Comparison of Commensal Escherichia coli Isolates from Adults and Young Children in Lubuskie Province, Poland: Virulence Potential, Phylogeny and Antimicrobial Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and E. coli Identification
2.2. Virulence Genotyping
2.3. Phylogenetic Typing
2.4. Antimicrobial Susceptibility Testing
2.5. Identification of Resistance Genes
2.6. Statistical Methods
3. Results
3.1. Frequency of Virulence Associated Genes
3.2. Distribution of Virulence Genes among E. coli Isolates According to the Age of Adults
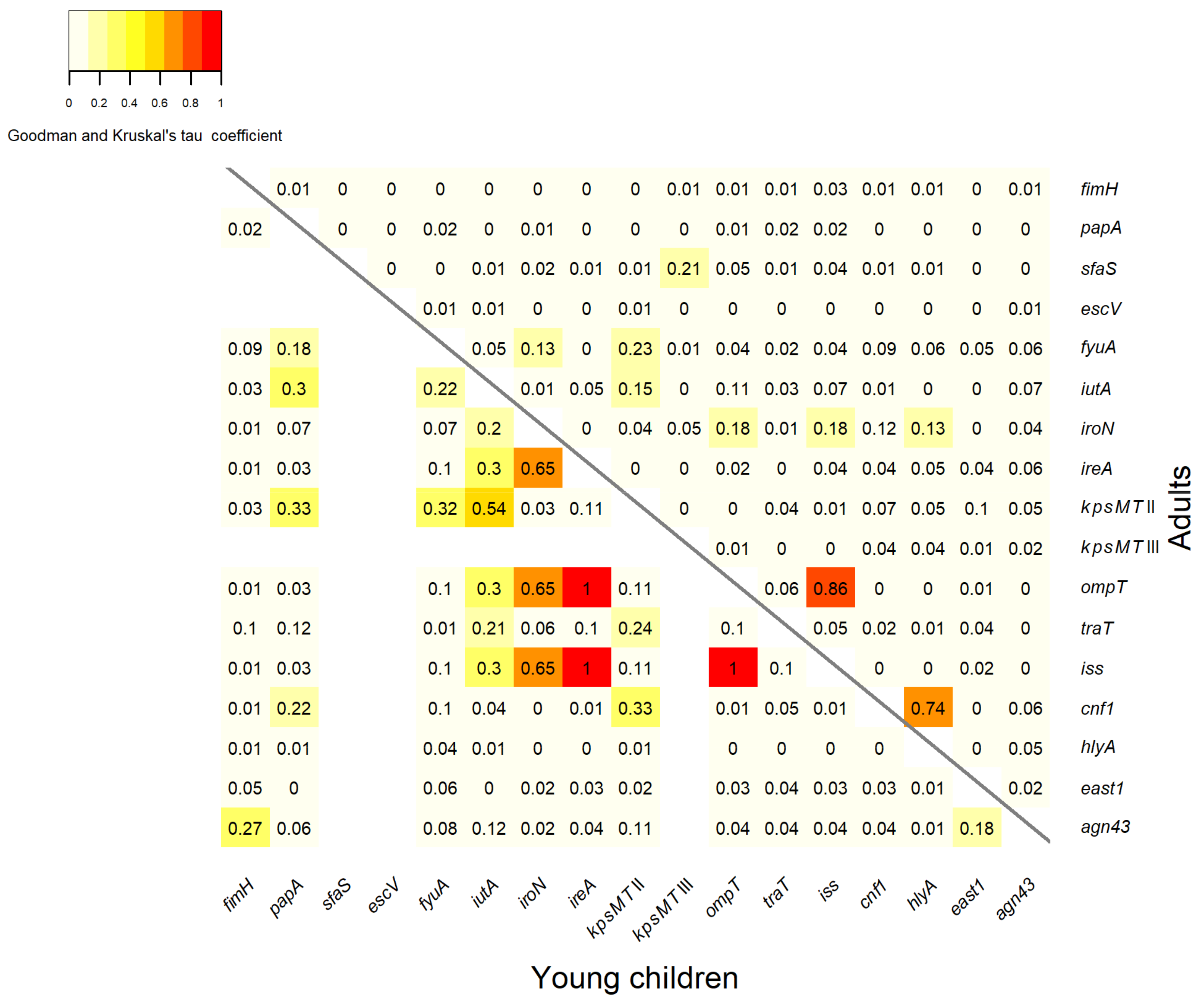
3.3. Association between Virulence Genes
3.4. Phylogenetic Structure of Commensal E. coli
3.5. Distribution of Virulence Genes in Relation to Phylogenetic Groups
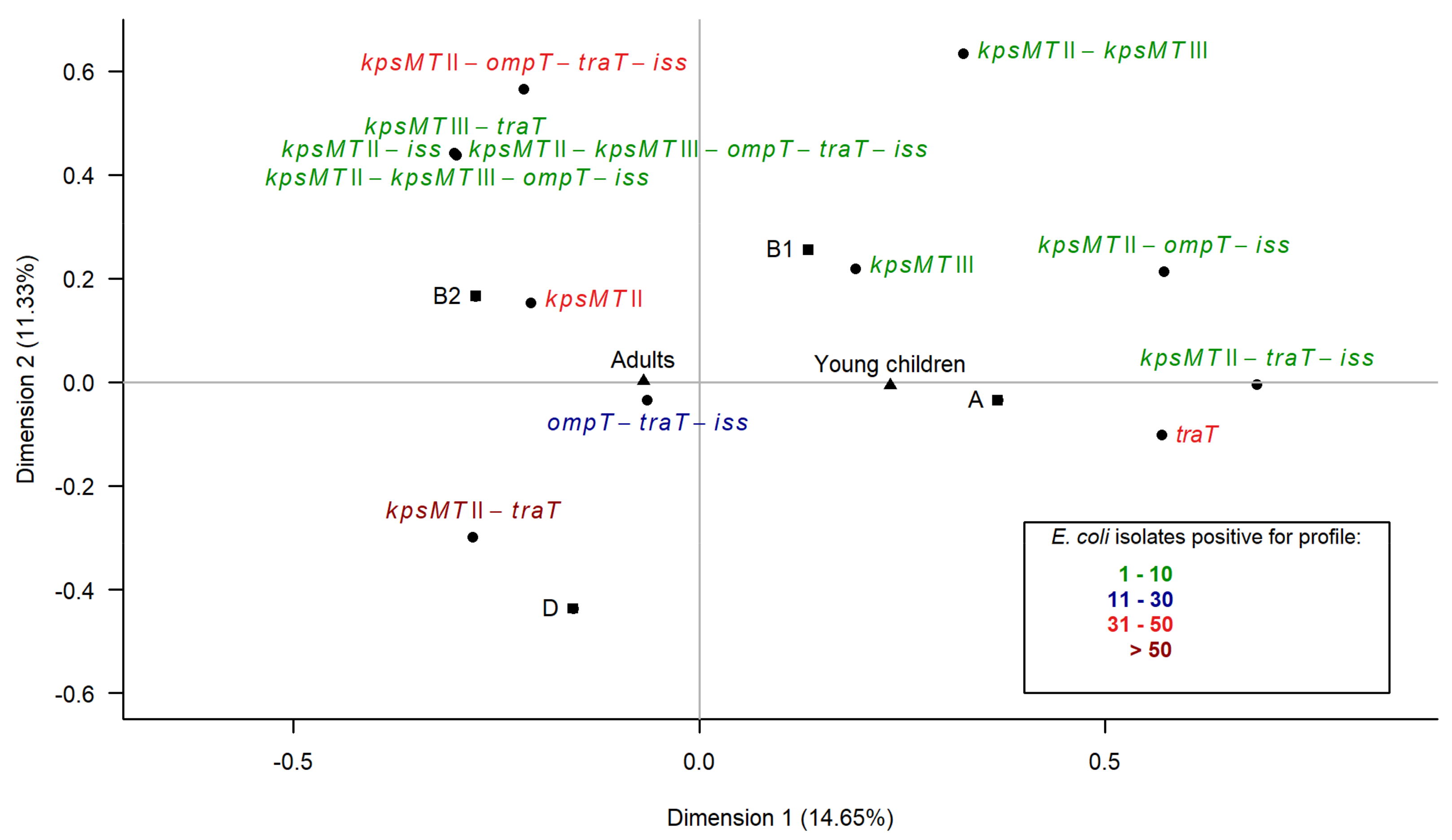
3.6. Multiple Correspondence Analysis
3.7. Prevalence of Antimicrobial Resistance
3.8. Detection of Resistance Determinants
3.9. Correlations between Virulence Genes and Antimicrobial Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.D.; Dobrindt, U. What defines extraintestinal pathogenic Escherichia coli? Int. J. Med. Microbiol. 2011, 301, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Kosek, M.; Bern, C.; Guerrant, R.L. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 2003, 81, 197–204. [Google Scholar] [PubMed]
- Russo, T.A.; Johnson, J.R. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes Infect. 2003, 5, 449–456. [Google Scholar] [CrossRef]
- Touchon, M.; Hoede, C.; Tenaillon, O.; Barbe, V.; Baeriswyl, S.; Bidet, P.; Bingen, E.; Bonacorsi, S. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet. 2009, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alteri, C.J.; Mobley, H.L. Escherichia coli physiology and metabolism dictates adaptation to diverse host microenvironments. Curr. Opin. Microbiol. 2012, 15, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, T.; Clermont, O.; Gouriou, S.; Picard, B.; Nassif, X.; Denamur, E.; Tenaillon, O. Extraintestinal virulence is a coincidental by-product of commensalism in B2 phylogenetic group Escherichia coli strains. Mol. Biol. Evol. 2007, 24, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Starčič, E.M.; Žgur-Bertok, D. Virulence potential for extraintestinal infections among commensal Escherichia coli isolated from healthy humans—The Trojan horse within our gut. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef]
