Toxicity of Beauveria bassiana-28 Mycelial Extracts on Larvae of Culex quinquefasciatus Mosquito (Diptera: Culicidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Culture
2.2. Commercial Microbial Insecticide B. bassiana-22
2.3. Morphological Identification of B. bassiana-28
2.4. Mass Culturing of B. bassiana-28
2.5. Crude Extraction from B. bassiana-28
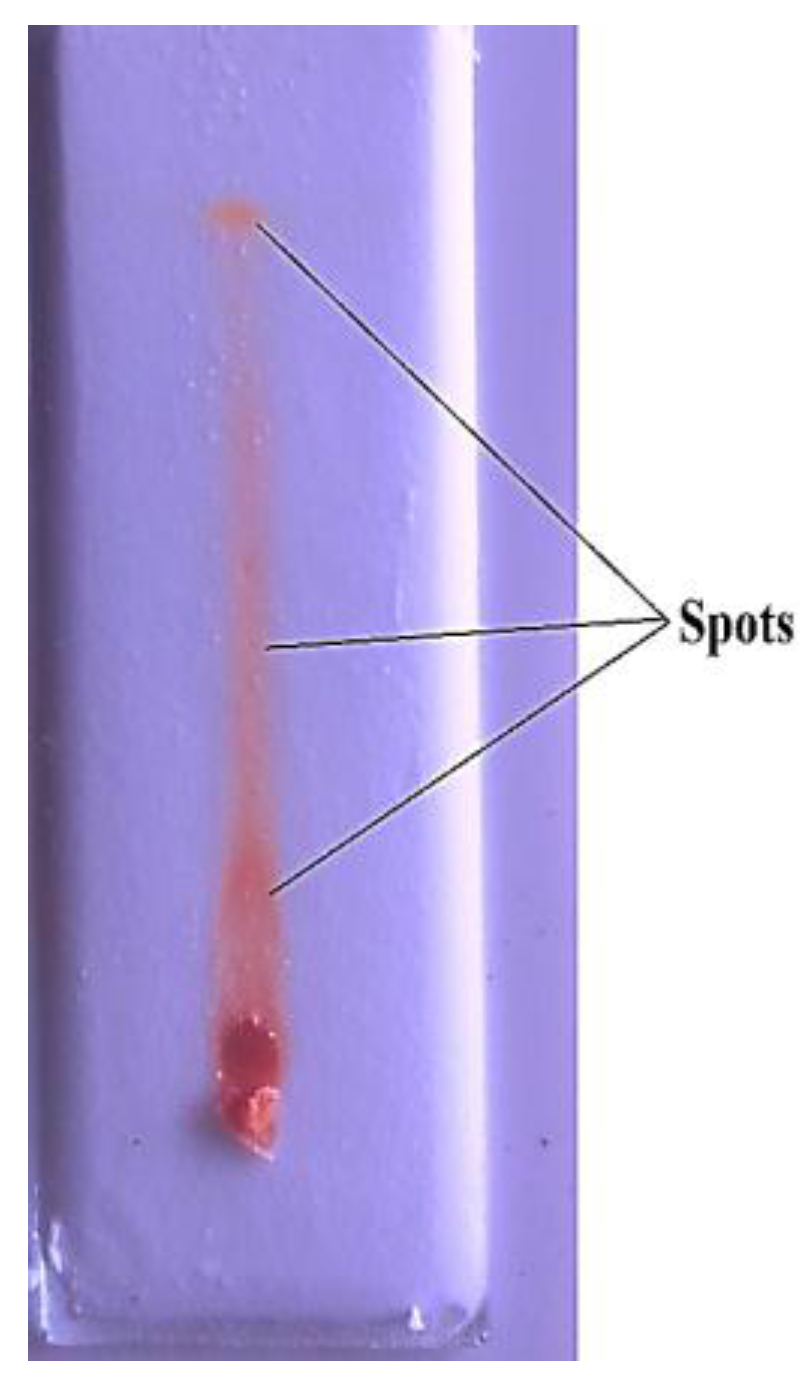
2.6. Thin Layer Chromatography
2.7. Mosquito Culture
2.8. Larval Bioassay
2.9. Pupal Toxicity Tests
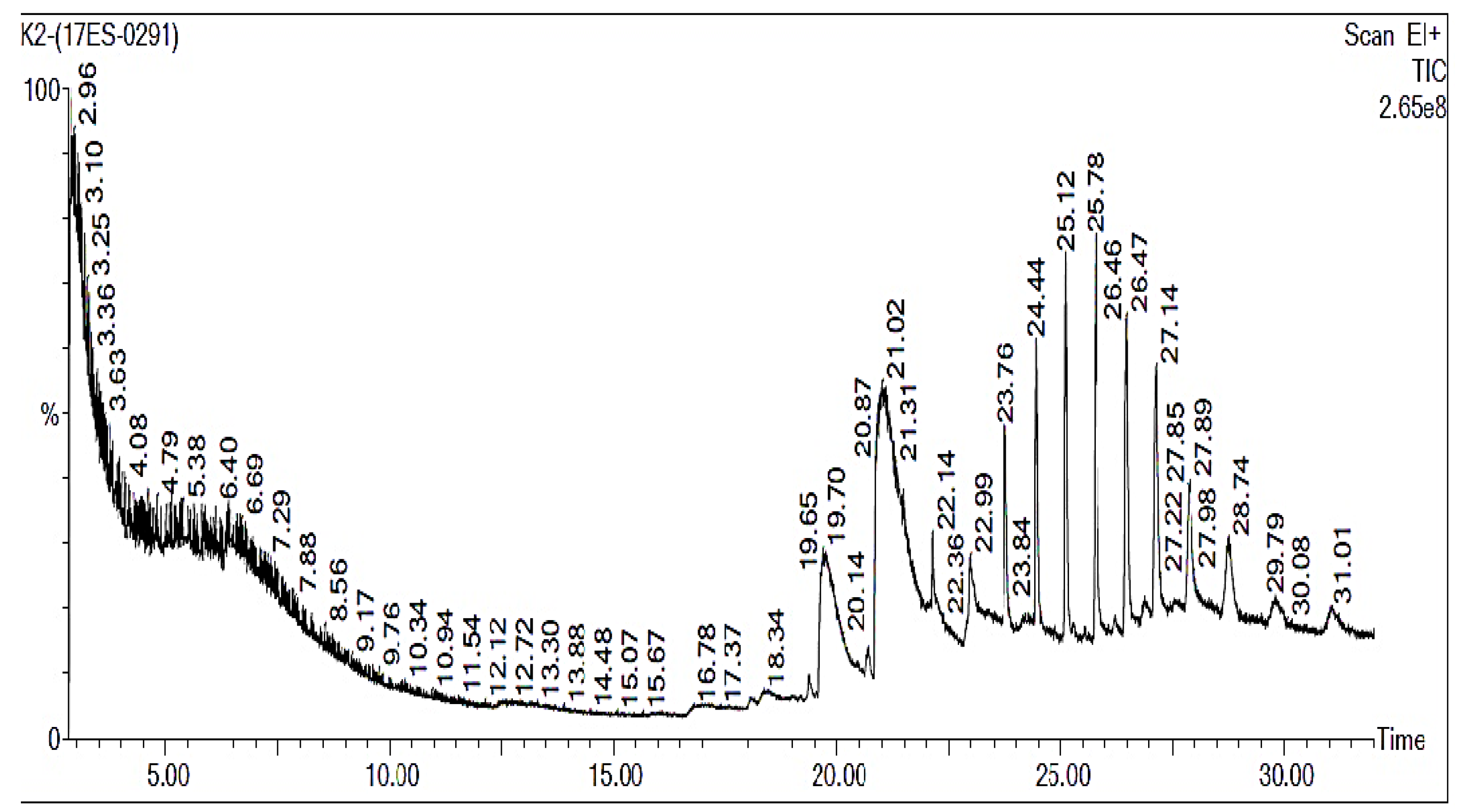
3. Gas Chromatography-Mass Spectrophotometer (GC-MS) Analysis
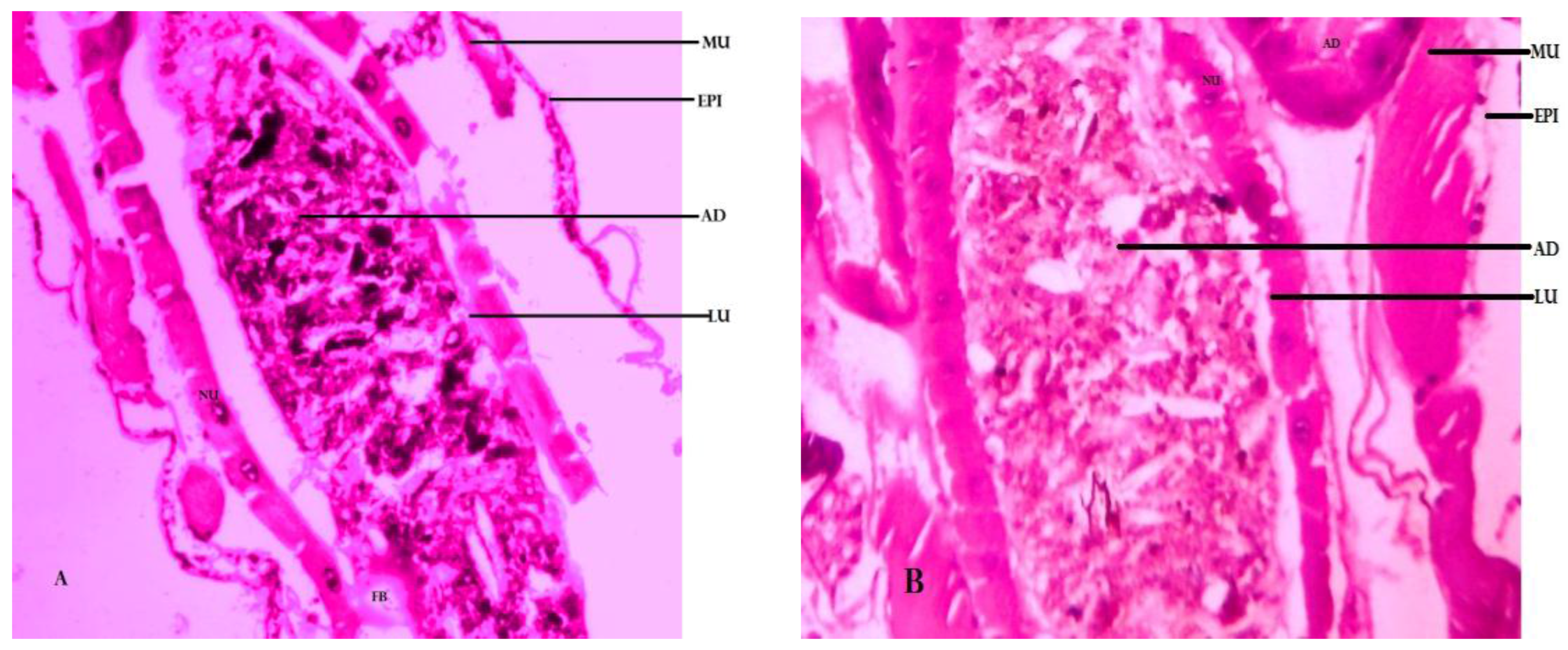
Histological Studies
4. Results
4.1. Larval Bioassay
4.2. Thin Layer Chromatography
4.3. Gas Chromatography-Mass Spectrometry Analysis of B. bassiana-28 Ethyl Acetate Mycelial Extract
4.4. Fourier transform infrared spectrum Analysis of B. bassiana-28 Ethyl Acetate Mycelial Extract
4.5. Histological Studies of Larvae of Cx. quinquefasciatus
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Programme to Eliminate Lymphatic Filariasis-Progress Report on Mass Drug Administration in 2016; Weekly Epidemiological Record; World Health Organization: Geneva, Switzerland, 2016; Volume 85, pp. 365–372. [Google Scholar]
- Knight, K.L.; Stone, A. A Catalogue of the Mosquitoes of the World (Diptera: Culicidae), 2nd ed.; Thomas Say Foundation; Entomological Society of America: College Park, MD, USA, 1997; p. 611. [Google Scholar]
- Solomon, T. Flavivirus encephalitis. N. Engl. J. Med. 2004, 351, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Amerasan, D.; Murugan, K.; Kovendan, K.; Mahesh Kumar, P.; Panneerselvam, C.; Subramaniam, J.; William, S.J.; Hwang, J.S. Adulticidal and repellent properties of Cassia tora Linn. (Family: Caesalpinaceae) against Culex quinquefasciatus, Aedes aegypti, and Anopheles stephensi. Parasitol. Res. 2012, 111, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y. Vector Mosquitoes of Filariasis in Japan. Trop. Med. Health 2011, 39, 39–45. [Google Scholar] [PubMed]
