Wetlands and Malaria in the Amazon: Guidelines for the Use of Synthetic Aperture Radar Remote-Sensing
Abstract
:1. Background of the Study
2. Purpose of the Study
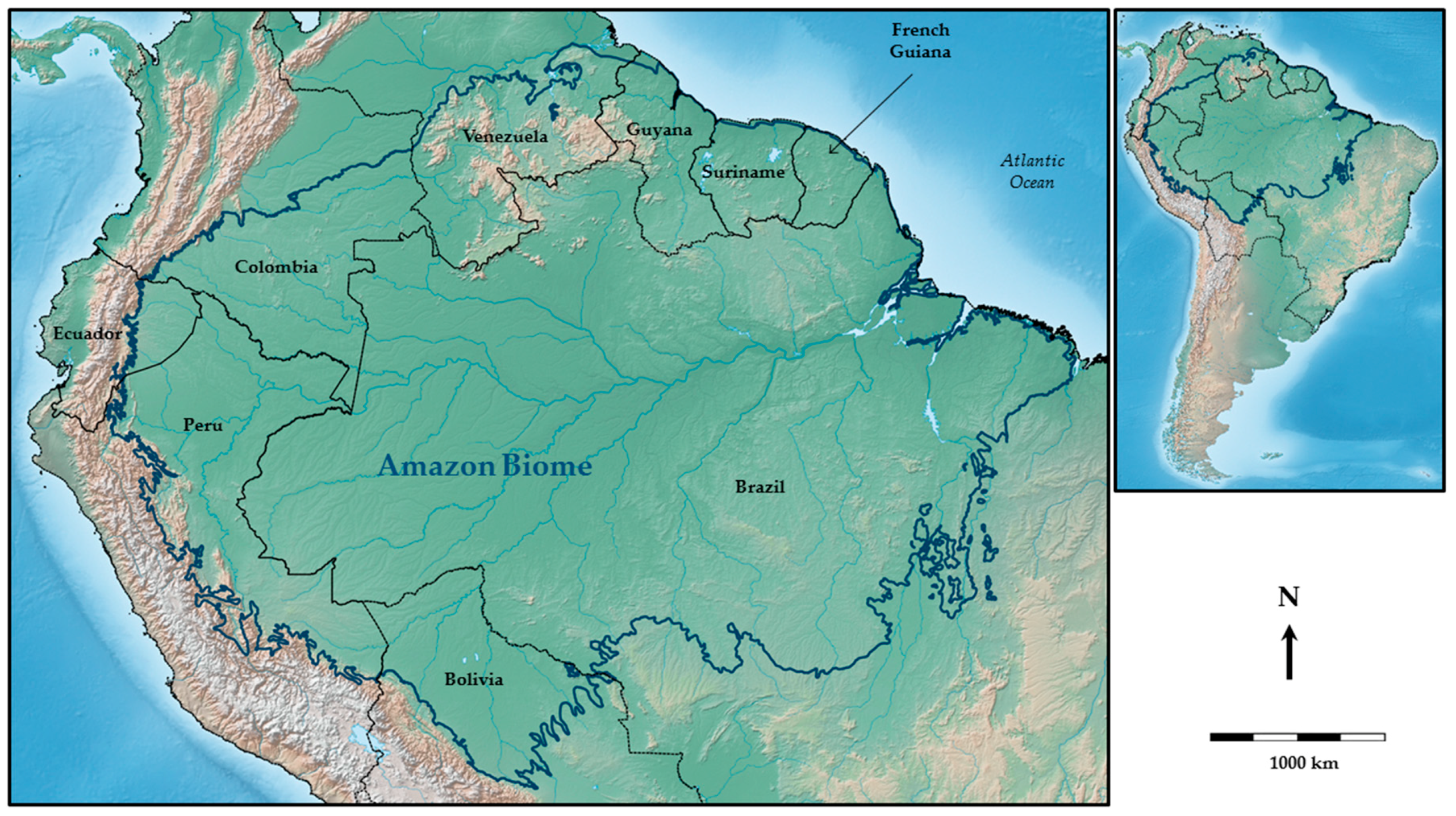
3. Study Area: The Amazon Region
4. Role of Wetlands in Malaria Epidemiology
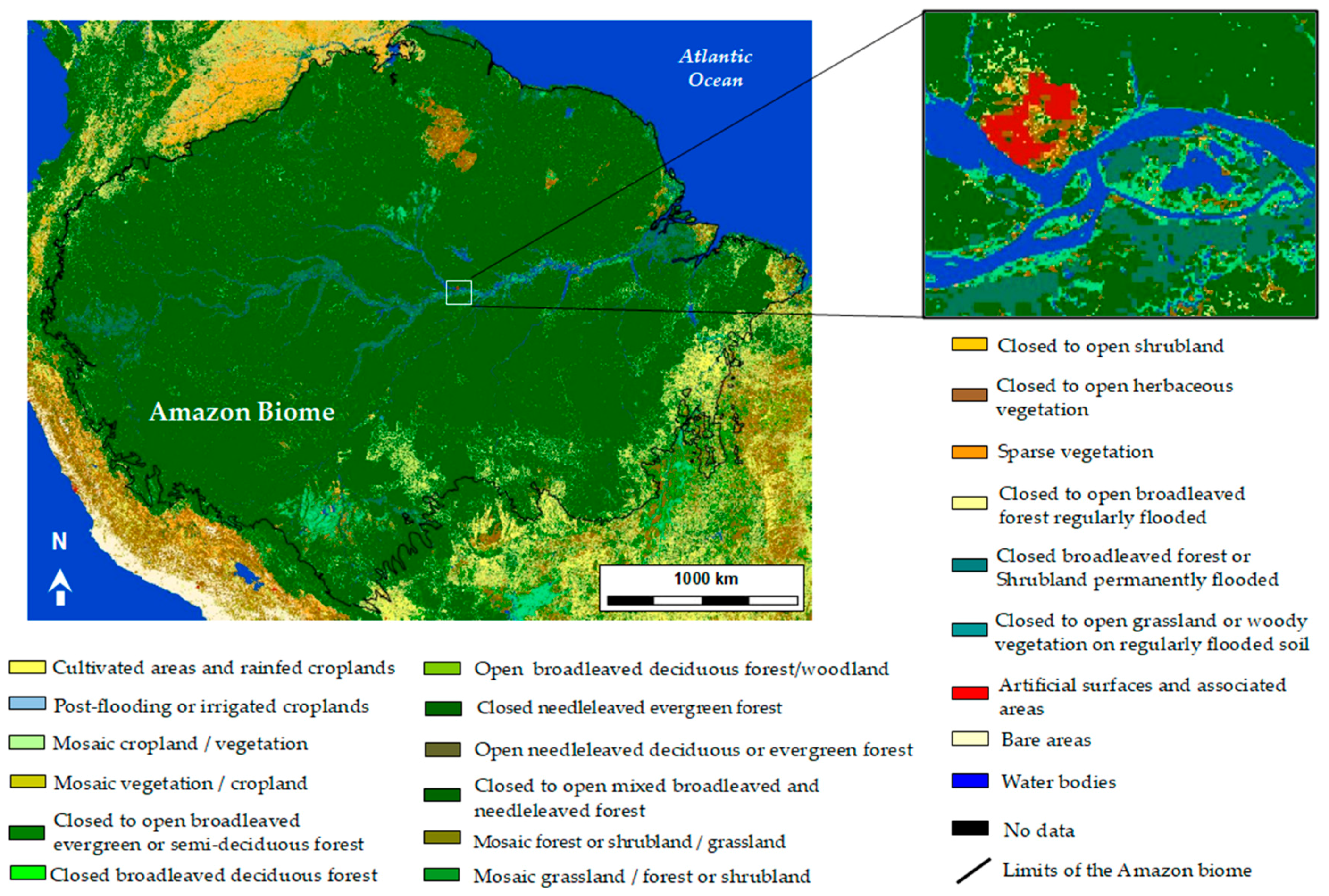
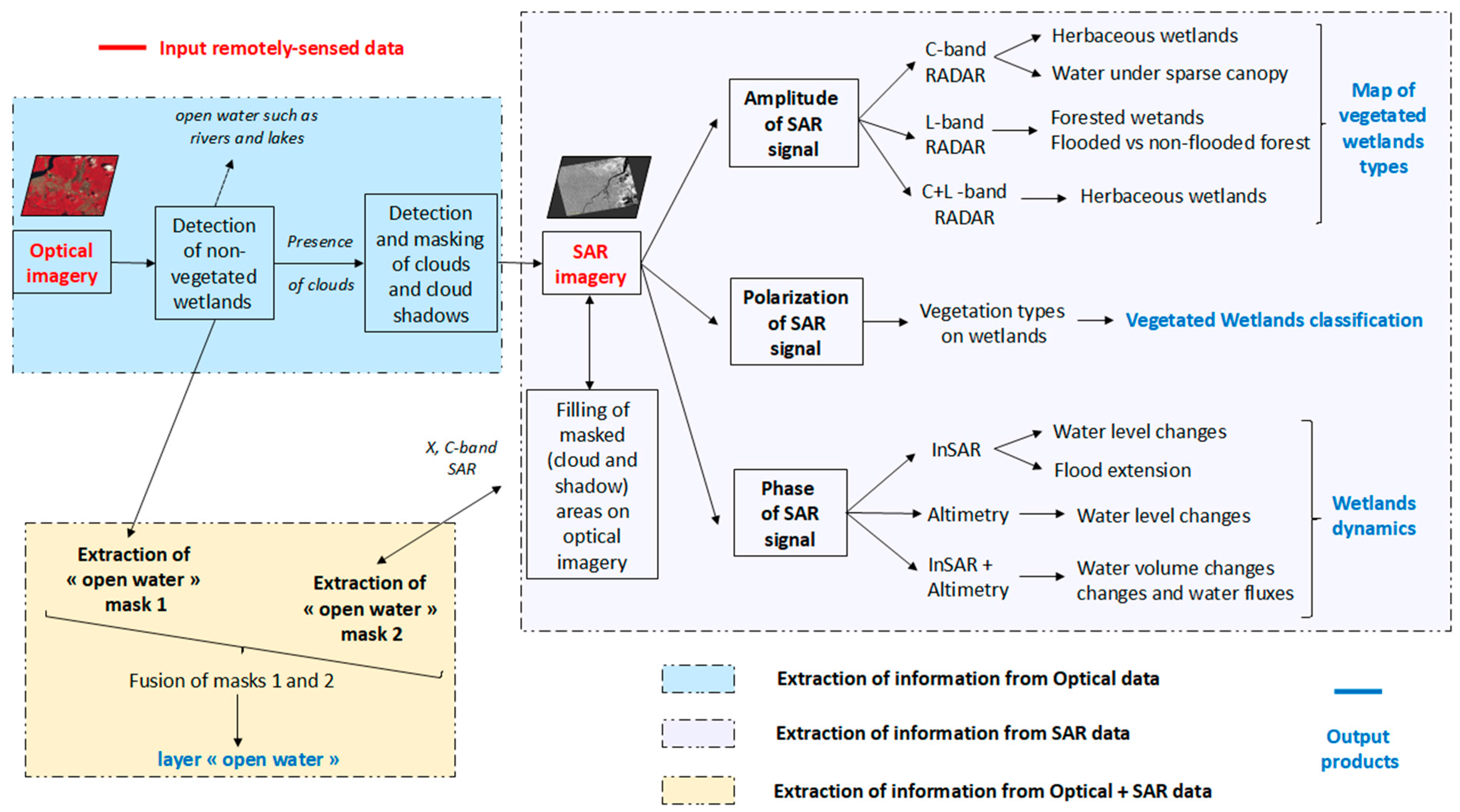
5. Remote-Sensing for the Classification of Wetlands in the Amazon
