Multi-Scale Clustering of Lyme Disease Risk at the Expanding Leading Edge of the Range of Ixodes scapularis in Canada
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
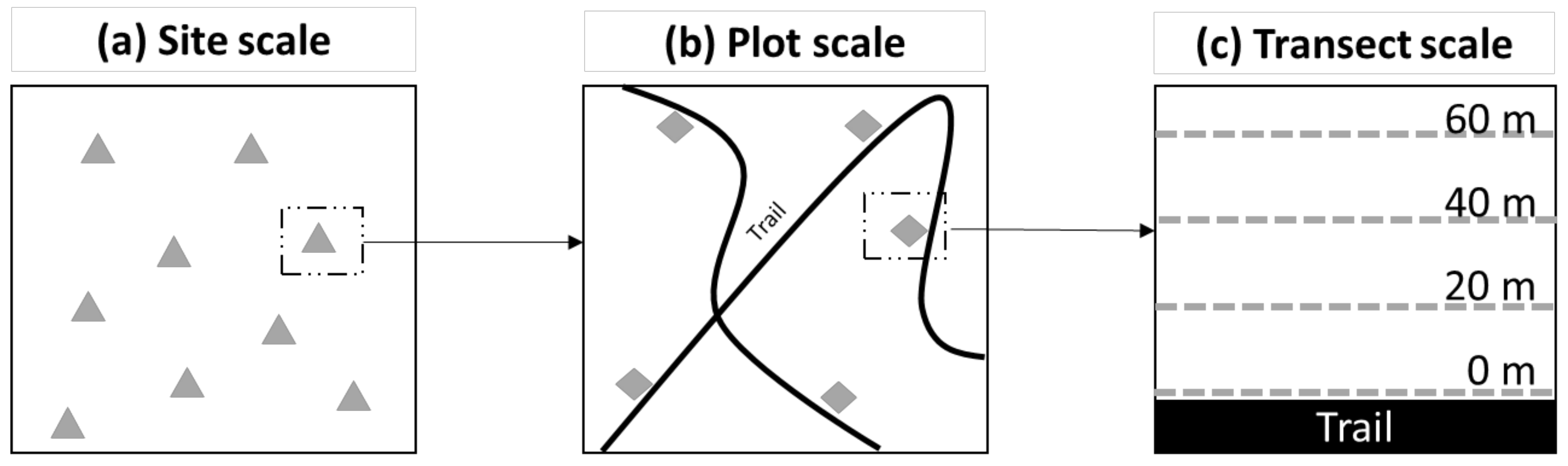
2.2. Sampling Protocol
2.3. Tick Collection
2.4. Environmental Factors
2.4.1. Site Scale
2.4.2. Plot Scale
2.4.3. Transect Scale
2.5. Statistical Analysis
2.5.1. Spatial Analysis
2.5.2. Regression Models
3. Results
3.1. Tick Collection
3.2. Spatial Distribution of Nymphs at Site Scale
3.3. Relationship between Environmental Factors and Nymph Density
4. Discussion
4.1. Regional Scale
4.2. Local Scale
4.3. Implications for the Management of Emerging Lyme Disease Risk
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eisen, R.J.; Eisen, L. Spatial modeling of human risk of exposure to vector-borne pathogens based on epidemiological versus arthropod vector data. J. Med. Entomol. 2008, 45, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.L. Distribution and abundance of Ixodes scapularis (acari: Ixodidae) in North America: Ecological processes and spatial analysis. J. Med. Entomol. 1998, 35, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.; Young, A.S.; Lega, J.; Andreadis, T.; Schurich, J.; Comrie, A. Projection of climate change influences on U.S. West Nile virus vectors. Earth Interact. 2015, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R.; Hanincová, K.; Barker, I.K.; Bigras-Poulin, M.; Charron, D.F.; Heagy, A.; Francis, C.M.; O’Callaghan, C.J.; Schwartz, I.; et al. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl. Environ. Microbiol. 2008, 74, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Eisen, R.J. Need for improved methods to collect and present spatial epidemiologic data for vectorborne diseases. Emerg. Infect. Dis. 2007, 13, 1816–1820. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L.; Castro, M.B.; Lane, R.S. Environmentally related variability in risk of exposure to Lyme disease spirochetes in northern California: Effect of climatic conditions and habitat type. Environ. Entomol. 2003, 32, 1010–1018. [Google Scholar] [CrossRef]
- Eisen, R.J.; Lane, R.S.; Fritz, C.L.; Eisen, L. Spatial patterns of Lyme disease risk in California based on disease incidence data and modeling of vector-tick exposure. Am. J. Trop. Med. Hyg. 2006, 75, 669–676. [Google Scholar] [PubMed]
- Gasmi, S.; Ogden, N.H.; Leighton, P.A.; Lindsay, L.R.; Thivierge, K. Analysis of the human population bitten by Ixodes scapularis ticks in Quebec, Canada: Increasing risk of Lyme disease. Ticks Tick-Borne Dis. 2016, 7, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Irace-Cima, A.; Adam-Poupart, A.; Ouhoummane, N.; Milord, F.; Thivierge, K. Rapport De Surveillance De La Maladie De Lyme Et Des Autres Maladies Transmises Par La Tique Ixodes Scapularis Au Québec Entre 2004 Et 2013; Institut National de Santé Publique du Québec: Québec City, QC, Canada, 2016; p. 8. [Google Scholar]
- Burgdorfer, W.; Barbour, A.; Hayes, S.; Benach, J.; Grunwaldt, E.; Davis, J. Lyme disease—A tick-borne spirochetosis? Science 1982, 216, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Reported Cases of Lyme Disease by Year, United States, 1995–2015. Available online: www.cdc.gov/lyme/stats/chartstables/casesbyyear.html (accessed on 25 September 2017).
