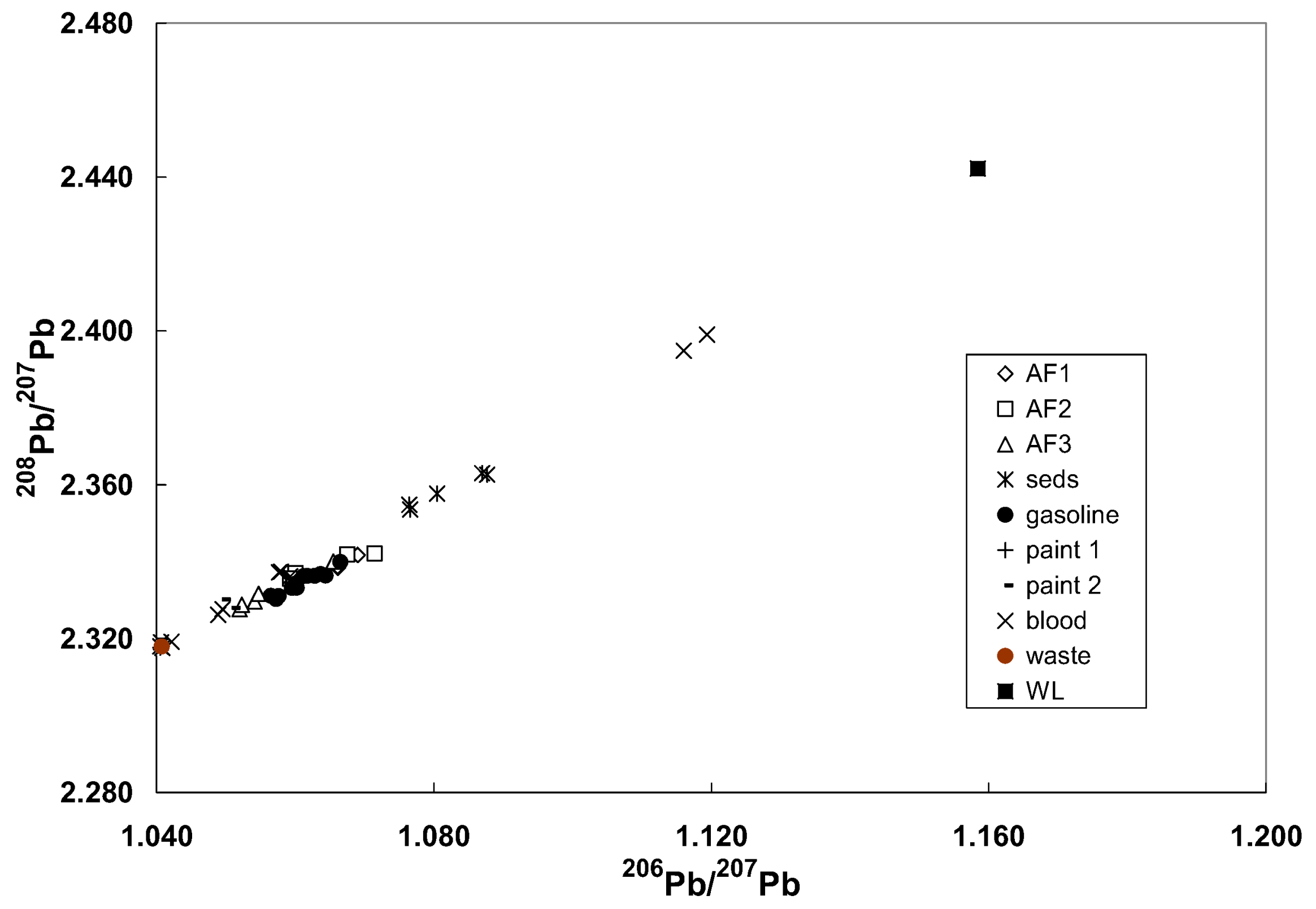
3.7. Example of Laboratory Problem
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barbaste, M.; Halicz, L.; Galy, A.; Medina, B.; Emteborg, H.; Adams, F.C.; Lobinski, R. Evolution of the accuracy of the determination of lead isotope ratios in wine by ICP MS using quadrupole, multicollector magnetic sector and time-of-flight analyzers. Talanta 2001, 54, 307–317. [Google Scholar] [CrossRef]
- Walczyk, T. TIMS versus multicollector-ICP-MS: Co-existence or struggle for survival? Anal. Bioanal. Chem. 2004, 378, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Kamenov, G.D.; Mueller, P.; Perfit, M. Optimization of mixed Pb-Tl solutions for high precision isotopic analyses by MC-ICP-MS. J. Anal. At. Spectrom. 2004, 19, 1262–1267. [Google Scholar] [CrossRef]
- Gulson, B. Discussion: Tracing changes in atmospheric sources of lead contamination using lead isotopic compositions in Australian red wine. Chemosphere 2016, 154, 40–47. [Google Scholar]
- Gulson, B.; Mizon, K.; Korsch, M.; Taylor, A. Changes in the lead isotopic composition of blood, diet and air in Australia over a decade: Globalization and implications for future isotopic studies. Environ. Res. 2006, 100, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Duzgoren-Aydin, N.S.; Weiss, A.L. Use and abuse of Pb-isotope fingerprinting technique and GIS mapping data to assess lead in environmental studies. Environ. Geochem. Health 2008, 30, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Gwiazda, R.H.; Smith, D.R. Lead isotopes as a supplementary tool in the routine evaluation of household lead hazards. Environ. Health Perspect. 2000, 108, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Manton, W.I.; Angle, C.R.; Stanek, K.L.; Reese, Y.R.; Kuehnemann, T.J. Acquisition and retention of lead by young children. Environ. Res. 2000, 82, 60–80. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, M.B. Isotopic characterization of various brands of corroding grade refined lead metal. Bull. Environ. Contam. Toxicol. 2000, 69, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, M.B.; Hall, G.S. Isotopic characterization of six major brands of white basic lead carbonate paint pigments. Bull. Environ. Contam. Toxicol. 2002, 69, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Ellam, R.M. The graphical presentation of lead isotope data for environmental source apportionment. Sci. Total Environ. 2010, 408, 3490–3492. [Google Scholar] [CrossRef] [PubMed]
- Ridley, I. Plumbo-Isotopy: The Measurement of Lead Isotopes by Multi-Collector Inductively Coupled Mass Spectrometry. Open File Report 2005–1393. Available online: https://pubs.er.usgs.gov/publication/ofr20051393 (accessed on 12 July 2016).