5.1. SAR Remote-Sensing for the Characterization of Wetlands
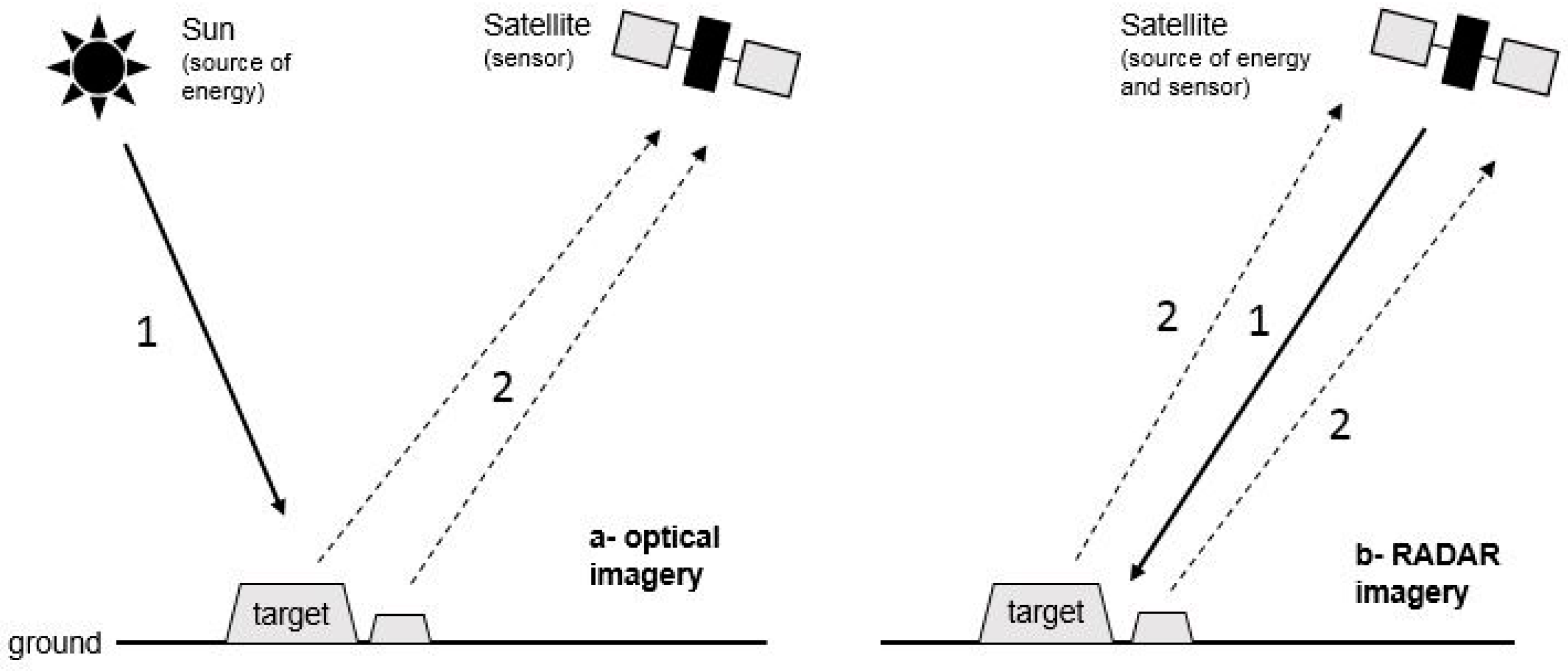
5.1.1. Synthetic Aperture Radar: Theoretical Considerations
- HH—for horizontal transmit and horizontal receive,
- VV—for vertical transmit and vertical receive,
- HV—for horizontal transmit and vertical receive, and
- VH—for vertical transmit and horizontal receive.