- Barker, I.K.; Surgeoner, G.A.; Artsob, H.; McEwen, S.A.; Elliott, L.A.; Campbell, G.D.; Robinson, J.T. Distribution of the Lyme disease vector, Ixodes dammini (acari: Ixodidae) and isolation of Borrelia burgdorferi in Ontario, Canada. J. Med. Entomol. 1992, 29, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.G.; Anderson, R.C. Ixodes scapularis say on white-tailed deer (Odocoileus virginianus) from long point, Ontario. J. Wildl. Dis. 1976, 12, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R.; Morshed, M.; Sockett, P.N. The emergence of Lyme disease in Canada. CMAJ 2009, 180, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; Vourc’h, G.; Cislo, P.; Hoen, A.G.; Melton, F.; Hamer, S.A.; Rowland, M.; Cortinas, R.; Hickling, G.J.; Tsao, J.I.; et al. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Glob. Ecol. Biogeogr. 2010, 19, 504–514. [Google Scholar]
- Pardanani, N.; Mather, T.N. Lack of spatial autocorrelation in fine-scale distributions of Ixodes scapularis (acari: Ixodidae). J. Med. Entomol. 2004, 41, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.J.; Boccia, T.M.; Varde, S.; Marcus, J.; Le, J.; Bucher, D.J.; Falco, R.C.; Schwartz, I. Geographic risk for lyme disease and human granulocytic ehrlichiosis in southern New York state. Appl. Environ. Microbiol. 1998, 64, 4663–4669. [Google Scholar] [PubMed]
- Brownstein, J.; Holford, T.R.; Fish, D. A climate-based model predicts the spatial distribution of the lyme disease vector Ixodes scapularis in the United States. Environ. Health Perspect. 2003, 111, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Diuk-Wasser, M.A.; Gatewood, A.G.; Cortinas, M.R.; Yaremych-Hamer, S.; Tsao, J.; Kitron, U.; Hickling, G.; Brownstein, J.S.; Walker, E.; Piesman, J.; et al. Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (acari: Ixodidae) in the United States. J. Med. Entomol. 2006, 43, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-scale distribution of Ixodes scapularis and Ixodes pacificus (acari: Ixodidae) in the continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A. Geostatistics and remote sensing as predictive tools of tick distribution: A cokriging system to estimate Ixodes scapularis (acari: Ixodidae) habitat suitability in the United States and Canada from advanced very high resolution radiometer satellite image. J. Med. Entomol. 1998, 35, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A. The relationships between habitat topology, critical scales of connectivity and tick abundance Ixodes ricinus in a heterogeneous landscape in northern Spain. Ecography 2003, 26, 661–671. [Google Scholar] [CrossRef]
- Allan, B.F.; Keesing, F.; Ostfeld, R. Effect of forest fragmentation on Lyme disease risk. Conserv. Biol. 2003, 17, 267–272. [Google Scholar] [CrossRef]
- Bunnell, J.E.; Price, S.D.; Das, A.; Shields, T.M.; Glass, G.E. Geographic information system and spatial analysis of adult Ixodes scapulris (acari: Ixodidae) in the middle Atlantic region of the USA. J. Med. Entomol. 2003, 40, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Cortinas, R.; Spomer, S.M. Occurrence and county-level distribution of ticks (acari: Ixodoidea) in Nebraska using passive surveillance. J. Med. Entomol. 2014, 51, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Koffi, J.K.; Leighton, P.A.; Pelcat, Y.; Trudel, L.; Lindsay, R.; Milord, F.; Ogden, N.H.; Lindsay, L.R. Passive surveillance for I. scapularis ticks: Enhanced analysis for early detection of emerging Lyme disease risk. J. Med. Entomol. 2012, 49, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, M.C.; Mather, T.N. Methods for evaluating Lyme disease risks using geographic information systems and geospatial analysis. J. Med. Entomol. 1996, 33, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, J.S.; Skelly, D.K.; Holford, T.R.; Fish, D. Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia 2005, 146, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.S.; Zhioua, E.; Mitra, S.; Fischer, J.; Buckley, P.A.; Verret, F.; Underwood, H.B.; Buckley, F.G. Woodland type and spatial distribution of nymphal Ixodes scapularis (acari: Ixodidae). Environ. Entomol. 2004, 33, 1266–1273. [Google Scholar] [CrossRef]