- Sabaté, M.; Moreno, E.; Pérez, T.; Andreu, A.; Prats, G. Pathogenicity island markers in commensal and uropathogenic Escherichia coli isolates. Clin. Microbiol. Infect. 2006, 12, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Koga, V.L.; Tomazetto, G.; Cyoia, P.S.; Neves, M.S.; Vidotto, M.C.; Nakazato, G.; Kobayashi, R.K.T. Molecular screening of virulence genes in extraintestinal pathogenic Escherichia coli isolated from human blood culture in Brazil. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.K.; Pinyon, J.L.; Anantham, S.; Hall, R.M. Commensal Escherichia coli of healthy humans: A reservoir for antibiotic-resistance determinants. J. Med. Microbiol. 2010, 59, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Bettelheim, K.A.; Lennox-King, S.M. The acquisition of Escherichia coli by new-born babies. Infection 1976, 4, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Fryklund, B.; Tullus, K.; Berglund, B.; Burman, L.G. Importance of the environmental and the faecal flora of infants, nursing staff and parents as sources of Gramnegative bacteria colonizing newborns in three neonatal wards. Infection 1992, 20, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzian, F.; Hesselmar, B.; Saalman, R.; Strannegard, I.L.; Aberg, N.; Wold, A.E.; Adlerberth, I. Escherichia coli in infants’ intestinal microflora: Colonization rate, strain turnover, and virulence gene carriage. Pediatr. Res. 2003, 54, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Stell, A.L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Picard, B.; Garcia, J.S.; Gouriou, S.; Duriez, P.; Brahimi, N.; Bingen, E.; Elion, J.; Denamur, E. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 1999, 67, 546–553. [Google Scholar] [PubMed]
- Johnson, J.R.; Oswald, E.; O’Bryan, T.T.; Kuskowski, M.A.; Spanjaard, L. Phylogenetic distribution of virulence-associated genes among Escherichia coli isolates associated with neonatal bacterial meningitis in the Netherlands. J. Infect. Dis. 2002, 185, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Caugant, D.A.; Levin, B.R.; Selander, R.K. Genetic diversity and temporal variation in the E. coli population of a human host. Genetics 1981, 98, 467–490. [Google Scholar] [PubMed]