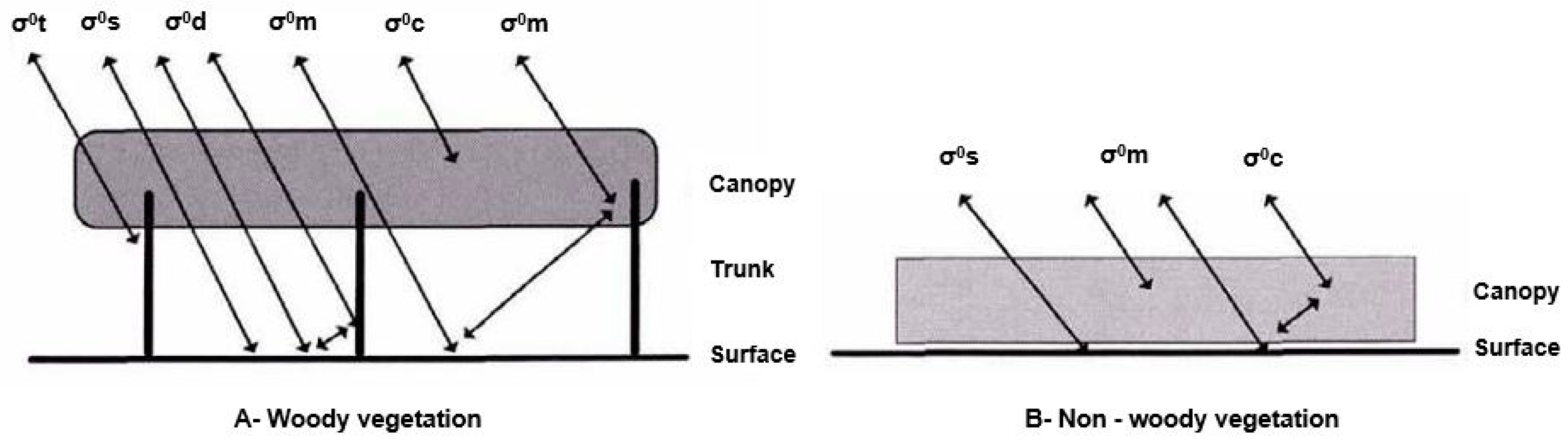
5.1.2. SAR and Wetland Typologies
Detection of Free Water by SAR Sensors
Detection of Water Covered in Herbaceous Vegetation
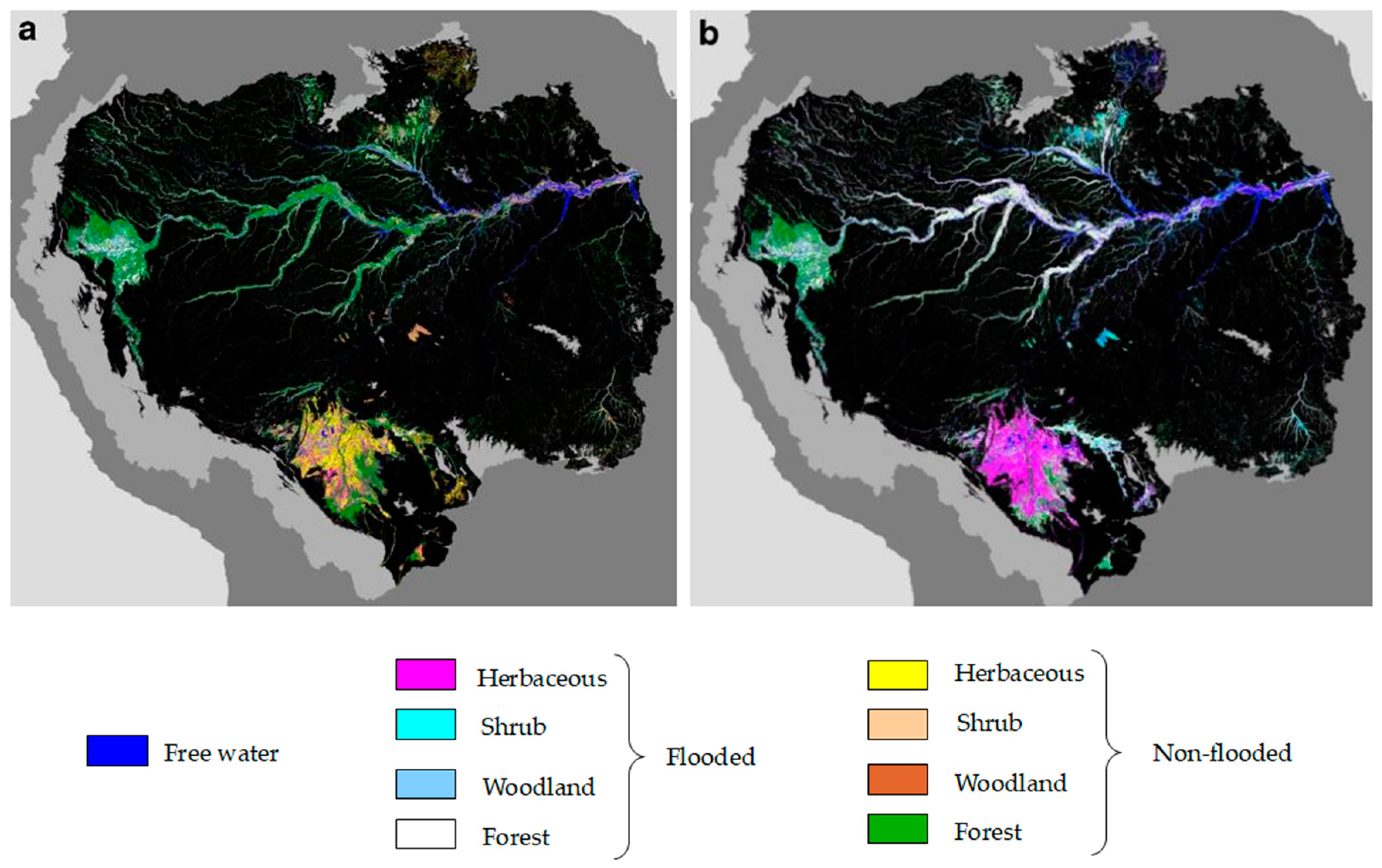
Detection of Water under Forest Canopy
Discriminate Wetland Classes Using SAR
Monitor Flooding Conditions and Water Level Variations in Wetlands Using SAR
Summary of Recommendations for the Use of SAR to Characterize Wetlands
5.2. SAR for Wetlzand Classification in the Amazon Region
6. SAR Remote-Sensing of Wetlands and Malaria Control in the Amazon
6.1. Previous Studies
6.2. From Wetland Characterization to Malaria Exposure Risk in the Tropics
- (1)
- Producing multi-temporal maps of wetlands and water collections is of prime importance in order to identify potential suitable habitats for the vectors of malaria (the hazard component of malaria risk). The identification of various types of water collections combined with exhaustive field sampling allows us to discriminate the types of wetlands more adapted to specific species of anopheles vectors [12]. Updating those maps over time, especially given the changes occurring between dry season and rainy season in tropical regions (changes on vegetation cover or in water extent, leaving extensive moist soils and fragmented pools when the wetland dries up), is primordial in order to survey how the geographical distribution of larval reservoirs evolves over time. Once potential larval reservoirs have been identified, the estimation of habitat suitability for vectors can be carried out through species distribution models for example [85]. Together with wetland mapping and the evolution of wetlands in space and time, the definition of vector control strategies relies on the estimation of wetlands’ larval production. This step, based on field sampling, is primordial in order to correctly assess the role of wetlands in malaria risk. The seasonality of environmental factors estimated using remotely sensed data echoes in the predictions of malaria seasonality (the combination of disease risk in space and time).