- Duffy, D.C.; Clark, D.D.; Campbell, S.R.; Gurney, S.; Perello, R.; Simon, N. Landscape patterns of abundance of Ixodes scapularis (acari: Ixodidae) on shelter island, New York. J. Med. Entomol. 1994, 31, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.H.; Fish, D.; Moy, F.H. Landscape features associated with Lyme disease risk in a suburban residential environment. Landsc. Ecol. 1998, 13, 27–36. [Google Scholar] [CrossRef]
- Ostfeld, R.S.; Hazler, K.R.; Cepeda, O.M. Temporal and spatial dynamics of Ixodes scapularis (acari: Ixodidae) in a rural landscape. J. Med. Entomol. 1996, 33, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Telford, S.R.; Urioste, S.S.; Spielman, A. Clustering of host-seeking nymphal deer ticks (Ixodes dammini) infected by lyme disase spirochetes (Borrelia burgdorferi). Am. J. Trop. Med. Hyg. 1992, 47, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Vourc’h, G.; Abrial, D.; Bord, S.; Jacquot, M.; Masséglia, S.; Poux, V.; Pisanu, B.; Bailly, X.; Chapuis, J.L. Mapping human risk of infection with Borrelia burgdorferi sensu lato, the agent of lyme borreliosis, in a periurban forest in France. Ticks Tick-Borne Dis. 2016, 7, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Leighton, P.A.; Koffi, J.K.; Pelcat, Y.; Lindsay, L.R.; Ogden, N.H. Predicting the speed of tick invasion: An empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J. Appl. Ecol. 2012, 49, 457–464. [Google Scholar] [CrossRef]
- Ogden, N.H.; Bigras-Poulin, M.; Hanincová, K.; Maarouf, A.; O’Callaghan, C.J.; Kurtenbach, K. Projected effects of climate change on tick phenology and fitness of pathogens transmitted by the North American tick Ixodes scapularis. J. Theor. Biol. 2008, 254, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R.; Beauchamp, G.; Charron, D.; Maarouf, A.; O’Callaghan, C.J.; Waltner-Toews, D.; Barker, I.K. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (acari: Ixodidae) in the laboratory and field. J. Med. Entomol. 2004, 41, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; St-Onge, L.; Barker, I.K.; Brazeau, S.; Bigras-Poulin, M.; Charron, D.F.; Francis, C.M.; Heagy, A.; Lindsay, L.R.; Maarouf, A.; et al. Risk maps for range expansion of the lyme disease vector, Ixodes scapularis, in Canada now and with climate change. Int. J. Health Geogr. 2008, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.; Walker, E.; Jones, C.; Paskewitz, S.; Cortinas, M.R.; Stancil, A.; Beck, L.; Bobo, M.; Kitron, U. Predicting the risk of lyme disease: Habitat suitability for Ixodes scapularis in the north central United States. Emerg. Infect. Dis. 2002, 8, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, L.R.; Mathison, S.W.; Barker, I.K.; McEwen, S.A.; Gillespie, T.J.; Surgeoner, G.A. Microclimate and habitat in relation to Ixodes scapularis (acari: Ixodidae) populations on long point, Ontario, Canada. J. Med. Entomol. 1999, 36, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Piesman, J.; Gern, L. Lyme borreliosis in Europe and North America. Parasitology 2004, 129, S191–S220. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Bouchard, C.; Kurtenbach, K.; Margos, G.; Lindsay, L.R.; Trudel, L.; Nguon, S.; Milord, F. Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of lyme disease risk emergence in Canada. Environ. Health Perspect. 2010, 118, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Gassner, F.; van Vliet, A.J.H.; Burgers, S.L.; Jacobs, F.; Verbaarschot, P.; Hovius, E.K.E.; Mulder, S.; Verhulst, N.O.; van Overbeek, L.S.; Takken, W. Geographic and temporal variations in population dynamics of Ixodes ricinus and associated Borrelia infections in the Netherlands. Vector-Borne Zoonotic Dis. 2011, 11, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Kitron, U.; Kazmierczak, J.J. Spatial analysis of the distribution of Lyme disease in Wisconsin. Am. J. Epidemiol. 1997, 145, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.; Keesing, F. Biodiversity and disease risk: The case of Lyme disease. Conserv. Biol. 2000, 14, 722–728. [Google Scholar] [CrossRef]