- Nowrouzian, F.; Adlerberth, I.; Wold, A.E. P fimbriae, capsule and aerobactin characterize colonic resident Escherichia coli. Epidemiol. Infect. 2001, 126, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzian, F.L.; Adlerberth, I.; Wold, A.E. Enhanced persistence in the colonic microbiota of Escherichia coli strains belonging to phylogenetic group B2: Role of virulence factors and adherence to colonic cells. Microbes Infect. 2006, 8, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E.; Zarazaga, M.; Sáenz, Y.; Briñas, L.; Torres, C. Mechanisms of antibiotic resistance in Escherichia coli isolates obtained from healthy children in Spain. Microb. Drug Resist. 2002, 8, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, Z.C.; Wang, M.H.; Huang, X.H.; Pan, Y.H.; Cao, Y.P. Antimicrobial resistance and integrons of commensal Escherichia coli strains from healthy humans in China. J. Chemother. 2014, 26, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Hannoun, C.; Adlerberth, I.; Wold, A.E. Colonization dynamics of ampicillin-resistant Escherichia coli in the infantile colonic microbiota. J. Antimicrob. Chemother. 2008, 62, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Páramo, P.; Grenet, K.; Le Menac’h, A.; Rode, L.; Salgado, E.; Amorin, C.; Gouriou, S.; Picard, B.; Rahimy, M.C.; Andremont, A.; et al. Large-scale population structure of human commensal Escherichia coli isolates. Appl. Environ. Microbiol. 2004, 70, 5698–5700. [Google Scholar] [CrossRef] [PubMed]
- Al-Mayahie, S.M.G.; Al-Khafajy, A.A.M.; Dosh, N.A.S.; Al-Rekabi, A.R.K.; Al-Atabie, A.G.N. Phylogenetic grouping of dominant fecal escherichia coli isolates from healthy males and females in Al-Kut/Wasit Province/Iraq. J. Bacteriol. Parasitol. 2015, 6. [Google Scholar] [CrossRef]
- Ostblom, A.; Adlerberth, I.; Wold, A.E.; Nowrouzian, F.L. Pathogenicity island markers, virulence determinants malX and usp, and the capacity of Escherichia coli to persist in infants’ commensal microbiotas. Appl. Environ. Microbiol. 2011, 77, 2303–2308. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Lauber, C.; Costello, E.K.; Lozupone, C.A.; Humphrey, G.; Berg-Lyons, D.; Caporaso, J.G.; Knights, D.; Clemente, J.C.; Nakielny, S.; et al. Cohabiting family members share microbiota with one another and with their dogs. Elife 2013, 16. [Google Scholar] [CrossRef] [PubMed]
