The Burden of COPD Morbidity Attributable to the Interaction between Ambient Air Pollution and Temperature in Chengdu, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Statistical Methods
2.3.1. Core Model Development
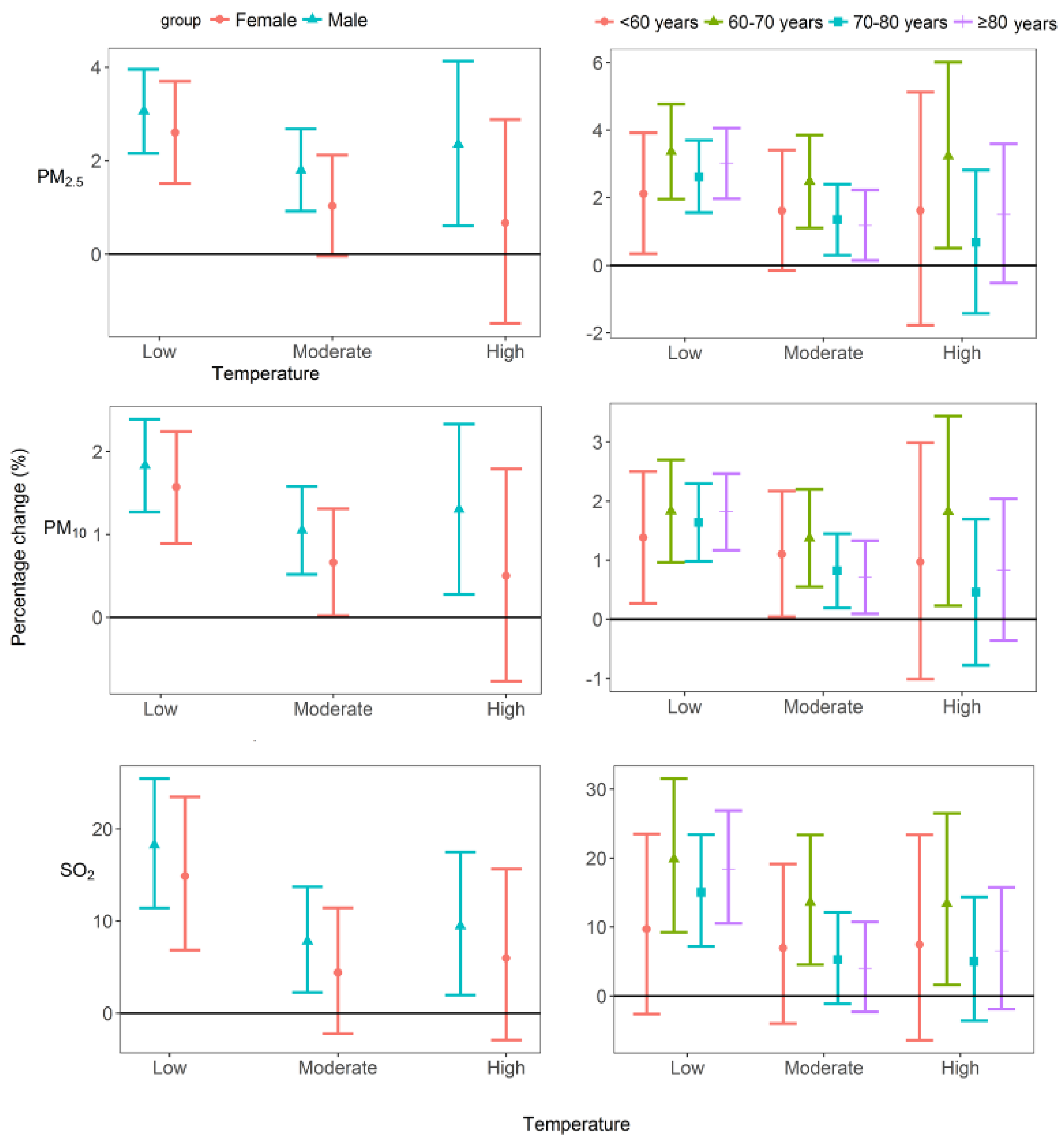
2.3.2. Analysis of Individual Effects
2.3.3. Analysis of Interaction Effects
2.3.4. Estimating COPD Morbidity Burden
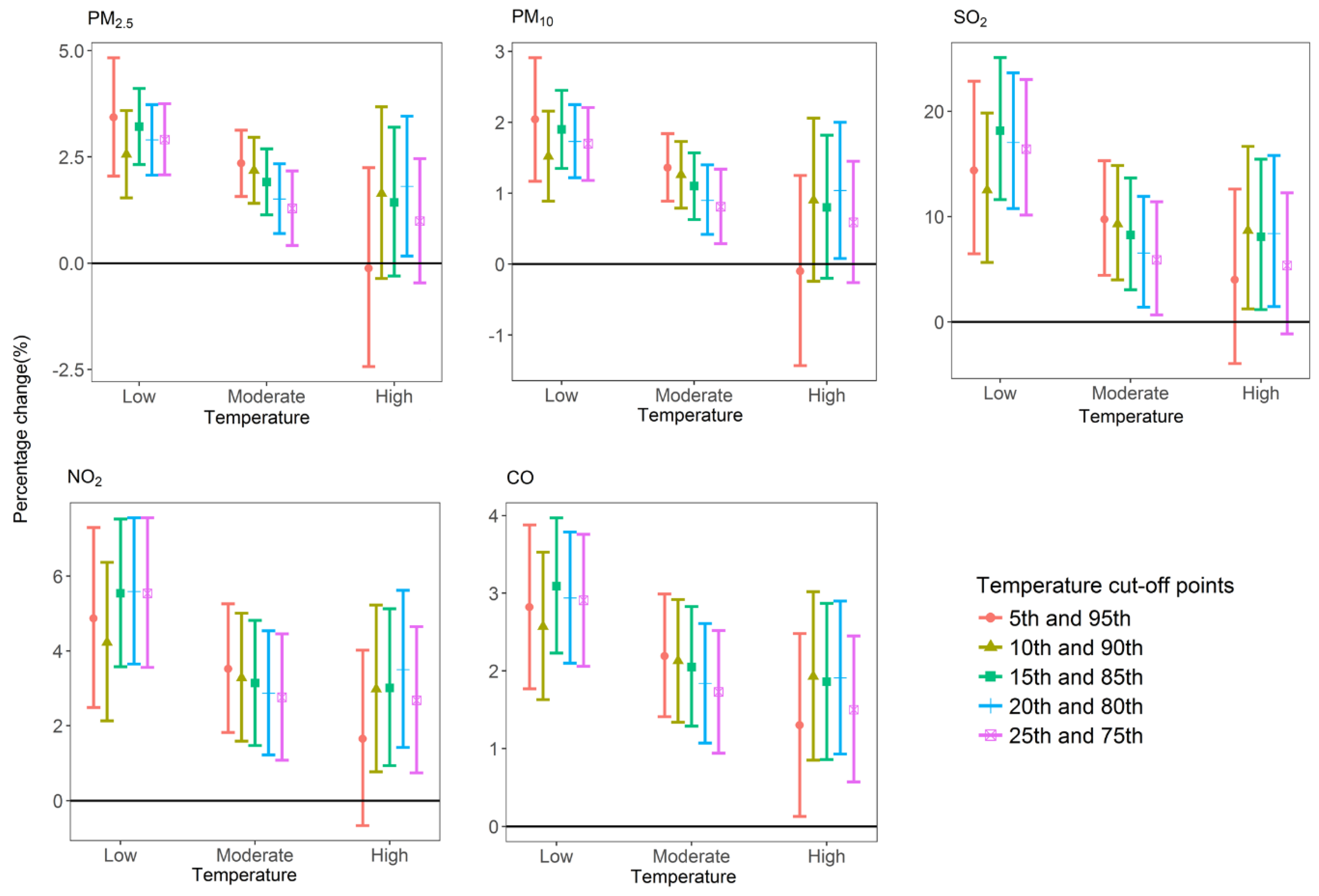
2.3.5. Sensitivity Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buist, A.S.; McBurnie, M.A.; Vollmer, W.M.; Gillespie, S.; Burney, P.; Mannino, D.M.; Menezes, A.M.; Sullivan, S.D.; Lee, T.A.; Weiss, K.B.; et al. International variation in the prevalence of COPD (the BOLD Study): A population-based prevalence study. Lancet 2007, 370, 741–750. [Google Scholar] [CrossRef]
- Zhong, N.; Wang, C.; Yao, W.; Chen, P.; Kang, J.; Huang, S.; Chen, B.; Wang, C.; Ni, D.; Zhou, Y.; et al. Prevalence of chronic obstructive pulmonary disease in China: A large, population-based survey. Am. J. Respir. Crit. Care Med. 2007, 176, 753–760. [Google Scholar] [CrossRef] [PubMed]
- WHO, Media Centre: CHORONIC Obstrutive Pulmonary Disease (COPD). 2017. Available online: http://www.who.int/mediacentre/factsheets/fs315/en/ (accessed on 29 December 2017).