- (2)
- Water level changes within water collections can be associated with heavy rain fall and flooding typical of tropical regions such as the Amazon. The dynamics of water stage imply dynamics in vector populations [86]. While some species of anopheles are adapted to either still, stagnant or turbulent water collections, some others have a specific affinity for non-disturbed waters. When water level decreases, the length of water collections margins increases, by the effect of pools fragmentation, combining dense vegetation and moist soils and therefore creating suitable conditions for the development of larval reservoirs [12]. Discriminating between areas covered by open water and areas with high soil moisture is a strong concern for entomologists since both types or areas can be larval reservoirs and their discrimination is not easy using satellite imagery (either optical or SAR images).
- (3)
- In the context of global change (either climate change of human-induced changes), land cover and wetland distributions are strongly modified, thereby causing changes in the geographical expansions of vector-borne diseases and exposing new populations to disease [87]. Efficient prevention and vector control depends on the availability of up-to-date data and land cover maps combined with malaria case databases (such as the SIVEP database in Brazil [88]) at the local and regional scales in order to support targeted interventions. In many countries, national malaria control programs lack detailed disease-risk maps to guide intervention and reinforce vector control. Such maps could: (i) optimize the use of larvicide in targeted areas where the presence of suitable candidate wetlands and population has been acknowledged; (ii) optimize the timing of insecticide distribution for impregnating bed nets; (iii) restrict the distribution of antimalarial drugs to periods of known disease risk; (iv) help defining local environmental engineering actions and (v) help defining more specific prevention messages.
6.3. Improving the Characterization of Wetlands to Improve Vector Control
6.4. Limitations of SAR Remote-Sensing for Public Health Topics Remain
6.4.1. Multi-Scale Problem
6.4.2. Lack of Ground Data
6.4.3. Public Health Actors’ Needs vs. Space Agencies’ Requirements
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). A Global Brief on Vector-Borne Diseases; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organization (WHO). World Malaria Report; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Gubler, D.J. Resurgent vector-borne diseases as a global health problem. Emerg. Infect. Dis. 1998, 4, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Gratz, N.G. Emerging and resurging vector-borne diseases. Annu. Rev. Entomol. 1999, 44, 51–75. [Google Scholar] [CrossRef] [PubMed]
- Machault, V.; Vignolles, C.; Borchi, F.; Vounatsou, P.; Briolant, S.; Lacaux, J.P.; Rogier, C. The use of remotely sensed environmental data in the study of Malaria. Geospat. Health 2011, 5, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, H.M.; Dornhaus, A.; Beeche, A.; Borgemeister, C.; Gottlieb, M.; Mulla, M.S.; Gimnig, J.E.; Fish, D.; Killeen, G.F. Ecology: A prerequisite for Malaria elimination and eradication. PLoS Med. 2010, 7, e1000303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, W.J.M.; Jetten, T.H.; Rotmans, J.; Niessen, L.W. Climate change and vector-borne diseases: A global modelling perspective. Glob. Environ. Chang. 1995, 5, 195–209. [Google Scholar] [CrossRef]
- Sutherst, R.W. Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev. 2004, 17, 136–173. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Vector-borne diseases. Revue Sci. Tech. 2009, 28, 583–588. [Google Scholar] [CrossRef]
- Hiwat, H.; Bretas, G. Ecology of Anopheles darlingi Root with respect to vector importance: A review. Parasites Vectors 2011, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Dusfour, I.; Corrêa, A.P.; Cruz, M.C.; Dessay, N.; Galardo, A.K.; Galardo, C.D.; Girod, R.; Gomes, M.S.; Gurgel, H.; et al. Land cover, land use and Malaria in the Amazon: A systematic literature review of studies using remotely sensed data. Malar. J. 2013, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Kabaria, C.W.; et al. The dominant Anopheles vectors of human Malaria in the Americas: Occurrence data, distribution maps and bionomic précis. Parasites Vectors 2010, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.S.; Honório, N.A.; Arruda, M.E. Temporal and spatial distribution of Malaria within an agricultural settlement of the Brazilian Amazon. J. Vector Ecol. 2011, 36, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Vittor, A.Y.; Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Lozano, W.S.; Pinedo-Cancino, V.; Patz, J.A. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum Malaria in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2006, 74, 3–11. [Google Scholar] [PubMed]
- Li, Z.; Roux, E.; Dessay, N.; Girod, R.; Stefani, A.; Nacher, M.; Moiret, A.; Seyler, F. Mapping a Knowledge-Based Malaria Hazard Index Related to Landscape Using Remote Sensing: Application to the Cross-Border Area between French Guiana and Brazil. Remote Sens. 2016, 8, 319. [Google Scholar] [CrossRef]
- Vittor, A.Y.; Pan, W.; Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Sanchez-Lozano, W.; Pinedo, V.V.; Salas-Cobos, E.; Flores, S.; et al. Linking deforestation to Malaria in the Amazon: Characterization of the breeding habitat of the principal Malaria vector, Anopheles darlingi. Am. J. Trop. Med. Hyg. 2009, 81, 5–12. [Google Scholar] [PubMed]