- Kimura, M.; Darbro, J.M.; Harrington, L.C. Avian malaria parasites share congeneric mosquito vectors. J. Parasitol. 2010, 96, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, G.; Shivakumar, M.S. Laboratory development of Permethrin resistance and cross-resistance pattern of Culex quinquefasciatus to other insecticides. Parasitol. Res. 2015, 114, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, R.; Shivakumar, M.S. Susceptibility status of Aedes aegypti (L.) (Diptera: Culicidae) to temephos from three districts of Tamil Nadu, India. J. Vector Borne Dis. 2015, 52, 159–165. [Google Scholar] [PubMed]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Flamini, G.; Canale, A.; Cioni, P.L.; Conti, B. Toxicity evaluation of different essential oil formulations against the Mediterranean Fruit Fly Ceratitis capitata (Wiedemann) (Diptera Tephritidae). Crop Prot. 2012, 42, 223–229. [Google Scholar] [CrossRef]
- Benelli, G.; Flamini, G.; Canale, A.; Molfetta, I.; Cioni, P.L.; Conti, B. Repellence of Hyptis suaveolens L. (Lamiaceae) whole essential oil and major constituents against adults of the granary weevil Sitophilus granarius (L.) (Coleoptera: Dryophthoridae). Bull. Insectol. 2012, 65, 177–183. [Google Scholar]
- Ramkumar, G.; Karthi, S.; Muthusamy, R.; Suganya, P.; Natarajan, D.; Kweka, E.J.; Shivakumar, M.S. Mosquitocidal Effect of Glycosmis pentaphylla Leaf Extracts against Three Mosquito Species (Diptera: Culicidae). PLoS ONE 2016, 11, e0158088. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, P.; Senthil-Nathan, S.; Shivakumar, M.S. Larvicidal, pupicidal and adult smoke toxic effects of Acanthospermum hispidum (DC) leaf crude extracts against mosquito vectors. Physiol. Mol. Plant Pathol. 2017, 101, 156–162. [Google Scholar] [CrossRef]