- Sears, H.J.; Brownlee, I.; Uchiyama, J.K. Persistence of individual strains of Escherichia coli in the intestinal tract of man. J. Bacteriol. 1950, 59, 293–301. [Google Scholar] [PubMed]
- Müller, D.; Hagedorn, P.; Brast, S.; Heusipp, G.; Bielaszewska, M.; Friedrich, A.W.; Karch, H.; Schmidt, M.A. Rapid identification and differentiation of clinical isolates of enteropathogenic Escherichia coli (EPEC), atypical EPEC, and Shiga toxin-producing Escherichia coli by a one-step multiplex PCR method. J. Clin. Microbiol. 2006, 44, 2626–2629. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Carlino, U.B.; Johnson, J.R. Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 2001, 69, 6209–6216. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; O’Bryan, T.T. Detection of the Escherichia coli group 2 polysaccharide capsule synthesis gene kpsM by a rapid and specific PCR-based assay. J. Clin. Microbiol. 2004, 42, 1773–1776. [Google Scholar] [CrossRef] [PubMed]
- Mellata, M.; Ameiss, K.; Mo, H.; Curtiss, R., 3rd. Characterization of the contribution to virulence of three large plasmids of avian pathogenic Escherichia coli chi7122 (O78:K80:H9). Infect. Immun. 2010, 78, 1528–1541. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Terai, A.; Yuri, K.; Kurazono, H.; Takeda, Y.; Yoshida, O. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 1995, 12, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Chapman, T.A.; Wu, X.Y.; Barchia, I.; Bettelheim, K.A.; Driesen, S.; Trott, D.; Wilson, M.; Chin, J.J. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl. Environ. Microbiol. 2006, 72, 4782–4795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restieri, C.; Garriss, G.; Locas, M.C.; Dozois, C.M. Autotransporter-encoding sequences are phylogenetically distributed among Escherichia coli clinical isolates and reference strains. Appl. Environ. Microbiol. 2007, 73, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- EUCAST Disk Diffusion Method for Antimicrobial Susceptibility Testing Version 5.0 (January 2015). Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/Manual_v_5.0_EUCAST_Disk_Test.pdf (accessed on 27 March 2018).