- Yin, P.; Wang, H.; Vos, T.; Li, Y.; Liu, S.; Liu, Y.; Liu, J.; Wang, L.; Naghavi, M.; Murray, C.J.; et al. A Subnational Analysis of Mortality and Prevalence of COPD in China from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Chest 2016, 150, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Fan, L.C.; Mao, B.; Yang, J.W.; Choi, A.M.K.; Cao, W.J.; Xu, J.F. Short-term Exposure to Ambient Fine Particulate Matter Increases Hospitalizations and Mortality in COPD: A Systematic Review and Meta-analysis. Chest 2016, 149, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Eisner, M.D.; Anthonisen, N.; Coultas, D.; Kuenzli, N.; Perez-Padilla, R.; Postma, D.; Romieu, I.; Silverman, E.K.; Balmes, J.R. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 693–718. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.S.; Barnes, P.J. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009, 374, 733–743. [Google Scholar] [CrossRef]
- Li, G.; Sun, J.; Jayasinghe, R.; Pan, X.C.; Zhou, M.; Wang, X.; Cai, Y.; Sadler, R.; Shaw, G.R. Temperature Modifies the Effects of Particulate Matter on Non-Accidental Mortality: A Comparative Study of Beijing, China and Brisbane, Australia. Public Health Res. 2012, 2, 21–27. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chau, T.T. An Association between Air Pollution and Daily Outpatient Visits for Respiratory Disease in a Heavy Industry Area. PLoS ONE 2013, 8, e75220. [Google Scholar] [CrossRef] [PubMed]
- Halonen, J.I.; Lanki, T.; Yli-Tuomi, T.; Kulmala, M.; Tiittanen, P.; Pekkanen, J. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax 2008, 63, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Guo, S.E.; Chi, M.C.; Chou, C.T.; Lin, Y.C.; Lin, C.M.; Chou, Y.L. Association between Atmospheric Fine Particulate Matter and Hospital Admissions for Chronic Obstructive Pulmonary Disease in Southwestern Taiwan: A Population-Based Study. Int. J. Environ. Res. Public Health 2016, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, W.; Li, S.; Zhang, Y.; Williams, G.; Huxley, R.; Ren, H.; Cao, W.; Guo, Y. The impact of ambient fine particles on influenza transmission and the modification effects of temperature in China: A multi-city study. Environ. Int. 2017, 98, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Goggins, W.B.; Chan, E.Y.Y.; Ng, E.; Ren, C.; Chen, L. Effect Modification of the Association between Short-term Meteorological Factors and Mortality by Urban Heat Islands in Hong Kong. PLoS ONE 2012, 7, e38551. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Woodward, A.; Hou, X.Y.; Zhu, T.; Zhang, J.; Brown, H.; Yang, J.; Qin, R.; Gao, J.; Gu, S. Modification of the effects of air pollutants on mortality by temperature: A systematic review and meta-analysis. Sci. Total Environ. 2017, 575, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.X.; Xiao, C.; Zhu, Y.; Li, J.; Yang, J.; Gu, S.; Xia, J.; Su, B.; Liu, Q.; Woodward, A. The interactive effects between high temperature and air pollution on mortality: A time-series analysis in Hefei, China. Sci. Total Environ. 2016, 575, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.H.; Wang, G.S.; Guo, Y.L.; Chang, S.C.; Wan, G.H. Urban air pollution and meteorological factors affect emergency department visits of elderly patients with chronic obstructive pulmonary disease in Taiwan. Environ. Pollut. 2017, 224, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.W.; Tam, W.; Wong, T.W.; Chan, D.P.; Tung, A.H.; Lai, C.K.; Hui, D.S. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax 2007, 62, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Yu, I.T.S.; Wang, X.; Tian, L.; Tse, L.A.; Wong, T.W. Season and humidity dependence of the effects of air pollution on COPD hospitalizations in Hong Kong. Atmos. Environ. 