- Senthil-Nathan, S.; Chung, P.G.; Murugan, K. Effect of botanicals and bacterial toxin on the gut enzyme of Cnaphalocrocis medinalis. Phytoparasitica 2004, 32, 433–443. [Google Scholar] [CrossRef]
- Malik, A.; Singh, N.; Satya, S. House Fly (Musca domestica): A review of control strategies for a challenging pest. J. Environ. Sci. Health Part B 2007, 42, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Ponsankar, A.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Thanigaivel, A.; Edwin, E.; Selin-Rani, S.; Kalaivani, K.; Hunter, W.B.; Alessandro, R.T.; Abel-Megeed, A.; et al. Target and non-target toxicity of botanical insecticide derived from Couroptia guianensis L. flower against generalist herbivore, Spodoptera litura Fab, and an earthworm, Eisenia foetida Savigny. Ecotoxicol. Environ. Saf. 2016, 133, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Govindrajan, M.; Jebamesan, A.; Reetha, D. Larvicidal effect of extracellular secondary metabolites of different fungi against the mosquito, Cx quinquefasciatus Say. Trop. Biomed. 2005, 22, 1–3. [Google Scholar]
- Selin-Rani, S.; Senthil-Nathan, S.; Thanigaivel, A.; Vasantha-Srinivasan, P.; Edwin, E.; Ponsankar, A.; Lija-Escaline, J.; Kalaivani, K.; Abdel-Megeed, A.; Hunter, W.B.; et al. Toxicity and physiological effect of quercetin on generalist herbivore, Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Chemosphere 2016, 165, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Prakash, S. Entomopathogenic fungus generated nanoparticles for enhancement of efficacy in Culex quinquefasciatus and Anopheles stephensi. Asian Pac. J. Trop. Dis. 2012, 2, 356–361. [Google Scholar] [CrossRef]