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 4.0; EUCAST: Basel, Switzerland, 2014. [Google Scholar]
- Clinical Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement; Document M100-S23; CLSI: Wayne, PA, USA, 2013. [Google Scholar]
- Ng, L.K.; Martin, I.; Alfa, M.; Mulvey, M. Multiplex PCR for the detection of tetracycline resistant genes. Mol. Cell. Probes 2001, 15, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Frech, G.; Kehrenberg, C.; Schwarz, S. Resistance phenotypes and genotypes of multiresistant Salmonella enterica subsp. enterica serovar Typhimurium var. Copenhagen isolates from animal sources. J. Antimicrob. Chemother. 2003, 51, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Kozak, G.K.; Boerlin, P.; Janecko, N.; Reid-Smith, R.J.; Jardine, C. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl. Environ. Microbiol. 2009, 75, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Cattoir, V.; Poirel, L.; Rotimi, V.; Soussy, C.J.; Nordmann, P. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J. Antimicrob. Chemother. 2007, 60, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Cao, V.; Lambert, T.; Nhu, D.Q.; Loan, H.K.; Hoang, N.K.; Arlet, G.; Courvalin, P. Distribution of extended-spectrum beta-lactamases in clinical isolates of Enterobacteriaceae in Vietnam. Antimicrob. Agents Chemother. 2002, 46, 3739–3743. [Google Scholar] [CrossRef] [PubMed]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [PubMed]
- Leimbach, A.; Hacker, J.; Dobrindt, U. E. coli as an all-rounder: the thin line between commensalism and pathogenicity. Curr. Top. Microbiol. Immunol. 2013, 358, 3–32. [Google Scholar] [PubMed]
- Nowrouzian, F.L.; Wold, A.E.; Adlerberth, I. Escherichia coli strains belonging to phylogenetic group B2 have superior capacity to persist in the intestinal microflora of infants. J. Infect. Dis. 2005, 191, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Dureja, C.; Mahajan, S.; Raychaudhuri, S. Phylogenetic distribution and prevalence of genes encoding class I Integrons and CTX-M-15 extended-spectrum β-lactamases in Escherichia coli isolates from healthy humans in Chandigarh, India. PLoS ONE 2014, 9, e112551. [Google Scholar] [CrossRef] [PubMed]
- Hacker, J.; Kaper, J.B. Pathogenicity islands and the evolution of microbes. Ann. Rev. Microbiol. 2000, 54, 641–679. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Nolan, L.K. Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol. Mol. Biol. Rev. 2009, 73, 750–774. [Google Scholar] [CrossRef] [PubMed]
- Lemaître, C.; Mahjoub-Messai, F.; Dupont, D.; Caro, V.; Diancourt, L.; Bingen, E.; Bidet, P.; Bonacorsi, S. A conserved virulence plasmidic region contributes to the virulence of the multiresistant Escherichia coli meningitis strain S286 belonging to phylogenetic group C. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Tivendale, K.A.; Noormohammadi, A.H.; Allen, J.L.; Browning, G.F. The conserved portion of the putative virulence region contributes to virulence of avian pathogenic Escherichia coli. Microbiology 2009, 155, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.G.; Silva, V.L.; Diniz, C.G. Occurrence and antimicrobial drug susceptibility patterns of commensal and diarrheagenic Escherichia coli in fecal microbiota from children with and without acute diarrhea. J. Microbiol. 