- Manguin, S.; Roberts, D.R.; Andre, R.G.; Rejmankova, E.; Hakre, S. Characterization of Anopheles darlingi (Diptera: Culicidae) larval habitats in Belize, Central America. J. Med. Entomol. 1996, 33, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Tadei, W.P.; Thatcher, B.D.; Santos, J.M.; Scarpassa, V.M.; Rodrigues, I.B.; Rafael, M.S. Ecologic observations on anopheline vectors of Malaria in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 1998, 59, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.S.M.; Arruda, M.E.; Gurgel, H.C.; Honorio, N.A. Spatial clustering and longitudinal variation of Anopheles darlingi (Diptera: Culicidae) larvae in a river of the Amazon: The importance of the forest fringe and of obstructions to flow in frontier Malaria. Bull. Entomol. Res. 2011, 101, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ribas, J.; Oliveira-Ferreira, J.; Rosa-Freitas, M.G.; Trilla, L.; Silva-do-Nascimento, T.F. New classification of natural breeding habitats for Neotropical anophelines in the Yanomami Indian Reserve, Amazon Region, Brazil and a new larval sampling methodology. Memórias do Instituto Oswaldo Cruz 2015, 110, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Rozendaal, J.A. Relations between Anopheles darlingi breeding habitats, rainfall, river level and Malaria transmission rates in the rain forest of Suriname. Med. Vet. Entomol. 1992, 6, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.H.; Castro, M.C. Agricultural colonization and Malaria on the Amazon frontier. Ann. N. Y. Acad. Sci. 2001, 954, 184–222. [Google Scholar] [CrossRef] [PubMed]
- Cline, B.L. New eyes for epidemiologists: Aerial photography and other remote sensing techniques. Am. J. Epidemiol. 1970, 92, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Pope, K.O.; Rejmankova, E.; Savage, H.M.; Arredondo-Jimenez, J.I.; Rodriguez, M.H.; Roberts, D.R. Remote sensing of tropical wetlands for Malaria control in Chiapas, Mexico. Ecol. Appl. 1994, 4, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Rejmankova, E.; Pope, K.O.; Roberts, D.R.; Lege, M.G.; Andre, R.G.; Greico, J.; Alonzo, Y. Characterization and detection of Anopheles vestitipennis and Anopheles punctimacula (Diptera: Culicidae) larval habitats in Belize with field survey and SPOT satellite imagery. J. Vect. Ecol. 1998, 23, 74–88. [Google Scholar]
- Correia, V.R.D.M.; Carvalho, M.S.; Sabroza, P.C.; Vasconcelos, C.H. Remote sensing as a tool to survey endemic diseases in Brazil. Cadernos de Saúde Pública 2004, 20, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, C.H.; Novo, E.M.L.; De, M.; Donalisio, M.R. Use of remote sensing to study the influence of environmental changes on Malaria distribution in the Brazilian Amazon. Cad. Saude Publica 2006, 22, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Arboleda, S.; Peterson, A.T. Mapping environmental dimensions of dengue fever transmission risk in the Aburrá Valley, Colombia. Int. J. Environ. Res. Public Health 2009, 6, 3040–3055. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Roux, E.; Fotsing, J.-M.; Carme, B. Studying relationships between environment and Malaria incidence in Camopi (French Guiana) through the objective selection of buffer-based landscape characterisations. Int. J. Health Geogr. 2011, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Snow, R.W.; Rogers, D.J. From predicting mosquito habitat to Malaria seasons using remotely sensed data: Practice, problems and perspectives. Parasitol. Today 1998, 14, 306–313. [Google Scholar] [CrossRef]
- Newton, A.C.; Hill, R.A.; Echeverría, C.; Golicher, D.; Rey Benayas, J.M.; Cayuela, L.; Hinsley, S.A. Remote sensing and the future of landscape ecology. Progress Phys. Geography 2009, 33, 528–546. [Google Scholar] [CrossRef]
- Hay, S.I. An Overview of Remote Sensing and Geodesy for Epidemiology and Public Health Application. Adv. Parasitol. 2000, 47, 1–35. [Google Scholar] [PubMed]
- De Loor, G.P. The dielectric properties of wet materials. IEEE Trans. Geosci. Remote Sens. 1983, 364–369. [Google Scholar] [CrossRef]
- Ross, S.G.; Thomson, M.C.; Pultz, T.; Mbogo, C.M.; Regens, J.L.; Swalm, C.; Beier, J.C. On the use of RADARSAT-1 for monitoring malaria risk in Kenya. In Retrieval of Bio-and Geo-Physical Parameters from SAR Data for Land Applications; University of Sheffield: Sheffield, UK, 2002. [Google Scholar]
- Kaya, S.; Sokol, J.; Pultz, T.J. Monitoring environmental indicators of vector-borne disease from space: A new opportunity for RADARSAT-2. Can. J. Remote Sens. 2004, 30, 560–565. [Google Scholar] [CrossRef]
- Herbreteau, V.; Salem, G.; Souris, M.; Hugot, J.P.; Gonzalez, J.P. Thirty years of use and improvement of remote sensing, applied to epidemiology: From early promises to lasting frustration. Health Place 2007, 13, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, H.M.; Abad-Franch, F.; Dias, J.C.P.; Junqueira, A.C.V.; Coura, J.R. Chagas disease in the Amazon Region. Mem. Inst. Oswaldo Cruz 2007, 102, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M.; Dinerstein, E. The Global 200: Priority ecoregions for global conservation. Ann. MO Bot. Garden 2002, 89, 199–224. [Google Scholar] [CrossRef]
- Hess, L.L.; Melack, J.M.; Novo, E.M.; Barbosa, C.C.; Gastil, M. Dual-season mapping of wetland inundation and vegetation for the central Amazon basin. Remote Sens. Environ. 2003, 87, 404–428. [Google Scholar] [CrossRef]
- Ozesmi, S.L.; Bauer, M.E. Satellite remote sensing of wetlands. Wetlands Ecol. Manag. 2002, 10, 381–402. [Google Scholar] [CrossRef]
- Helie, R. Canadian Wetland Inventory. 2004. Available online: www.wetkit.net/modules/4/index.php (accessed on 5 March 2018).