- Mnyone, L.L.; Kirby, M.J.; Mpingwa, M.W.; Lwetoilera, D.W.; Knols, B.G.J.; Takken, W.; Koenraadt, C.J.M.; Russel, T.L. Infection of Anopheles gambiae mosquitoes with entomopathogenic fungi: Effect of host age and blood feeding stage. Parasitol. Res. 2011, 108, 317–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukhari, T.; Takken, W.; Koenraadt, C.J.M. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasites Vectors 2011, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Prakash, S. Evaluation of culture filtrates of Culicinomyces clavisporus: Myco adulticides for Culex quinquefasciatus, Aedes aegypti and Anopheles stephensi. Parasitol. Res. 2010, 110, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Shukla, R.; Singh, P.; Kumar, A.; Mishra, P.K.; Dubey, N.K. Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. Int. J. Food Microbiol. 2010, 142, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Pimmental, D.; Perkins, J.H. Pest Control: Cultural and Environmental Aspects; AAAS Symposium; Westview Press: Boulder, CO, USA, 1980; p. 243. [Google Scholar]
- Nguyen, N.T.H.; Borgemeister, C.; Poehling, H.M.; Zimmermann, G. Laboratory investigations on the potential of entomopathogenic fungi for biocontrol of Helicoverpa armigera (Lepidoptera: Noctuidae) larvae and pupae. Biocontrol. Sci. Technol. 2007, 17, 853–864. [Google Scholar] [CrossRef]
- Godonou, I.; James, B.; Atcha-Ahowe, C.; Vodouhe, S.; Kooyman, C.; Ahanchede, A.; Korie, S. Potential of Beauveria bassiana and Metarhizium anisopliae isolates from Benin to control Plutella xylostella L. (Lepidoptera: Plutellidae). Crop Prot. 2009, 28, 220–224. [Google Scholar] [CrossRef]