- Komarek, M.; Ettler, V.; Chrastny, V.; Mihaljevic, M. Lead isotopes in environmental sciences: A review. Environ. Int. 2008, 34, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B. Stable lead isotopes in environmental health with emphasis on human investigations. Sci. Total Environ. 2008, 400, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V.; Komarkova, M.; Jehlicka, J.; Coufal, P.; Hradil, D.; Machovic, V.; Delorme, F. Leaching of lead metallurgical slag in citric solutions—Implications for disposal and weathering in soil environments. Chemosphere 2004, 57, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Vanhaecke, F.; Moens, L. Overcoming spectral overlap in isootpic analysis via single- and multi-collector ICP-mass spectrometry. Anal. Bioanal. Chem. 2004, 378, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Ettler, V.; Mihaljevid, M.; Sebek, O.; Molek, M.; Grygar, T.; Zeman, J. Geochemical and Pb isotopic evidence for sources and dispersal of metal contamination in stream sediments from the mining and smelting district of Pribram, Czech Republic. Environ. Pollut. 2006, 142, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Shotyk, W.; Rausch, N.; Outridge, P.M.; Krachler, M. Isotopic evolution of atmospheric Pb from metallurgical processing in flin flon, manitoba: Retrospective analysis using peat cores from bogs. Environ. Pollut. 2016, 218, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, M.B. Stable isotopes of lead for source identification. J. Toxicol. Clin. Toxicol. 1995, 33, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Cumming, G.L.; Richards, J.R. Ore lead isotope ratios in a continuously changing Earth. Earth Planet. Sci. Lett. 1975, 28, 155–171. [Google Scholar] [CrossRef]
- Stacey, J.S.; Kramers, J.D. Approximation of terrestrial lead isotope evolution by a two stage model. Earth Planet. Sci. Lett. 1975, 26, 207–221. [Google Scholar] [CrossRef]
- Patterson, C.C. Age of meteorites and the earth. Geochim. Cosmochim. Acta 1956, 10, 230–237. [Google Scholar] [CrossRef]
- Bacon, J.R.; Jones, K.C.; McGrath, S.P.; Johnston, A.E. Isotopic character of lead deposited from the atmosphere at a grassland site in the U.K. since 1860. Environ. Sci. Technol. 1996, 30, 2511–2518. [Google Scholar] [CrossRef]
- Gulson, B.L. Lead Isotopes in Mineral Exploration; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Kelepertzis, E.; Komarek, M.; Argyraki, A.; Sillerova, H. Metal(oid) distribution and Pb isotopic signatures in the urban environment of Athens, Greece. Environ. Pollut. 2016, 213, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Komarek, M.; Chrastny, V.; Ettler, V.; Tlustos, P. Evaluation of extraction/digestion techniques used to determine lead isotopic composition in forest soils. Anal. Bioanal. Chem. 2006, 385, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Woodhead, J.D.; Horstwood, M.S.A.; Cottle, J.M. Advances in isotope ratio determination by LA-ICP-MS. Elements 2016, 12, 317–322. [Google Scholar] [CrossRef]
- Fillion, M.; Blais, J.M.; Yumvihoze, E.; Nakajima, M.; Workman, P.; Osborne, G.; Chan, H.M. Identification of environmental sources of lead exposure in Nunavut (Canada) using stable isotope analyses. Environ. Int. 2014, 71, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Binkowski, L.J.; Meissner, W.; Trzeciak, M.; Izevbekhai, K.; Barker, J. Lead isotope ratio measurements as indicators for the source of lead poisoning in mute swans (Cygnus olor) wintering in puck bay (northern Poland). Chemosphere 2016, 164, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Krachler, M.; Le Roux, G.; Kober, B.; Shotyk, W. Optimising accuracy and precision of lead isotope measurement (206Pb, 207Pb, 208Pb) in acid digests of peat with ICP-MS using individual mass corrections. J. Anal. At. Spectrom. 2004, 19, 354–361. [Google Scholar] [CrossRef]