- Piesman, J.; Spielman, A. Host-associations and seasonal abundance of immature Ixodes dammini (acarina, Ixodidae) in southeastern Massachusetts. Ann. Entomol. Soc. Am. 1979, 72, 829–832. [Google Scholar] [CrossRef]
- Schwarz, A.; Maier, W.A.; Kistemann, T.; Kampen, H. Analysis of the distribution of the tick Ixodes ricinus L. (acari: Ixodidae) in a nature reserve of western Germany using geographic information systems. Int. J. Hyg. Environ. Health 2009, 212, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Leonard, E.; Koffi, J.K.; Pelcat, Y.; Peregrine, A.; Chilton, N.; Rochon, K.; Lysyk, T.; Lindsay, L.R.; Ogden, N.H. The increasing risk of Lyme disease in Canada. Can. Vet. J. 2013, 2015, 693–699. [Google Scholar]
- Ferrouillet, C.; Fortin, A.; Milord, F.; Serhir, B.; Thivierge, K.; Ravel, A.; Tremblay, C. Proposition D’un Programme De Surveillance Intégré Pour La Maladie De Lyme Et Les Autres Maladies Transmises Par La Tique Ixodes Scapularis Au Québec; Institut National de Santé Publique du Québec: Québec City, QC, Canada, 2014; p. 95. [Google Scholar]
- Bouchard, C.; Beauchamp, G.; Leighton, P.A.; Lindsay, L.R.; Bélanger, D.; Ogden, N.H. Does high biodiversity reduce the risk of Lyme disease invasion? Parasites Vectors 2013, 6, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Adam-Poupart, A.; Milord, F.; Irace-Cima, A. Maladie De Lyme: Cartographie Du Risque D’acquisition De La Maladie; Institut National de Santé Publique du Québec: Québec City, QC, Canada, 2017; p. 6. [Google Scholar]
- Bouchard, C.; Beauchamp, G.; Nguon, S.; Trudel, L.; Milord, F.; Lindsay, L.R.; Bélanger, D.; Ogden, N.H. Associations between Ixodes scapularis ticks and small mammal hosts in a newly endemic zone in southeastern Canada: Implications for Borrelia burgdorferi transmission. Ticks Tick-Borne Dis. 2011, 2, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Census Program. Available online: http://www12.statcan.gc.ca (accessed on 5 April 2017).
- Environmental Systems Research Institute (ESRI). Arcgis. Version 10.3.1; Environmental Systems Research Institute: Redlands, CA, USA, 2015. [Google Scholar]
- Nguon, S.; Milord, F.; Ogden, N.H.; Trudel, L.; Lindsay, R.; Bouchard, C. Étude Épidémiologique Sur Les Zoonoses Transmises Par Les Tiques Dans Le Sud-Ouest Du Québec—2008; Institut de Santé Publique du Québec: Ville de Québec, QC, Canada, 2010. [Google Scholar]
- Clifford, C.M.; Anastos, G.; Elbl, A. The larval ixodid ticks of the eastern United States. Entomol. Soc. Am. 1961, 2, 215–244. [Google Scholar]
- Keirans, J.E.; Clifford, C.M. The genus Ixodes in the United States: A scanning electron microscope study and key to the adults. J. Med. Entomol. Suppl. 1978, 2, 1–149. [Google Scholar] [CrossRef] [PubMed]
- Keirans, J.E.; Hutcheson, H.J.; Durden, L.A.; Klompen, J.S.H. Ixodes (Ixodes) scapularis (Acari: Ixodidae): Redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J. Med. Entomol. 1996, 33, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Trudel, L.; Artsob, H.; Barker, I.K.; Beauchamp, G.; Charron, D.F.; Drebot, M.A.; Galloway, T.D.; O’Handley, R.; Thompson, R.A.; et al. Ixodes scapularis ticks collected by passive surveillance in Canada: Analysis of geographic distribution and infection with lyme borreliosis agent Borrelia burgdorferi. J. Med. Entomol. 2006, 43, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Conditions Métérologiques Et Climatiques Passées. Données Historiques. Available online: http://climat.meteo.gc.ca/historical_data/search_historic_data_f.html (accessed on 5 January 2015).