- Mnyone, L.L.; Kirby, M.J.; Lwetoijera, D.W.; Mpingwa, M.W.; Simfukwe, E.T.; Knols, B.G.J.; Takken, W.; Russell, T.L. Anopheline and culicine mosquitoes are not repelled by surfaces treated with the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Parasites Vectors 2010, 3, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajek, A.E.; Wraight, S.P.; Vandenberg, J.D. Control of arthropods using pathogenic fungi. In Bio-Exploitation of Filamentous Fungi; Fungal Diversity Research Series; Pointing, S.B., Hyde, K.D., Eds.; Fungal Diversity Press: Hong Kong, China, 2001; Volume 6, pp. 309–347. [Google Scholar]
- Howard, A.F.V.; Guessan, R.N.; Koenraadt, C.J.M.; Asidi, A.; Farenhorst, M.; Akogbéto, M.; Thomas, M.B.; Knols, B.G.J.; Takken, W. The entomopathogenic fungus Beauveria bassiana reduces instantaneous blood feeding in wild multi-insecticide-resistant Culex quinquefasciatus mosquitoes in Benin, West Africa. Parasites Vectors 2010, 3, 87. [Google Scholar] [CrossRef] [PubMed]
- Ragavendran, C.; Natarajan, D. Insecticidal potency of Aspergillus terreus against larvae and pupae of three mosquito species Anopheles stephensi, Culex quinquefasciatus, and Aedes aegypti. Environ. Sci. Pollut. Res. 2015, 22, 17224–17237. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Communicable Disease Control, Prevention and Eradication; WHO Pesticide Evaluation Scheme. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; WHO/CDS/WHOPES/GCDPP/1.3; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Achonduh, O.A.; Tondje, P.R. First report of pathogenicity of Beauveria bassiana RBL 1034 to the malaria vector, Anopheles gambiae s.l. (Diptera; Culicidae) in Cameroon. Afr. J. Biotechnol. 2008, 7, 931–935. [Google Scholar]
- Inglis, D.G.; Johnson, D.L.; Goettel, M.S. Effects of temperature and thermoregulation on mycosis by Beauveria bassiana in grasshoppers. Biol. Control 1996, 7, 131–139. [Google Scholar] [CrossRef]