- Fournier, R.A.; Grenier, M.; Lavoie, A.; Hélie, R. Towards a strategy to implement the Canadian Wetland Inventory using satellite remote sensing. Can. J. Remote Sens. 2007, 33 (Suppl. 1), S1–S16. [Google Scholar] [CrossRef]
- Lulla, K. The Landsat satellites and selected aspects of physical geography. Progress Phys. Geography 1983, 7, 1–45. [Google Scholar] [CrossRef]
- Hardisky, M.A.; Gross, M.F.; Klemas, V. Remote sensing of coastal wetlands. Bioscience 1986, 36, 453–460. [Google Scholar] [CrossRef]
- Lee, K.H.; Lunetta, R.S. Wetland detection methods. In Wetland and Environmental Application of GIS; Lyon, J.G., McCarthy, J., Eds.; Lewis Publishers: New York, NY, USA, 1996. [Google Scholar]
- Kasischke, E.S.; Bourgeau-Chavez, L.L. Monitoring South Florida wetlands using ERS-1 SAR imagery. Photogramm. Eng. Remote Sens. 1997, 63, 281–291. [Google Scholar]
- Wang, Y.; Davis, F.W.; Melack, J.M.; Kasischke, E.S.; Christensen, N.L., Jr. The effects of changes in forest biomass on radar backscatter from tree canopies. Remote Sens. 1995, 16, 503–513. [Google Scholar] [CrossRef]
- Dobson, M.C.; Ulaby, F.T.; Pierce, L.E.; Sharik, T.L.; Bergen, K.M.; Kellndorfer, J.; Kendra, J.R.; Li, E.; Lin, Y.C.; Sarabandi, K.; et al. Estimation of forest biophysical characteristics in northern Michigan with SIR-C/X-SAR. IEEE Trans. Geosci. Remote Sens. 1995, 33, 877–895. [Google Scholar] [CrossRef]
- Hess, L.L.; Melack, J.M.; Simonett, D.S. Radar detection of flooding beneath the forest canopy: A review. Int. J. Remote Sens. 1990, 11, 1313–1325. [Google Scholar] [CrossRef]
- Kasischke, E.S.; Melack, J.M.; Craig Dobson, M. The use of imaging radars for ecological applications—A review. Remote Sens. Environ. 1997, 59, 141–156. [Google Scholar] [CrossRef]
- Henderson, F.M.; Lewis, A.J. Radar detection of wetland ecosystems: A review. Int. J. Remote Sens. 2008, 29, 5809–5835. [Google Scholar] [CrossRef]
- Ramsey, E.W., III. Radar remote sensing of wetlands. In Remote Sensing Change Detection: Environmental Monitoring Methods and Applications; Ann Arbor Press: Ann Arbor, MI, USA, 1998. [Google Scholar]
- White, L.; Brisco, B.; Dabboor, M.; Schmitt, A.; Pratt, A. A collection of SAR methodologies for monitoring wetlands. Remote Sens. 2015, 7, 7615–7645. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.; Silva, T.S.; Evans, T.L. Wetland Classification. Remote Sensing of Natural Resources; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Martinis, S.; Kuenzer, C.; Wendleder, A.; Huth, J.; Twele, A.; Roth, A.; Dech, S. Comparing four operational SAR-based water and flood detection approaches. Int. J. Remote Sens. 2015, 36, 3519–3543. [Google Scholar] [CrossRef]
- Townsend, P.A. Mapping seasonal flooding in forested wetlands using multi-temporal Radarsat SAR. Photogramm. Eng. Remote Sens. 2001, 67, 857–864. [Google Scholar]
- Townsend, P.A. Estimating forest structure in wetlands using multitemporal SAR. Remote Sens. Environ. 2002, 79, 288–304. [Google Scholar] [CrossRef]
- Li, G.; Lu, D.; Moran, E.; Dutra, L.; Batistella, M. A comparative analysis of ALOS PALSAR L-band and RADARSAT-2 C-band data for land-cover classification in a tropical moist region. ISPRS J. Photogramm. Remote Sens. 2012, 70, 26–38. [Google Scholar] [CrossRef]
- Bourgeau-Chavez, L.L.; Riordan, K.; Powell, R.B.; Miller, N.; Nowels, M. Improving Wetland Characterization with Multi-Sensor, Multi-Temporal SAR and Optical/Infrared Data Fusion. Available online: https://www.intechopen.com/books/advances-in-geoscience-and-remote-sensing/improving-wetland-characterization-with-multi-sensor-multi-temporal-sar-and-optical-infrared-data-fu (accessed on 5 March 2018).
- Bourgeau-Chavez, L.L.; Kasischke, E.S.; Brunzell, S.M.; Mudd, J.P.; Smith, K.B.; Frick, A.L. Analysis of space-borne SAR data for wetland mapping in Virginia riparian ecosystems. Int. J. Remote Sens. 2001, 22, 3665–3687. [Google Scholar] [CrossRef]
- Karszenbaum, H.; Kandus, P.; Martinez, J.M.; Le Toan, T.; Tiffenberg, J.; Parmuchi, G. ERS-2, RADARSAT SAR Backscattering Characteristics of the Paraná River Delta Wetland, Argentina; ESA Publication SP-461: Gothenburg, Sweden, 2000. [Google Scholar]
- Lang, M.W.; Kasischke, E.S.; Prince, S.D.; Pittman, K.W. Assessment of C-band synthetic aperture radar data for mapping and monitoring Coastal Plain forested wetlands in the Mid-Atlantic Region, USA. Remote Sens. Environ. 2008, 112, 4120–4130. [Google Scholar] [CrossRef]
- Patel, P.; Srivastava, H.S.; Navalgund, R.R. Use of synthetic aperture radar polarimetry to characterize wetland targets of Keoladeo National Park, Bharatpur, India. Curr. Sci. 2009, 97, 529–537. [Google Scholar]
- Brisco, B.; Kapfer, M.; Hirose, T.; Tedford, B.; Liu, J. Evaluation of C-band polarization diversity and polarimetry for wetland mapping. Can. J. Remote Sens. 2011, 37, 82–92. [Google Scholar] [CrossRef]
- Schmitt, A.; Brisco, B.; Kaya, S.; Roth, A.; Mueller, A. Wetlands monitoring with Polarimetric SAR change detection methods. In Proceedings of the 34th International Symposium for Remote Sensing of the Environment (ISRSE), Sydney, Australia, 10–15 April 2011; pp. 10–15. [Google Scholar]
- Alsdorf, D.; Melack, J.; Dunne, T.; Mertes, L.; Hess, L.; Smith, L. Interferometric radar measurements of water level changes on the Amazon floodplain. Nature 2000, 404, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Wdowinski, S.; Kim, S.; Amelung, F.; Dixon, T. Wetland InSAR: A New Space-Based Hydrological Monitoring Tool of Wetlands Surface Water Level Changes; GlobWetland Symposium Proceedings: Frescati, Italy, 2006; p. 6. [Google Scholar]
- Wdowinski, S.; Kim, S.W.; Amelung, F.; Dixon, T.H.; Miralles-Wilhelm, F.; Sonenshein, R. Space-based detection of wetlands’ surface water level changes from L-band SAR interferometry. Remote Sens. Environ. 2008, 112, 681–696. [Google Scholar] [CrossRef]
- Hong, S.H.; Wdowinski, S.; Kim, S.W. Evaluation of TerraSAR-X observations for wetland InSAR application. IEEE Trans. Geosci. Remote Sens. 2010, 48, 864–873. [Google Scholar] [CrossRef]
- Hong, S.H.; Wdowinski, S.; Kim, S.W.; Won, J.S. Multi-temporal monitoring of wetland water levels in the Florida Everglades using interferometric synthetic aperture radar (InSAR). Remote Sens. Environ. 2010, 114, 2436–2447. [Google Scholar] [CrossRef]