- Kylander, M.E.; Weiss, D.J.; Kober, B. Two high resolution terrestrial records of atmospheric Pb deposition from New Brunswick, Canada and Loch Laxford, Scotland. Sci. Total Environ. 2009, 407, 1644–1657. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Shotyk, W.; Boyle, E.A.; Kramers, J.D.; Appleby, P.G.; Cheburkin, A.K. Comparative study of the temporal evolution of atmospheric lead deposition in Scotland and eastern Canada using blanket peat bogs. Sci. Total Environ. 2002, 292, 7–18. [Google Scholar] [CrossRef]
- Weiss, D.; Shotyk, W.; Appleby, P.; Kramers, J.; Cheburkin, A. Atmospheric Pb deposition since the industrial revolution recorded by five Swiss peat profiles: Enrichment factors, fluxes, isootpic compositon and sources. Environ. Sci. Technol. 1999, 33, 1340–1352. [Google Scholar] [CrossRef]
- Gulson, B.; Korsch, M.; Matisons, M.; Douglas, C.; Gillam, L.; McLaughlin, V. Windblown lead carbonate as the main source of lead in blood of children from a seaside community: An example of local birds as “canaries in the mine”. Environ. Health Perspect. 2009, 117, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B.; Korsch, M.; Winchester, W.; Devenish, M.; Hobbs, T.; Main, C.; Smith, G.; Rosman, K.; Howearth, L.; Burn-Nunes, L.; et al. Successful application of lead isotopes in source apportionment, legal proceedings, remediation and monitoring. Environ. Res. 2012, 112, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B.; Chiaradia, M.; Davis, J.; O’Connor, G. Impact on the environment from steel bridge paint deterioration using lead isotopic tracing, paint compositions and soil deconstruction. Sci. Total Environ. 2016, 550, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Bollhöfer, A.; Rosman, K.J.R. Isotopic source signatures for atmospheric lead: The Northern, Hemisphere. Geochim. Cosmochim. Acta 2001, 65, 1727–1740. [Google Scholar] [CrossRef]
- Gulson, B.; Mizon, K. Lead isotopes as a tool for gossan assessment in base metal exploration. Geochem. Explor. 1979, 11, 299–320. [Google Scholar] [CrossRef]
- Cui, J.; Wu, X. An experimental investigation on lead isotopic fractionation during metallurgical processes. Archaeometry 2011, 53, 205–214. [Google Scholar] [CrossRef]