- Clark, T.B.; Kellen, W.; Fukuda, T.; Lindegren, J.E. Field and laboratory studies on the pathogenicity of the fungus Beauveria bassiana to three genera of mosquitoes. J. Invertebr. Pathol. 1968, 11, 1–7. [Google Scholar] [CrossRef]
- Blanford, S.; Chan, B.H.K.; Jenkins, N.; Sim, D.; Turner, R.J.; Read, A.F.; Thomas, M.B. Fungal pathogen reduces potential for malaria transmission. Science 2005, 308, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Kikankie, C.K.; Brooke, B.D.; Knols, B.G.J.; Koekemoer, L.L.; Farenhorst, M.; Hunt, R.H.; Thomas, M.B.; Coetzee, M. The infectivity of the entomopathogenic fungus Beauveria bassiana to insecticide-resistant and susceptible Anopheles arabiensis mosquitoes at two different temperatures. Malar. J. 2010, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, G.; Madhanraj, P.; Panneerselvam, S. A studies on the compounds and its antifungal potentiality of fungi isolated from paddy field soils of Jenbagapuram Village, Thanjavur District, and South India. Asian J. Pharm. Res. 2011, 1, 19–21. [Google Scholar]



| Mosquito Species | Larval Stages | Concentration (mg/L) | Mortality (%) ± S.D. | LC50 (LCL-UCL) mg/L | LC90 (LCL-UCL) mg/L | χ2 (df) 3 |
|---|---|---|---|---|---|---|
| Cx. quinquefasciatus | 1st Instar | Control | 2.5 ± 0.12 | 11.538 (4.061–20.308) | 16.155 (6.575–26.375) | 4.276 |
| 25 | 15.24 ± 0.8 | |||||
| 50 | 43.33 ± 1.0 | |||||
| 100 | 53.33 ± 1.0 | |||||
| 150 | 71.66 ± 2.5 | |||||
| 200 | 83.33 ± 1.5 | |||||
| 250 | 91.66 ± 0.5 | |||||
| 2nd Instar | Control | 2.1 ± 0.11 | 6.953 (1.158–15.718) | 10.790 (2.345–21.689) | 3.089 | |
| 25 | 17.12 ± 1.0 | |||||
| 50 | 43.33 ± 2.5 | |||||
| 100 | 53.33 ± 1.0 | |||||
| 150 | 61.66 ± 1.0 | |||||
| 200 | 78.33 ± 2.0 | |||||
| 250 | 83.33 ± 1.0 | |||||
| 3rd Instar | Control | 1.8 ± 0.10 | 5.841 (1.151–12.787) | 8.337 (1.993–16.673) | 2.978 | |