2013, 76, 74–80. [Google Scholar] [CrossRef]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Walker, J.; Samet, J.M.; Zeger, S.L.; Dominici, F. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 U.S. counties, 1999–2005. Am. J. Epidemiol. 2008, 168, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Chen, W.; Pan, J.J.; Duan, Z.Q.; Han, X.; Tan Kun, C.W.; Pan, J.J.; Duan, Z.Q.; Han, X. Analysis on medical record first page data quality from Sichuan health statistics reporting system. Soft Sci. Health 2015, 29, 637–641. [Google Scholar]
- Dockery, D.W.; Luttmann-Gibson, H.; Rich, D.Q.; Link, M.S.; Mittleman, M.A.; Gold, D.R.; Koutrakis, P.; Schwartz, J.D.; Verrier, R.L. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ. Health Perspect. 2005, 113, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, G.; Zhao, D.; Xie, X.; Wei, Z.; Wang, W.; Wang, M.; Li, G.; Liu, W.; Sun, J. Relationship between fine particulate air pollution and ischaemic heart disease morbidity and mortality. Heart 2015, 101, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xiang, X.; Juan, J.; Sun, K.; Song, J.; Cao, Y.; Hu, Y. Fine particulate air pollution and hospital visits for asthma in Beijing, China. Environ. Pollut. 2017, 230, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Di, Q.; Dai, L.; Wang, Y.; Zanobetti, A.; Choirat, C.; Schwartz, J.D.; Dominici, F. Association of Short-term Exposure to Air Pollution With Mortality in Older Adults. JAMA 2017, 318, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environmental Protection of the People’s Republic of China, Ambient Air Quality Standards. 2016. Available online: http://kjs.mep.gov.cn/hjbhbz/bzwb/dqhjbh/dqhjzlbz/201203/t20120302_224165.htm (accessed on 10 January 2017).
- Junninen, H.; Niska, H.; Tuppurainen, K.; Ruuskanen, J.; Kolehmainen, M. Methods for imputation of missing values in air quality data sets. Atmos. Environ. 2004, 38, 2895–2907. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, S.; Yu, Q.; Huo, X.; Ming, X.; Wang, J.; Zhou, Y.; Peng, Z.; Zhang, H.; Cui, X. Short-term effects of ambient air pollution on pediatric outpatient visits for respiratory diseases in Yichang city, China. Environ. Pollut. 2017, 227, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Shukri, Y.A.; Noraziana, M.N.; Al, A.M.M. Estimation of missing values in air pollution data using single imputation techniques. Scienceasia 2008, 34, 341–345. [Google Scholar]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Li, N.; Wang, Z.; Liu, Y.; Chen, X.; Chu, Y.; Li, X.; Zhu, Z.; Tian, L.; Xiang, H. The short-term effects of air pollutants on respiratory disease mortality in Wuhan, China: Comparison of time-series and case-crossover analyses. Sci. Rep. 2017, 7, 40482. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D. Statistical Methods for Environmental Epidemiology with R; Springer: New York, NY, USA, 2008. [Google Scholar]
- Gu, Y.; Lin, H.; Liu, T.; Xiao, J.; Zeng, W.; Li, Z.; Lv, X.; Ma, W. The Interaction between Ambient PM10 and NO2 on Mortality in Guangzhou, China. Int. J. Environ. Res. Public Health 2017, 14, 1381. [Google Scholar] [CrossRef] [PubMed]
- WHO, Ambient (Outdoor) Air Quality and health. 2014. Available online: http://www.who.int/mediacentre/factsheets/fs313/en/ (accessed on 29 January 2017).
- Li, L.; Yang, J.; Song, Y.F.; Chen, P.Y.; Ou, C.Q. The burden of COPD mortality due to ambient air pollution in Guangzhou, China. Sci. Rep. 2016, 6, 25900. [Google Scholar] [CrossRef] [PubMed]
- Olivares, E.; Wagner, V. China: Air Quality Standards. Available online: http://transportpolicy.net/index.php?title=China:_Air_Quality_Standards (accessed on 29 January 2016).