2011, 49, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Lim, Y.S.; Park, M.S.; Kim, S.H.; Kang, Y.H. Prevalence of antibiotic resistance in Escherichia coli fecal isolates from healthy persons and patients with diarrhea. Osong Public Health Res. Perspect. 2011, 2, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Dyar, O.J.; Hoa, N.Q.; Trung, N.V.; Phuc, H.D.; Larsson, M.; Chuc, N.T.; Lundborg, C.S. High prevalence of antibiotic resistance in commensal Escherichia coli among children in rural Vietnam. BMC Infect. Dis. 2012, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Logue, C.M.; Johnson, J.R.; Kuskowski, M.A.; Sherwood, J.S.; Barnes, H.J.; DebRoy, C.; Wannemuehler, Y.M.; Obata-Yasuoka, M.; Spanjaard, L.; et al. Associations between multidrug resistance, plasmid content, and virulence potential among extraintestinal pathogenic and commensal Escherichia coli from humans and poultry. Foodborne Pathog. Dis. 2012, 9, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Leite-Martins, L.R.; Mahú, M.I.; Costa, A.L.; Mendes, A.; Lopes, E.; Mendonça, D.M.; Niza-Ribeiro, J.J.; de Matos, A.J.; da Costa, P.M. Prevalence of antimicrobial resistance in enteric Escherichia coli from domestic pets and assessment of associated risk markers using a generalized linear mixed model. Prev. Vet. Med. 2014, 117, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Koczura, R.; Semkowska, A.; Mokracka, J. Integron-bearing Gram-negative bacteria in lake waters. Lett. Appl. Microbiol. 2014, 59, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Proia, L.; Anzil, A.; Subirats, J.; Borrego, C.; Farrè, M.; Llorca, M.; Balcázar, J.L.; Servais, P. Antibiotic resistance along an urban river impacted by treated wastewaters. Sci. Total Environ. 2018, 628–629, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kinkelaar, D.; Huang, Y.; Li, Y.; Li, X.; Wang, H.H. Acquired antibiotic resistance: Are we born with it? Appl. Environ. Microbiol. 2011, 77, 7134–7141. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Manuzon, M.; Lehman, M.; Wan, K.; Luo, H.; Wittum, T.E.; Yousef, A.; Bakaletz, L.O. Food commensal microbes as a potentially important avenue in transmitting antibiotic resistance genes. FEMS Microbiol. Lett. 2006, 254, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.H. Tetracycline resistance associated with commensal bacteria from representative ready-to-consume deli and restaurant foods. J. Food Prot. 2010, 73, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Wold, A.E.; Adlerberth, I. Antibiotic resistance is linked to carriage of papC and iutA virulence genes and phylogenetic group D background in commensal and uropathogenic Escherichia coli from infants and young children. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 721–729. [Google Scholar] [CrossRef] [PubMed]


 p < 0.001,
p < 0.001,  p < 0.01,
p < 0.01,  p < 0.05. The values significantly lower than among the other groups are indicated as follows:
p < 0.05. The values significantly lower than among the other groups are indicated as follows:  p < 0.001,
p < 0.001,  p < 0.01,
p < 0.01,  p < 0.05.
p < 0.05.
 p < 0.001,
p < 0.001,  p < 0.01,
p < 0.01,  p < 0.05. The values significantly lower than among the other groups are indicated as follows:
p < 0.05. The values significantly lower than among the other groups are indicated as follows:  p < 0.001,
p < 0.001,  p < 0.01,
p < 0.01,  p < 0.05.
p < 0.05.