- Fish, D. Population ecology of Ixodes dammini. In Ecology and Environmental Management of Lyme Disease; Ginsberg, H.S., Ed.; Rutgers University Press: New Brunswick, NB, Canada, 1993; pp. 25–42. [Google Scholar]
- Piesman, J.; Dolan, M.C. Protection against Lyme disease spirochete transmission provided by prompt removal of nymphal Ixodes scapularis (acari: Ixodidae). J. Med. Entomol. 2002, 39, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 2010, 24, 189–206. [Google Scholar] [CrossRef]
- Ord, J.K.; Getis, A. Local spatial autocorrelation statistics: Distributional issues and an application. Geogr. Anal. 2010, 27, 286–306. [Google Scholar] [CrossRef]
- Anselin, L. Local indicators of spatial association-lisa. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Oliveau, S. Autocorrélation spatiale: Leçons du changement d’échelle. L’espace Géographique 2010, 1, 51–64. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing, version 3.2.4; R Development Core Team: Vienna, Austria, 2016. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; p. 574. [Google Scholar]
- Dohoo, I.R.; Martin, W.; Stryhn, H.E. Veterinary Epidemiologic Research, 2nd ed.; VER Inc.: Charlottetown, PEI, Canada, 2009; p. 865. [Google Scholar]
- Dormann, C.F.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Gudrun, C.; Davies, R.G.; Hirzel, A.; Jetz, W.; Kissling, W.D.; et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer-Verlag: New York, NY, USA, 2002. [Google Scholar]
- Bouchard, C. Éco-épidémiologie de la maladie de Lyme dans le sud-ouest du québec. In Etude Des Facteurs Environnementaux Associés à Son Établissement; Université de Montréal: Saint Hyacinthe, QC, Canada, 2013. [Google Scholar]
- Ogden, N.H.; Mechai, S.; Margos, G. Changing geographic ranges of ticks and tick-borne pathogens: Drivers, mechanisms and consequences for pathogen diversity. Front. Cell. Infect. Microbiol. 2013, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dennis, D.T.; Nekomoto, T.S.; Victor, J.C.; Paul, W.S.; Piesman, J. Reported distribution of Ixodes scapularis and Ixodes pacificus (acari: Ixodidae) in the United States. J. Med. Entomol. 1998, 35, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Wong, D.; Shelus, V.; Eisen, R.J. What is the risk for exposure to vector-borne pathogens in United States national parks? J. Med. Entomol. 2013, 50, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Ostfeld, R.; Glass, G.E.; Keesing, F. Spatial epidemiology: An emerging (or re-emerging) discipline. Trends Ecol. Evol. 2005, 20, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Madhav, N.K.; Brownstein, J.S.; Tsao, J.I.; Fish, D. A dispersal model for the range expansion of blacklegged tick (acari: Ixodidae). J. Med. Entomol. 2004, 41, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A. Ecologic niche modeling and spatial patterns of disease transmission. Emerg. Infect. Dis. 2006, 12, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peavey, C.A.; Lane, R.S. Density and spatial distribution of Ixodes pacificus (acari: Ixodidae) in two recreational areas in north coastal California. Am. J. Trop. Med. Hyg. 2000, 62, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Bowen, G.S.; Lakat, M.F.; Parkin, W.E.; Shisler, J.K. Geographical distribution and density of Ixodes dammini (acari: Ixodidae) and relationship to Lyme disease transmission in New Jersey. Yale J. Biol. Med. 1984, 57, 669–675. [Google Scholar] [PubMed]
- Werden, L. Factors Affecting the Abundance of Blacklegged Ticks (Ixodes Scapularis) and the Prevalence of Borrelia Burgdorferi in Ticks and Small Mammals in the Thousand Islands Region; University of Guelph: Guelph, ON, Canada, 2012. [Google Scholar]