- Evans, J.; Van, D.A.; Martin, R.V.; Burnett, R.; Rainham, D.G.; Birkett, N.J.; Krewski, D. Estimates of global mortality attributable to particulate air pollution using satellite imagery. Environ. Res. 2013, 120, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Hwang, S.A.; Kinney, P.L.; Lin, S. Seasonal and temperature modifications of the association between fine particulate air pollution and cardiovascular hospitalization in New York state. Sci. Total Environ. 2017, 578, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.C.; Lee, J.T.; Kim, H.; Ha, E.H.; Schwartz, J.; Christiani, D.C. Effects of air pollutants on acute stroke mortality. Environ. Health Perspect. 2002, 110, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Carraway, M.S.; Madden, M.C. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J. Toxicol. Environ. Health Part B Crit. Rev. 2012, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.S.; Chang, C.C.; Yang, C.Y. Fine particulate air pollution and hospital admissions for chronic obstructive pulmonary disease: A case-crossover study in Taipei. Int. J. Environ. Res. Public Health 2013, 10, 6015–6026. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, L.; Engling, G.; Zhang, R.; Yang, Y.; Cao, J.; Zhu, C.; Wang, Q.; Luo, L. Chemical composition of PM 2.5 in an urban environment in Chengdu, China: Importance of springtime dust storms and biomass burning. Atmos. Res. 2013, 122, 270–283. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Cao, J.; Hsu, S.C.; Xia, X.; Zhang, Z.; Lin, Z.; Cheng, T.; Zhang, R. Characterization and source apportionment of aerosol light extinction in Chengdu, southwest China. Atmos. Environ. 2014, 95, 552–562. [Google Scholar] [CrossRef]
- Qiao, X.; Jaffe, D.; Tang, Y.; Bresnahan, M.; Song, J. Evaluation of air quality in Chengdu, Sichuan Basin, China: Are China’s air quality standards sufficient yet? Environ. Monit. Assess.t 2015, 187, 250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, S.; Lang, L.; Huang, C.; Ma, W.; Lin, H. Ambient fine and coarse particulate matter pollution and respiratory morbidity in Dongguan, China. Environ. Pollut. 2017, 222, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, R.; Wang, W.; Xu, X.; Ha, S.; Kan, H. Does ambient CO have protective effect for COPD patient? Environ. Res. 2015, 136, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Traffic Management Bureau of the Public Security Ministry. For the First Time, the Number of Cars Surpassed 200 Million with Over 360 Million Drivers. 2017. Available online: http://www.mps.gov.cn/n2255040/n4908728/c5682934/content.html (assessed on 17 May 2017).
- Yang, G.H.; Ma, J.M.; Liu, N.; Zhou, L.N. Smoking and passive smoking in Chinese, 2002. Chin. J. Epidemiol. 2005, 26, 77–83. [Google Scholar]