- Hong, S.H.; Wdowinski, S. Evaluation of the quad-polarimetric Radarsat-2 observations for the wetland InSAR application. Can. J. Remote Sens. 2012, 37, 484–492. [Google Scholar] [CrossRef]
- Kim, J.W.; Lu, Z.; Lee, H.; Shum, C.K.; Swarzenski, C.M.; Doyle, T.W.; Baek, S.H. Integrated analysis of PALSAR/Radarsat-1 InSAR and ENVISAT altimeter data for mapping of absolute water level changes in Louisiana wetlands. Remote Sens. Environ. 2009, 113, 2356–2365. [Google Scholar] [CrossRef]
- Lee, J.S.; Pottier, E. Polarimetric Radar Imaging: From Basics to Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Veloso, H.P.; Goes-Filho, L. Brazilian Phytogeography: Physiognomic-Ecological Classification of Neotropical Vegetation; RADAMBRASIL Project: Salvador, Brazil, 1982. [Google Scholar]
- Sgrenzaroli, M.; Baraldi, A.; De Grandi, G.D.; Eva, H.; Achard, F. A novel approach to the classification of regional-scale radar mosaics for tropical vegetation mapping. IEEE Trans. Geosci. Remote Sens. 2004, 42, 2654–2669. [Google Scholar] [CrossRef]
- Souza Filho, P.W.M.; Paradella, W.R. Use of RADARSAT-1 fine mode and Landsat-5 TM selective principal component analysis for geomorphological mapping in a macrotidal mangrove coast in the Amazon Region. Can. J. Remote Sens. 2005, 31, 214–224. [Google Scholar] [CrossRef]
- Santos da Silva, J.; Calmant, S.; Seyler, F.; Lee, H.; Shum, C. Mapping of the extreme stage variations using ENVISAT altimetry in the Amazon basin rivers. Int. Water Technol. J. 2012, 2, 14–25. [Google Scholar]
- Evans, T.L.; Costa, M. Landcover classification of the Lower Nhecolândia subregion of the Brazilian Pantanal Wetlands using ALOS/PALSAR, RADARSAT-2 and ENVISAT/ASAR imagery. Remote Sens. Environ. 2013, 128, 118–137. [Google Scholar] [CrossRef]
- Liesenberg, V.; Gloaguen, R. Evaluating SAR polarization modes at L-band for forest classification purposes in Eastern Amazon, Brazil. Int. J. Appl. Earth Obs. Geoinformat. 2013, 21, 122–135. [Google Scholar] [CrossRef]
- Chapman, B.; McDonald, K.; Shimada, M.; Rosenqvist, A.; Schroeder, R.; Hess, L. Mapping regional inundation with spaceborne L-Band SAR. Remote Sens. 2015, 7, 5440–5470. [Google Scholar] [CrossRef]
- Hess, L.L.; Melack, J.M.; Affonso, A.G.; Barbosa, C.; Gastil-Buhl, M.; Novo, E.M. Wetlands of the lowland Amazon basin: Extent, vegetative cover, and dual-season inundated area as mapped with JERS-1 Synthetic Aperture Radar. Wetlands 2015, 35, 745–756. [Google Scholar] [CrossRef]
- Olson, S.H. Links between Climate, Malaria, and Wetlands in the Amazon Basin. Emerg. Infect. Dis. 2009, 15, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Catry, T.; Dessay, N.; Roux, E.; Mahé, E.; Seyler, F. Multi-sensor data fusion for identifying Malaria environmental features. In Proceedings of the 2016 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Beijing, China, 10–16 July 2016; IEEE: New York, NY, USA; pp. 2529–2532. [Google Scholar]
- Li, Z.; Catry, T.; Dessay, N.; Roux, E.; Seyler, F. Mapping soil typologies using geomorphologic features extracted from DEM and SAR data: A environmental factor affecting Malaria transmission in the Amazon. In Proceedings of the 2016 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Beijing, China, 10–16 July 2016; IEEE: New York, NY, USA; pp. 3140–3143. [Google Scholar]
- Moua, Y.; Roux, E.; Girod, R.; Dusfour, I.; De Thoisy, B.; Seyler, F.; Briolant, S. Distribution of the Habitat Suitability of the Main Malaria Vector in French Guiana Using Maximum Entropy Modeling. J. Med. Entomol. 2016, 54, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Wolfarth, B.R.; Filizola, N.; Tadei, W.P.; Durieux, L. Epidemiological analysis of Malaria and its relationships with hydrological variables in four municipalities of the State of Amazonas, Brazil. Hydrol. Sci. J. 2013, 58, 1495–1504. [Google Scholar] [CrossRef]
- Cotter, C.; Sturrock, H.J.; Hsiang, M.S.; Liu, J.; Phillips, A.A.; Hwang, J.; Gueye, C.S.; Fullman, N.; Gosling, R.D.; Feachem, R.G. The changing epidemiology of Malaria elimination: New strategies for new challenges. Lancet 2013, 382, 900–911. [Google Scholar] [CrossRef]
- Wiefels, A.; Wolfarth-Couto, B.; Filizola, N.; Durieux, L.; Mangeas, M. Accuracy of the Malaria epidemiological surveillance system data in the state of Amazonas. Acta Amazon. 2016, 46, 383–390. [Google Scholar] [CrossRef]
- Valle, D.; Lima, J.M.T. Large-scale drivers of Malaria and priority areas for prevention and control in the Brazilian Amazon region using a novel multi-pathogen geospatial model. Malar. J. 2014, 13, 443. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Menach, A.; Pothin, E.; Eisele, T.P.; Gething, P.W.; Eckhoff, P.A. Mapping multiple components of malaria risk for improved targeting of elimination interventions. Malar. J. 2017, 16, 459. [Google Scholar] [CrossRef] [PubMed]
- Alimi, T.O.; Fuller, D.O.; Quinones, M.L.; Xue, R.D.; Herrera, S.V.; Arevalo-Herrera, M.; Ulrich, J.N.; Qualls, W.A.; Beier, J.C. Prospects and recommendations for risk mapping to improve strategies for effective Malaria vector control interventions in Latin America. Malar. J. 2015, 14, 519. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, C.; Roux, E.; Ceccato, P.; Gosselin, P.; Monteiro, A.M.; de Matos, V.P.; Xavier, D.R. An observatory to gather and disseminate information on the health-related effects of environmental and climate change. Pan Am. J. Public Health 2016, 40, 167–173. [Google Scholar]


| Mosquito Species | Light Intensity | Salinity | Turbidity | Water Movement | Vegetation | Vector Status | Distribution |
|---|---|---|---|---|---|---|---|
| An. darlingi | low | low | clear water | variable | yes | primary | Entire Amazon region |
| An. nuneztovari | variable | low | clear water | variable | yes | secondary | Brazilian Amazon, Venezuela |
| An. marajoara | variable | low | clear water | variable | yes | secondary | Brazilian Amazon, Venezuela |
| An. albitarsis | high | low | variable | still or stagnant | yes | secondary | Brazilian Amazon, Venezuela |
| An. braziliensis | low | low | clear water | variable | yes | secondary | Brazilian amazon exclusively |