| Functional Category Virulence Gene | E. coli Pathotype | Number (%) of E. coli Isolates with Virulence Genes | Test of Independence p-Value | |
|---|---|---|---|---|
| Adults | Young Children | |||
| n = 296 | n = 86 | |||
| Adhesins | ||||
| fimH | ExPEC | 286 (96.6) | 76 (88.4) | 0.005 * |
| papA | ExPEC | 54 (18.2) | 10 (11.6) | 0.1482 |
| sfaS | ExPEC | 4 (1.4) | 0 | 0.5787 |
| escV | EPEC, EHEC | 1 (0.3) | 0 | 1 |
| bfpB | EPEC | 0 | 0 | - |
| Iron acquisition | ||||
| fyuA | ExPEC | 225 (76) | 36 (41.9) | <0.0001 * |
| iutA | ExPEC | 184 (62.2) | 17 (19.8) | <0.0001 * |
| iroN | ExPEC | 110 (37.2) | 4 (4.7) | <0.0001 * |
| ireA | ExPEC | 87 (29.4) | 6 (7) | <0.0001 * |
| Protectins | ||||
| kpsMT II | ExPEC | 201 (67.9) | 16 (18.6) | <0.0001 * |
| -K1 | ExPEC | 158 (53.4) | 10 (11.6) | <0.0001 * |
| -K2 | ExPEC | 192 (64.9) | 15 (17.4) | <0.0001 * |
| -K5 | ExPEC | 125 (42.2) | 10 (11.6) | <0.0001 * |
| kpsMT III | ExPEC | 18 (6.1) | 0 | 0.02 * |
| ompT | ExPEC | 62 (21) | 6 (7) | 0.003 * |
| traT | ExPEC | 190 (64.2) | 37 (43) | 0.0004 * |
| iss | ExPEC | 70 (23.6) | 6 (7) | 0.0007 * |
| Toxins | ||||
| cnf1 | ExPEC, NTEC | 64 (21.6) | 6 (7) | 0.002 * |
| hlyA | ExPEC | 50 (16.2) | 4 (2.3) | 0.004 * |
| east1 | EAEC | 28 (9.5) | 23 (25.6) | <0.0001 * |
| ehxA | EPEC, EHEC | 0 | 0 | - |
| stx1 | EHEC | 0 | 0 | - |
| stx2 | EHEC | 0 | 0 | - |
| eltA | ETEC | 0 | 0 | - |
| estI | ETEC | 0 | 0 | - |
| estII | ETEC | 0 | 0 | - |
| Biofilm formation | ||||
| agn43 | ExPEC | 240 (81.1) | 58 (67.4) | 0.007 * |
| -a | ExPEC | 106 (35.8) | 12 (14) | 0.0001 * |
| -b | ExPEC | 73 (24.7) | 4 (4.7) | <0.0001 * |
| -K12 | ExPEC | 176 (59.5) | 53 (61.6) | 0.7179 |
| Functional Category | Virulence Genes | Number (%) of E. coli Isolates with Virulence Genes within Phylogenetic Groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B1 | B2 | D | ||||||
| Adults | Young Children | Adults | Young Children | Adults | Young Children | Adults | Young Children | ||
| n = 68 | n = 60 | n = 26 | n = 10 | n = 138 | n = 12 | n = 64 | n = 4 | ||
| Adhesins | fimH | 62 (91.2) | 50 (83.3) | 26 (100) | 10 (100) | 134 (97.1) | 12 (100) | 64 (100) | 4 (100) |
| papA | 0 | 0 | 4 (15.4) | 0 | 32 (23.2) | 6 (50) * | 18 (28.1) | 4 (100) *** | |
| sfaS | 0 | 0 | 0 | 0 | 4 (2.9) | 0 | 0 | 0 | |
| escV | 0 | 0 | 1 (0.3) | 0 | 0 | 0 | 0 | 0 | |
| Iron acquisition | fyuA | 32 (47.1) ** | 12 (20) | 20 (76.9) | 10 (100) | 131 (94.9) | 10 (83.3) | 42 (65.6) | 4 (100) |
| iutA | 30 (44.1) *** | 2 (3.3) | 14 (53.8) | 4 (40) | 96 (69.6) | 7 (58.3) | 44 (68.8) | 4 (100) | |
| iroN | 4 (5.9) | 0 | 14 (53.8) | 2 (20) | 88 (63.8) *** | 0 | 4 (6.2) | 2 (50) * | |
| ireA | 24 (35.3) *** | 0 | 6 (23.1) | 4 (40) | 40 (29) * | 0 | 17 (26.6) | 2 (50) | |
| Protectins | kpsMT II | 22 (32.4) *** | 0 | 14 (53.8) | 4 (40) | 117 (84.8) | 10 (83.3) | 48 (75) | 2 (50) |
| kpsMT III | 2 (2.9) | 0 | 2 (7.7) | 0 | 12 (8.7) | 0 | 2 (3.1) | 0 | |