- Chen, G.; Zhang, Y.; Zhang, W.; Li, S.; Williams, G.; Marks, G.B.; Jalaludin, B.; Abramson, M.J.; Luo, F.; Yang, D. Attributable risks of emergency hospital visits due to air pollutants in China: A multi-city study. Environ. Pollut. 2017, 228, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Wang, S.; Ai, J.; Gui, K.; Duan, B.; Zhao, Q.; Zhang, X.; Jiang, W.; Sun, Y. Heavy pollution episodes, transport pathways and potential sources of PM2.5 during the winter of 2013 in Chengdu (China). Sci. Total Environ. 2017, 584–585, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Zeger, S.L.; Thomas, D.; Dominici, F.; Samet, J.M.; Schwartz, J.; Dockery, D.; Cohen, A. Exposure measurement error in time-series studies of air pollution: Concepts and consequences. Environ. Health Perspect. 2000, 108, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.P.; Yu, G.P.; Liu, H.; Ma, X.M.; Wang, J.; Kong, G.L.; Li, Y.; Ma, W.; Cui, Y.; Xu, B.; et al. Control costs, enhance quality, and increase revenue in three top general public hospitals in Beijing, China. PLoS ONE 2013, 8, e72166. [Google Scholar] [CrossRef] [PubMed]

| Mean ± SD | Minimum | Percentiles | Maximum | |||
|---|---|---|---|---|---|---|
| 25 | 50 | 75 | ||||
| Daily COPD HAs (n) | 75 ± 32 | 15 | 52 | 70 | 94 | 194 |
| COPD HAs by sex (n) | ||||||
| male | 48 ± 21 | 9 | 33 | 45 | 62 | 126 |
| female | 27 ± 13 | 3 | 18 | 25 | 33 | 86 |
| COPD HAs by age (n) | ||||||
| ≥80 | 29 ± 13 | 2 | 19 | 27 | 37 | 88 |
| 70–80 | 25 ± 12 | 4 | 17 | 23 | 32 | 69 |
| 60–70 | 15 ± 7 | 1 | 9 | 13 | 19 | 43 |
| <60 | 6 ± 3 | 1 | 4 | 6 | 8 | 21 |
| Air pollution levels * | ||||||
| PM2.5 (μg/m3 ) | 57.29 ± 36.75 | 7.33 | 30.62 | 46.48 | 73.52 | 232.45 |
| PM10 (μg/m3 ) | 94.73 ± 57.07 | 12.77 | 53.43 | 77.50 | 124.09 | 339.20 |
| SO2 (μg/m3 ) | 13.80 ± 5.61 | 3.53 | 9.56 | 12.75 | 16.94 | 34.83 |
| NO2 (μg/m3 ) | 50.49 ± 15.21 | 13.86 | 39.22 | 48.05 | 59.60 | 105.74 |
| O3 (μg/m3 ) | 96.73 ± 55.77 | 5.60 | 53.00 | 86.20 | 136.60 | 290.40 |
| CO (mg/m3 ) | 1.07 ± 0.35 | 0.40 | 0.82 | 0.99 | 1.23 | 2.69 |
| Meteorological measures | ||||||
| Temperature (°C) | 16.99 ± 7.10 | −1.10 | 10.40 | 18.00 | 23.00 | 30.20 |
| Relative Humidity (%) | 80.46 ± 8.87 | 42.98 | 74.64 | 80.80 | 87.47 | 98.30 |
| Pollutants § | Temperature a | ||
|---|---|---|---|
| Low | Moderate | High | |
| PM2.5 | 2.90 (2.07, 3.73) * | 1.51 (0.70, 2.34) * | 1.81 (0.17, 3.46) * |
| +SO2 | 2.68 (1.83, 3.54) * | 1.26 (0.41, 2.11) * | 1.56 (−0.09, 3.23) |
| +NO2 | 2.53 (1.67, 3.39) * | 1.11 (0.26, 1.96) * | 1.21 (−0.45, 2.89) |
| +CO | 2.25 (1.37, 3.13) * | 0.81 (−0.06, 1.69) | 0.76 (−0.93, 2.46) |
| PM10 | 1.73 (1.22, 2.25) * | 0.90 (0.42, 1.40) * | 1.04 (0.08, 2.00) * |
| +SO2 | 1.60 (1.06, 2.13) * | 0.74 (0.22, 1.26) * | 0.89 (−0.08, 1.86) |
| +CO | 1.31 (0.75, 1.86) * | 0.46 (−0.08, 1.00) | 0.42 (−0.57, 1.43) |
| SO2 | 17.04 (10.77, 23.66) * | 6.54 (1.41, 11.93) * | 8.40 (1.46, 15.82) * |
| +PM2.5 | 11.22 (4.70, 18.14) * | 1.79 (−3.58, 7.47) | 2.80 (−4.23, 10.36) |
| +PM10 | 11.54 (5.08, 18.39) * | 1.80 (−3.55, 7.46) | 2.74 (−4.29, 10.28) |
| +NO2 | 13.14 (6.81, 19.85) * | 2.76 (−2.51, 8.32) | 3.87 (−3.07, 11.30) |
| +CO | 11.83 (5.59, 18.44) * | 1.77 (−3.41, 7.24) | 2.48 (−4.40, 9.85) |
| NO2 | 5.59 (3.65, 7.56) * | 2.87 (1.22, 4.54) * | 3.50 (1.42, 5.62) * |
| +PM2.5 | 3.42 (1.17, 5.72) * | 0.94 (−1.00, 2.92) | 1.35 (−1.01, 3.76) |
| +SO2 | 4.82 (2.70, 6.98) * | 2.10 (0.24, 4.00) * | 2.73 (0.48, 5.02) * |