| An. oswaldoi | low | low | clear water | variable | yes | secondary | Brazilian Amazon, Colombia, Peru, Venezuela |
| An. trianulatus | low | low | clear water | variable | yes | secondary | Brazilian Amazon, central and south America |
| SAR Satellite | Space Agency | In Operation Since | Spatial Resolution (m) | Revisit Period (days) | Scene Size (km2) | Band | Polarization | Cost |
|---|---|---|---|---|---|---|---|---|
| Sentinel 1A and 1B | ESA | 2014 and 2016 | 5 to 20 | 5 to 12 | 20 × 20 to 250 × 200 | C | dual | free |
| TerraSAR-X—TanDEM-X—PAZ | DLR | 2007, 2010 and 2014 | 0.5 to 40 | 4 to 7 | 4 × 8 to 270 × 200 | X | full | not free |
| Cosmo-SkyMed | ISA | 2007 | 1 to 100 | 16 | 10 × 10 to 200 × 200 | X | dual | not free |
| Radarsat-2 | CSA | 2007 | 1 to 100 | 24 | 25 × 25 to 500 × 500 | C | full | not free |
| ALOS-2 | JAXA | 2014 | 1 to 100 | 14 | 25 × 25 to 500 × 500 | L | full | not free |
| Risat-1 | ISRO | 2012 | 1 to 50 | 25 | 10 × 10 to 225 × 225 | C | full | not free |
| Kompsat-5 | KARI | 2013 | 1 to 20 | 28 | 5 × 5 to 100 × 100 | X | single | not free |
| SAOCOM-1A and 1B | ANCSA | to be launched in 2018 | 7 to 100 | 8 to 16 | 50 × 50 to 400 × 400 | L | full | not free |
| Radarsat Constellation | CSA | to be launched in 2018 | 1 to 100 | 1 to 4 | 25 × 25 to 500 × 500 | C | full | not free |
| BIOMASS | ESA | to be launched in 2020 | 50 to 200 | 17 | 50 × 50 | P | full | not free |
| SWOT | CNES, NASA, CSA, UKSA | to be launched in 2021 | 50 to 1000 | 16 | 100 km wide-swath | Ka | single | not free |
| Cosmo-SkyMed SG | ISA | to be launched in 2018 | 1 to 40 | 16 | 10 × 10 to 100 × 3000 | X | full | not free |
| NISAR | NASA, ISRO | to be launched in 2020 | 3 to 50 | 12 | 10 × 10 to 200 × 200 | S, L | full | not free |
| SAR Properties and Techniques | Free Water | Herbaceous Wetland | Forested Wetland | Herbaceous vs. Forested Wetland | Water Level |
|---|---|---|---|---|---|
| SAR Properties | |||||
| Wavelength band | X | C | L or C + L | C + L | |
| Polarization | VV | HH | VV or HH | VH or HV or combination | HH, VH or HV |
| Incidence angle | |||||
| SAR Techniques | |||||
| Backscatter intensity | Thresholding, texture and segmentation | Multi-temporal approach | Multi-temporal and multi-incidence approach | Seasonal approach | |
| InSAR | Low temporal baselines | ||||
| Altimetry | Alone or combined with InSAR or backscatter | ||||
| Polarimetry | HH + HH/HV decomposition Freeman-Dourden and Claude Poitier decompositions | Alpha angle and multi-temporal approach | |||
| Recommended (Free Access Data) | |||||
| Sentinel 1 | Sentinel 1 | Sentinel 1, ALOS-PALSAR | Sentinel 1, ALOS-PALSAR | Sentinel 1, ALOS-PALSAR | |
| RADAR Technology | Sensor | Main Results | Product | Reference |
|---|---|---|---|---|
| Airborne SAR | Classification of vegetation and wetlands | Periodic wetland maps | [74] | |
| C- and L-band SAR | SIR-C | Discrimination between flooded and non-flooded forest in the Amazon | Extent of flooded forest in the Amazon basin | [49] |
| L-band SAR | JERS-1 | Mapping of forest wetlands using L-band SAR | Maps of wetland extent in the central Amazon region | [39] |
| C- and L-band SAR | ALOS PALSAR + RADARSAT-2 | Usefulness of the C-band for the characterization of herbaceous wetlands and improvement of classification accuracy by combining C- and L-bands | Pixel-based classification of wetlands | [58] |
| SAR interferometry + altimetry | JERS-1 + TOPEX/POSEIDON | Detection of centimeter scale variations of the water surface height in lake Balbina | [67] | |
| L-band SAR | JERS-1 | Backscatter values and radar texture for floodplain forest, mangrove forest, flooded forests, dense canopy forest | Regional vegetation and wetland maps | [75] |
| C-band SAR | RADARSAT-1 | Backscatter values and radar texture for classification of various types of mangroves | Mangrove mapping | [76] |
| L-band SAR | ALOS-PALSAR | Classification of flooded vegetation using the segmentation of a multi-temporal, dual polarization image stack and temporal backscattering signatures | Classification of wetland habitats | [39] |
| Altimetry | ENVISAT | Time and space variations in water stored in or circulating through rivers, floodplains, wetlands and lakes in the major sub-basins of the Amazon basin | Water storage and circulation in the Amazon basin | [77] |
| C- and L-band SAR | ALOS PALSAR + RADARSAT-2 | Map ecosystems and create a 3-level lake distribution map of the Lower Nhecolàndia subregion in the Brazilian Pantanal | First line spatial resolution classification showing the spatial distribution of terrestrial and aquatic habitats for the entire subregion of Lower Nhecolàndia | [78] |
| L-band SAR | ALOS-PALSAR | Quad-polarization performs better for forest classification and classification accuracy improves when SAR and optical imagery are combined | Forest classification in the Amazon | [79] |
| L-band SAR | ALOS-PALSAR | Classification if the state of inundation of the entire Amazon using ScanSAR mode | Inundation maps of the Amazon basin | [80] |
| L-band SAR | ALOS-PALSAR | Classification of wetland areas into five land-cover classes using dual-season backscattering values | Extent of flooded areas in the Amazon basin during low and high water seasons | [81] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catry, T.; Li, Z.; Roux, E.; Herbreteau, V.; Gurgel, H.; Mangeas, M.; Seyler, F.; Dessay, N. Wetlands and Malaria in the Amazon: Guidelines for the Use of Synthetic Aperture Radar Remote-Sensing. Int. J. Environ. Res. Public Health 2018, 15, 468. https://doi.org/10.3390/ijerph15030468
Catry T, Li Z, Roux E, Herbreteau V, Gurgel H, Mangeas M, Seyler F, Dessay N. Wetlands and Malaria in the Amazon: Guidelines for the Use of Synthetic Aperture Radar Remote-Sensing. International Journal of Environmental Research and Public Health. 2018; 15(3):468. https://doi.org/10.3390/ijerph15030468
Chicago/Turabian StyleCatry, Thibault, Zhichao Li, Emmanuel Roux, Vincent Herbreteau, Helen Gurgel, Morgan Mangeas, Frédérique Seyler, and Nadine Dessay. 2018. "Wetlands and Malaria in the Amazon: Guidelines for the Use of Synthetic Aperture Radar Remote-Sensing" International Journal of Environmental Research and Public Health 15, no. 3: 468. https://doi.org/10.3390/ijerph15030468