- Brinkerhoff, R.J.; Gilliam, W.F.; Gaines, D. Lyme disease, Virginia, USA, 2000–2011. Emerg. Infect. Dis. 2014, 20, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Rand, P.W.; Lubelczyk, C.; Lavigne, G.R.; Elias, S.; Holman, M.S.; Lacombe, E.H.; Smith, R.P., Jr. Deer density and the abundance of Ixodes scapularis (acari: Ixodidae). J. Med. Entomol. 2003, 40, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fons, F.; Gilbert, L. The role of deer as vehicles to move ticks, Ixodes ricinus, between contrasting habitats. Int. J. Parasitol. 2010, 40, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Swei, A.; Meentemeyer, R.; Briggs, C.J. Influence of abiotic and environmental factors on the density and infection prevalence of Ixodes pacificus (acari: Ixodidae) with Borrelia burgdorferi. J. Med. Entomol. 2011, 48, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zeman, P.; Benes, C. Spatial distribution of a population at risk: An important factor for understanding the recent rise in tick-borne diseases (Lyme borreliosis and tick-borne encephalitis in the Czech Republic). Ticks Tick-Borne Dis. 2013, 4, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Pfäffle, M.; Littwin, N.; Muders, S.V.; Petney, T.N. The ecology of tick-borne diseases. Int. J. Parasitol. 2013, 43, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Boeckmann, M.; Joyner, T.A. Old health risks in new places? An ecological niche model for I. ricinus tick distribution in Europe under a changing climate. Health Place 2014, 30, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Eisen, R.J.; Chang, C.C.; Mun, J.; Lane, R.S. Acarologic risk of exposure to Borrelia burgdorferi spirochaetes: Long-term evaluations in north-western California, with implications for lyme borreliosis risk-assessment models. Med. Vet. Entomol. 2004, 18, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Talleklint-Eisen, L.; Lane, R.S. Spatial and temporal variation in the density of Ixodes pacificus (acari: Ixodidae) nymphs. Environ. Entomol. 2000, 29, 272–280. [Google Scholar] [CrossRef]
- Boehnke, D.; Brugger, K.; Pfäffle, M.; Sebastian, P.; Norra, S.; Petney, T.; Oehme, R.; Littwin, N.; Lebl, K.; Raith, J.; et al. Estimating Ixodes ricinus densities on the landscape scale. Int. J. Health Geogr. 2015, 14, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z.; Halouzka, J.; Juricová, Z. Host-seeking activity of ixodid ticks in relation to weather variables. J. Vector Ecol. 2003, 28, 159–165. [Google Scholar] [PubMed]
- Schurich, J.A.; Kumar, S.; Eisen, L.; Moore, C.G. Modeling Culex tarsalis abundance on the northern Colorado Front Range using a landscape-level approach. J. Am. Mosq. Control Assoc. 2014, 30, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.-W.; Hildreth, M.B.; Vanroekel, D.L.; Wimberly, M.C. Weather and land cover influences on mosquito populations in Sioux Falls, South Dakota. J. Med. Entomol. 2011, 48, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Bigras-Poulin, M.; O’Callaghan, C.J.; Barker, I.K.; Lindsay, L.R.; Maarouf, A.; Smoyer-Tomic, K.E.; Waltner-Toews, D.; Charron, D. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int. J. Parasitol. 2005, 35, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Estrada-Sánchez, A.; Estrada-Sánchez, D. Methodological caveats in the environmental modelling and projections of climate niche for ticks, with examples for Ixodes ricinus (ixodidae). Vet. Parasitol. 2015, 208, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Barker, I.K.; Beauchamp, G.; Brazeau, S.; Charron, D.F.; Maarouf, A.; Morshed, M.G.; O’Callaghan, C.J.; Thompson, R.A.; Waltner-Toews, D.; et al. Investigation of ground level and remote-sensed data for habitat classification and prediction of survival of Ixodes scapularis in habitats of southeastern Canada. J. Med. Entomol. 2006, 43, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A.; Wilson, M.L.; Levine, J.F.; Piesman, J. Ecology of Ixodes dammini-borne human babesiosis and lyme disease. Annu. Rev. Entomol. 1985, 30, 439–460. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.D.M.; Taylor, J.L.; Randolph, S.E. Tick (Ixodes ricinus) abundance and seasonality at recreational sites in the UK: Hazards in relation to fine-scale habitat types revealed by complementary sampling methods. Ticks Tick-Borne Dis. 2011, 2, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Tack, W.; Madder, M.; de Frenne, P.; Vanhellemont, M.; Gruwez, R.; Verheyen, K. The effects of sampling method and vegetation type on the estimated abundance of Ixodes ricinus ticks in forests. Exp. Appl. Acarol. 2011, 54, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.P.; Lubelczyk, C.B.; Rand, P.W.; Lacombe, E.H.; Holman, M.S.; Smith, R.P.; Eleanor, H. Deer browse resistant exotic-invasive understory: An indicator of elevated human risk of exposure to Ixodes scapularis (acari: Ixodidae) in southern coastal Maine woodlands. J. Med. Entomol. 2006, 43, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hartemink, N.; Speybroeck, N.; Vanwambeke, S.O. Consequences of landscape fragmentation on Lyme disease risk: A cellular automata approach. PLoS ONE 2012, 7, e39612. [Google Scholar] [CrossRef] [PubMed]
- Werden, L.; Barker, I.K.; Bowman, J.; Gonzales, E.K.; Leighton, P.A.; Lindsay, L.R.; Jardine, C.M. Geography, deer, and host biodiversity shape the pattern of Lyme disease emergence in the thousand islands archipelago of Ontario, Canada. PLoS ONE 2014, 9, e85640. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.I.; White, P.C.L.; Critchley, C.H. Roe deer Capreolus capreolus behaviour affects density estimates from distance sampling surveys. Mamm. Rev. 2004, 34, 315–319. [Google Scholar] [CrossRef]
- Falco, R.C.; Fish, D. Potential for exposure to tick bites in recreational parks in a Lyme disease endemic area. Am. J. Public Health 1989, 79, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Adam-Poupart, A.; Milord, F.; Thivierge, K. Proposition D’un Programme Pour La Surveillance Intégrée De La Maladie De Lyme Et Des Autres Maladies Transmises Par La Tique Ixodes Scapularis Au Québec—Mise à jour 2015; Institut National de Santé Publique du Québec: Québec City, QC, Canada, 2015; p. 45. [Google Scholar]
- Ogden, N.H.; Koffi, J.K.; Lindsay, L.R. Assessment of a screening test to identify Lyme disease risk. Can. Commun. Dis. Rep. 2014, 40, 1–5. [Google Scholar]
- Daniels, T.J.; Falco, R.C.; Fish, D. Estimating population size and drag sampling efficiency for the blacklegged tick (acari: Ixodidae). J. Med. Entomol. 2000, 37, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Eisen, R.J. Using geographic information systems and decision support systems for the prediction, prevention, and control of vector-borne diseases. Annu. Rev. Entomol. 2011, 56, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Green, L.; Costero, A. Lyme disease. A Canadian perspective. Can. Fam. Phys. 1993, 39, 581–591. [Google Scholar]

| Scale | Explanatory Variable | Value | Min | 1st Qu. | Median | Mean | 3rd Qu. | Max | Source |
|---|---|---|---|---|---|---|---|---|---|
| Site (n = 50) | Sampling distance | Continuous (m) | 650 | 1612 | 1912 | 1794 | 2100 | 2600 | Field |
| Elevation | Continuous (m) | 13.58 | 49.21 | 61.69 | 94.39 | 116.29 | 384.00 | GPS | |
| Annual degree days > 0 °C | Continuous (°C) | 2132 | 3268 | 3331 | 3265 | 3370 | 3489 | [60] | |
| Total annual precipitation | Continuous (mm) | 506 | 848 | 933 | 940 | 1064 | 1258 | [60] | |
| Litter depth | Continuous (cm) | 1.00 | 3.00 | 4.00 | 3.76 | 5.00 | 8.00 | Field | |
| Percentage of canopy cover | Category | 0%: 0; 25%: 1; 75%: 24; 100%: 13 | Field | ||||||
| Percentage covered by ground vegetation | Category | 0%: 10; 25%: 15; 75%: 12; 100%: 9 | Field | ||||||
| Percentage covered by shrubs | Category | 0%: 18; 25%: 18; 75%: 9; 100%: 4 | Field | ||||||
| Percentage covered by trees | Category | 0%: 2; 25%: 18; 75%: 29; 100%: 1 | Field | ||||||
| Wetlands | Yes/No | Yes: 16; No: 34 | Field | ||||||
| Woody debris on forest floor | Yes/No | Yes: 20; No: 30 | Field | ||||||