| +CO | 3.48 (1.37, 5.64) * | 0.89 (−0.96, 2.77) | 1.19 (−1.08, 3.52) |
| CO # | 2.94 (2.10, 3.79) * | 1.84 (1.07, 2.61) * | 1.91 (0.93, 2.90) * |
| +PM2.5 | 2.30 (1.38, 3.22) * | 1.25 (0.41, 2.10) * | 1.25 (0.20, 2.31) * |
| +PM10 | 2.34 (1.43, 3.25) * | 1.26 (0.43, 2.11) * | 1.26 (0.21, 2.31) * |
| +SO2 | 2.69 (1.81, 3.58) * | 1.57 (0.75, 2.40) * | 1.64 (0.62, 2.67) * |
| +NO2 | 2.51 (1.64, 3.39) * | 1.38 (0.57, 2.20) * | 1.39 (0.37, 2.42) * |
| Target Levels (μg/m3 ) * | Temperature Level | PM2.5 | PM10 | SO2 | |||
|---|---|---|---|---|---|---|---|
| AF (%, 95% CI) | AN (No., 95% CI) | AF (%, 95% CI) | AN (No., 95% CI) | AF (%, 95% CI) | AN (No., 95% CI) | ||
| WHO (25, 50, 20) | Low | 17.30 (12.39, 22.19) | 2260 (1619, 2900) | 14.72 (10.38, 19.06) | 1939 (1368, 2511) | 1.14 (0.74, 1.54) | 150 (98, 203) |
| Moderate | 4.77 (2.21, 7.33) | 1533 (710, 2356) | 4.16 (1.92, 6.41) | 1338 (616, 2060) | 0.08 (0.02, 0.14) | 25 (5, 44) | |
| High | 2.10 (0.21, 4.00) | 194 (19, 369) | 1.45 (0.12, 2.80) | 134 (11, 258) | 0 (0, 0) | 0 (0, 0) | |
| Overall | 7.33 (4.31, 10.34) | 3987 (2348, 5625) | 6.26 (3.66, 8.86) | 3411 (1995, 4829) | 0.32 (0.19, 0.45) | 175 (103, 247) | |
| China grade II (75, 150, 150) | Low | 5.89 (4.22, 7.57) | 770 (552, 989) | 3.35 (2.36, 4.33) | 441 (311, 570) | 0 (0, 0) | 0 (0, 0) |
| Moderate | 0.64 (0.30, 0.99) | 206 (96, 317) | 0.28 (0.13, 0.44) | 91 (42, 140) | 0 (0, 0) | 0 (0, 0) | |
| High | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | |
| Overall | 1.79 (1.19, 2.40) | 976 (648, 1306) | 0.98 (0.65, 1.30) | 532 (353, 710) | 0 (0, 0) | 0 (0, 0) | |
| 50% China grade II (37.5, 75,75) | Low | 13.73 (9.84, 17.62) | 1794 (1286, 2303) | 10.60 (7.48, 13.73) | 1397 (986, 1809) | 0 (0, 0) | 0 (0, 0) |
| Moderate | 3.08 (1.43, 4.74) | 991 (459, 1523) | 2.41 (1.11, 3.71) | 774 (356, 1192) | 0 (0, 0) | 0 (0, 0) | |
| High | 0.65 (0.07, 1.24) | 60 (6, 114) | 0.31 (0.02, 0.60) | 29 (2, 55) | 0 (0, 0) | 0 (0, 0) | |
| Overall | 5.23 (3.22, 7.24) | 2845 (1751, 3940) | 4.03 (2.46, 5.60) | 2200 (1344, 3056) | 0 (0, 0) | 0 (0, 0) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, H.; Tan, K.; Long, F.; Wang, L.; Yu, H.; Deng, R.; Long, H.; Zhang, Y.; Pan, J. The Burden of COPD Morbidity Attributable to the Interaction between Ambient Air Pollution and Temperature in Chengdu, China. Int. J. Environ. Res. Public Health 2018, 15, 492. https://doi.org/10.3390/ijerph15030492
Qiu H, Tan K, Long F, Wang L, Yu H, Deng R, Long H, Zhang Y, Pan J. The Burden of COPD Morbidity Attributable to the Interaction between Ambient Air Pollution and Temperature in Chengdu, China. International Journal of Environmental Research and Public Health. 2018; 15(3):492. https://doi.org/10.3390/ijerph15030492
Chicago/Turabian StyleQiu, Hang, Kun Tan, Feiyu Long, Liya Wang, Haiyan Yu, Ren Deng, Hu Long, Yanlong Zhang, and Jingping Pan. 2018. "The Burden of COPD Morbidity Attributable to the Interaction between Ambient Air Pollution and Temperature in Chengdu, China" International Journal of Environmental Research and Public Health 15, no. 3: 492. https://doi.org/10.3390/ijerph15030492