- Wu, J.; Liu, D.; Xie, Q.; Wang, J. Biological fractionation of lead isotopes in sprague-dawley rats lead poisoned via the respiratory tract. PLoS ONE 2012, 7, e52462. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, J.; Ouyang, L.; Wang, J. Variations in lead isotopic abundances in sprague-dawley rat tissues: Possible reason of formation. PLoS ONE 2014, 9, e89805. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, M.B.; Wetherill, G.W.; Kopple, J.D. Kinetic analysis of lead metabolism in healthy humans. J. Clin. Investig. 1976, 58, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Leggett, R.W. An age-specific kinetic model of lead metabolism in humans. Environ. Health Perspect. 1993, 101, 598–616. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, E.J. Physiologically based models for bone-seeking elements. Iv. Kinetics of lead disposition in humans. Toxicol. Appl. Pharmacol. 1993, 118, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Osterloh, J.D.; Flegal, A.R. Use of endogenous, stable lead isotopes to determine release of lead from the skeleton. Environ. Health Perspect. 1996, 104, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Manton, W.I. Sources of lead in blood. Identification by stable isotopes. Arch. Environ. Health 1977, 32, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Manton, W.I. Total contribution of airborne lead to blood lead. Br. J. Ind. Med. 1985, 42, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Gulson, B.L.; Mahaffey, K.R.; Mizon, K.J.; Korsch, M.J.; Cameron, M.A.; Vimpani, G. Contribution of tissue lead to blood lead in adult female subjects based on stable lead isotope methods. J. Lab. Clin. Med. 1995, 125, 703–712. [Google Scholar] [PubMed]
- Gulson, B.L.; Jameson, C.W.; Mahaffey, K.R.; Mizon, K.J.; Korsch, M.J.; Vimpani, G. Pregnancy increases mobilization of lead from maternal skeleton. J. Lab. Clin. Med. 1997, 130, 51–62. [Google Scholar] [CrossRef]
- Gulson, B.L.; Mahaffey, K.R.; Jameson, C.W.; Mizon, K.J.; Korsch, M.J.; Cameron, M.A.; Eisman, J.A. Mobilization of lead from the skeleton during the postnatal period is larger than during pregnancy. J. Lab. Clin. Med. 1998, 131, 324–329. [Google Scholar] [CrossRef]
- Smith, D.R.; Osterloh, J.D.; Niemeyer, S.; Flegal, A.R. Stable isotope labeling of lead compartments in rats with ultralow lead concentrations. Environ. Res. 1992, 57, 190–207. [Google Scholar] [CrossRef]
- Chiaradia, M.; Gulson, B.L.; James, M.; Jameson, C.W.; Johnson, D. Identification of secondary lead sources in the air of an urban environment. Atmos. Environ. 1997, 31, 3511–3521. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulson, B.; Kamenov, G.D.; Manton, W.; Rabinowitz, M. Concerns about Quadrupole ICP-MS Lead Isotopic Data and Interpretations in the Environment and Health Fields. Int. J. Environ. Res. Public Health 2018, 15, 723. https://doi.org/10.3390/ijerph15040723
Gulson B, Kamenov GD, Manton W, Rabinowitz M. Concerns about Quadrupole ICP-MS Lead Isotopic Data and Interpretations in the Environment and Health Fields. International Journal of Environmental Research and Public Health. 2018; 15(4):723. https://doi.org/10.3390/ijerph15040723
Chicago/Turabian StyleGulson, Brian, George D. Kamenov, William Manton, and Michael Rabinowitz. 2018. "Concerns about Quadrupole ICP-MS Lead Isotopic Data and Interpretations in the Environment and Health Fields" International Journal of Environmental Research and Public Health 15, no. 4: 723. https://doi.org/10.3390/ijerph15040723