| Season | Category | Spring: 11; Summer: 32; Autumn: 7 | Field | ||||||
| Plot (n = 63) | Elevation | Continuous (m) | 11.18 | 34.73 | 84.72 | 107.20 | 184.00 | 300.50 | GPS |
| Local temperature | Continuous (°C) | 15.17 | 19.32 | 22.79 | 22.79 | 25.91 | 32.49 | Data logger | |
| Local relative humidity | Continuous (%) | 42.65 | 55.22 | 63.16 | 65.74 | 77.65 | 88.55 | Data logger | |
| Width of trail | Continuous (m) | 1.40 | 2.30 | 3.10 | 3.02 | 3.60 | 6.30 | Field | |
| Type of trail | Category | Soil: 17; Wood chips: 2; Gravel/Asphalt: 44 | Field | ||||||
| Season | Category | Spring: 24; Summer: 39 | Field | ||||||
| Transect (n = 251) | Litter depth | Continuous (cm) | 0.00 | 1.00 | 2.00 | 2.81 | 4.00 | 12.00 | Field |
| Litter of leaves | Yes/No | Yes: 11; No: 240 | Field | ||||||
| Litter of conifer needles | Yes/No | Yes: 42; No: 209 | Field | ||||||
| No litter (bare soil) | Yes/No | Yes: 15; No: 236 | Field | ||||||
| Ground vegetation (e.g., grass) | Yes/No | Yes: 59; No: 192 | Field | ||||||
| Medium vegetation (e.g., ferns) | Yes/No | Yes: 69; No: 182 | Field | ||||||
| Tall vegetation (e.g., shrub) | Yes/No | Yes: 20; No: 231 | Field | ||||||
| Very tall vegetation (e.g., mature trees) | Yes/No | Yes: 246; No: 5 | Field | ||||||
| Unit | Year | Number of Units with Ticks/Total Number of Units | Sampling Area per Unit | Nymphs Collected /1000 m2 | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Min | Max | ||||
| Site | 2014 | 43/50 (86%) | 650 to 2600 m2 | 4.83 | 11.72 | 1.50 | 0 | 43.81 |
| Plot | 2013 | 45/63 (71%) | 400 m2 | 12.45 | 15.10 | 5.00 | 0 | 62.50 |
| Transect | 2013 | 133/251 (53%) | 100 m2 | 12.50 | 18.90 | 0.00 | 0 | 110.0 |
| Model 1 | Estimate | Std. Error | z Value | Pr (>|z|) |
|---|---|---|---|---|
| Intercept | −5.449 | 0.663 | −8.209 | <0.001 |
| Season | ||||
| Summer vs. Spring | −1.054 | 0.488 | −2.157 | 0.031 |
| Autumn vs. Spring | −1.589 | 0.678 | −2.343 | 0.019 |
| Autumn vs. Summer | −0.535 | 0.615 | −0.870 | 0.384 |
| Elevation * | 0.711 | 0.397 | 1.789 | 0.073 |
| Elevation2 * | −0.966 | 0.332 | −2.907 | 0.003 |
| Litter depth * | 0.460 | 0.196 | 2.341 | 0.019 |
| Autocovariate term | 0.001 | 0.0004 | 2.968 | 0.002 |
| Model 2 | Estimate | Std. Error | z Value | Pr (>|z|) |
|---|---|---|---|---|
| Intercept | 0.806 | 0.554 | 1.455 | 0.145 |
| Distance from trail * | ||||
| 20 m vs. 0 m | 0.519 | 0.190 | 2.730 | 0.006 |
| 40 m vs. 0 m | 0.435 | 0.193 | 2.249 | 0.024 |
| 60 m vs. 0 m | 0.624 | 0.187 | 3.331 | <0.001 |
| Type of trail | ||||
| wood chips vs. soil | −0.966 | 0.619 | −1.562 | 0.118 |
| gravel/asphalt vs. soil | −0.537 | 0.258 | −2.078 | 0.037 |
| gravel/asphalt vs. wood | 0.429 | 0.573 | 0.749 | 0.454 |
| Relative humidity ** | 0.212 | 0.110 | 1.925 | 0.054 |
| Relative humidity2 ** | −0.267 | 0.109 | −2.441 | 0.014 |
| Season | ||||
| summer vs. spring | −0.413 | 0.189 | −2.182 | 0.029 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ripoche, M.; Lindsay, L.R.; Ludwig, A.; Ogden, N.H.; Thivierge, K.; Leighton, P.A. Multi-Scale Clustering of Lyme Disease Risk at the Expanding Leading Edge of the Range of Ixodes scapularis in Canada. Int. J. Environ. Res. Public Health 2018, 15, 603. https://doi.org/10.3390/ijerph15040603
Ripoche M, Lindsay LR, Ludwig A, Ogden NH, Thivierge K, Leighton PA. Multi-Scale Clustering of Lyme Disease Risk at the Expanding Leading Edge of the Range of Ixodes scapularis in Canada. International Journal of Environmental Research and Public Health. 2018; 15(4):603. https://doi.org/10.3390/ijerph15040603
Chicago/Turabian StyleRipoche, Marion, Leslie Robbin Lindsay, Antoinette Ludwig, Nicholas H. Ogden, Karine Thivierge, and Patrick A. Leighton. 2018. "Multi-Scale Clustering of Lyme Disease Risk at the Expanding Leading Edge of the Range of Ixodes scapularis in Canada" International Journal of Environmental Research and Public Health 15, no. 4: 603. https://doi.org/10.3390/ijerph15040603