| ompT | 8 (11.8) ** | 0 | 4 (15.4) | 4 (40) | 46 (33.3) ** | 0 | 4 (6.2) | 2 (50) * | |
| traT | 34 (50) * | 18 (30) | 10 (38.5) | 4 (40) | 98 (71) | 11 (91.7) | 48 (75) | 4 (100) | |
| iss | 12 (17.6) *** | 0 | 4 (15.4) | 4 (40) | 50 (36.2) ** | 0 | 4 (6.2) | 2 (50) * | |
| Toxins | cnf1 | 2 (2.9) | 0 | 10 (38.5) * | 0 | 50 (36.2) | 6 (50) | 2 (3.1) | 0 |
| hlyA | 2 (2.9) | 4 (6.7) | 10 (38.5) * | 0 | 38 (27.5) * | 0 | 0 | 0 | |
| east1 | 8 (11.8) | 21 (35) ** | 2 (7.7) | 0 | 6 (4.3) | 2 (16.7) | 12 (18.8) | 0 | |
| Biofilm formation | agn43 | 36 (52.9) | 40 (66.7) | 20 (76.9) * | 4 (40) | 124 (89.9) | 10 (83.3) | 60 (93.8) | 4 (100) |
| Antimicrobial Agent | Number (%) of E. coli Isolates | Test of Independence p-Value * | |
|---|---|---|---|
| Adults | Young Children | ||
| n = 296 | n = 86 | ||
| Ampicillin | 113 (38.2) | 27 (31.4) | 0.2506 |
| Amoxicillin/Clavulanic acid | 40 (13.5) | 3 (3.5) | 0.0096 * |
| Piperacillin | 72 (24.3) | 5 (5.8) | 0.0002 * |
| Cephalothin | 86 (29.1) | 24 (27.9) | 0.8362 |
| Cefuroxime | 20 (6.8) | 3 (3.5) | 0.3152 |
| Cefotaxime | 4 (1.4) | 2 (2.7) | 0.6206 |
| Streptomycin | 137 (46.3) | 19 (22.1) | <0.0001 * |
| Gentamicin | 32 (10.8) | 9 (10.5) | 0.9273 |
| Amikacin | 30 (10.1) | 7 (8.1) | 0.5818 |
| Tetracycline | 63 (21.3) | 17 (19.8) | 0.7610 |
| Doxycycline | 46 (15.5) | 11 (12.8) | 0.5287 |
| Trimethoprim/sulfamethoxazole | 33 (11.1) | 12 (14) | 0.4776 |
| Chloramphenicol | 18 (6.1) | 0 | 0.0171 |
| Nalidixic acid | 39 (13.2) | 8 (9.3) | 0.3358 |
| Norfloxacin | 8 (2.7) | 2 (2.3) | 1 |
| Ciprofloxacin | 8 (2.7) | 2 (2.3) | 1 |
| Nitrofurantoin | 12 (4.1) | 3 (3.5) | 1 |
| Antimicrobial susceptibility characteristic | |||
| R | 224 (75.7) | 47 (54.7) | 0.0002 * |
| MDR | 90 (30.4) | 12 (14) | 0.0024 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bok, E.; Mazurek, J.; Myc, A.; Stosik, M.; Wojciech, M.; Baldy-Chudzik, K. Comparison of Commensal Escherichia coli Isolates from Adults and Young Children in Lubuskie Province, Poland: Virulence Potential, Phylogeny and Antimicrobial Resistance. Int. J. Environ. Res. Public Health 2018, 15, 617. https://doi.org/10.3390/ijerph15040617
Bok E, Mazurek J, Myc A, Stosik M, Wojciech M, Baldy-Chudzik K. Comparison of Commensal Escherichia coli Isolates from Adults and Young Children in Lubuskie Province, Poland: Virulence Potential, Phylogeny and Antimicrobial Resistance. International Journal of Environmental Research and Public Health. 2018; 15(4):617. https://doi.org/10.3390/ijerph15040617
Chicago/Turabian StyleBok, Ewa, Justyna Mazurek, Andrzej Myc, Michał Stosik, Magdalena Wojciech, and Katarzyna Baldy-Chudzik. 2018. "Comparison of Commensal Escherichia coli Isolates from Adults and Young Children in Lubuskie Province, Poland: Virulence Potential, Phylogeny and Antimicrobial Resistance" International Journal of Environmental Research and Public Health 15, no. 4: 617. https://doi.org/10.3390/ijerph15040617