| 25 | 21.45 ± 1.2 | |||||
| 50 | 63.33 ± 0.5 | |||||
| 100 | 73.33 ± 0.5 | |||||
| 150 | 83.33 ± 1.5 | |||||
| 200 | 93.33 ± 2.5 | |||||
| 250 | 96.66 ± 0.5 | |||||
| 4th Instar | Control | 2.1 ± 0.18 | 3.581 (2.254–18.730) | 5.265 (3.437–23.043) | 3.421 | |
| 25 | 37.23 ± 1.3 | |||||
| 50 | 71.66 ± 1.5 | |||||
| 100 | 78.33 ± 2.0 | |||||
| 150 | 86.66 ± 1.0 | |||||
| 200 | 93.33 ± 0.5 | |||||
| 250 | 100.00 ± 1.5 | |||||
| Pupa | Control | 2.7 ± 0.19 | 9.041 (2.975–16.369) | 12.104 (4.532–20.504) | 3.404 | |
| 25 | 25.42 ± 1.2 | |||||
| 50 | 61.66 ± 1.5 | |||||
| 100 | 78.33 ± 3.0 | |||||
| 150 | 86.66 ± 1.0 | |||||
| 200 | 93.33 ± 0.5 | |||||
| 250 | 100.00 ± 0.0 |
| Mosquito Species | Larval Stages | Concentration (mg/L) | Mortality (%) ± S.D. | LC50 (LCL-UCL) mg/L | LC90 (LCL-UCL) mg/L | χ2 (df) 3 |
|---|---|---|---|---|---|---|
| Cx. quinquefasciatus | 1st Instar | Control | 2.5 ± 0.10 | 10.523 (3.237–19.576) | 15.843 (5.871–26.807) | 0.774 |
| 25 | 18.02 ± 0.9 | |||||
| 50 | 41.66 ± 1.5 | |||||
| 100 | 60.00 ± 1.5 | |||||
| 150 | 78.33 ± 1.0 | |||||
| 200 | 88.33 ± 0.5 | |||||
| 250 | 98.33 ± 0.5 | |||||
| 2nd Instar | Control | 1.5 ± 0.00 | 6.840 (1.819–16.649) | 11.792 (2.069–24.581) | 0.721 | |
| 25 | 26.11 ± 0.8 | |||||
| 50 | 36.66 ± 2.0 | |||||
| 100 | 58.33 ± 1.5 | |||||
| 150 | 63.33 ± 1.0 | |||||
| 200 | 71.66 ± 1.0 | |||||
| 250 | 83.33 ± 2.5 | |||||
| 3rd Instar | Control | 2.0 ± 0.18 | 4.616 (1.010–11.781) | 7.631 (1.293–16.992) | 0.704 | |
| 25 | 28.32 ± 1.0 | |||||
| 50 | 55.00 ± 0.5 | |||||
| 100 | 73.33 ± 0.5 | |||||
| 150 | 81.66 ± 1.0 | |||||
| 200 | 91.66 ± 2.5 | |||||
| 250 | 98.33 ± 1.0 | |||||
| 4th Instar | Control | 2.3 ± 0.0 | 2.674 (1.343–8.466) | 4.605 (2.068–12.449) | 0.036 | |
| 25 | 34.12 ± 1.0 | |||||
| 50 | 61.66 ± 1.5 | |||||
| 100 | 81.66 ± 0.5 | |||||
| 150 | 91.66 ± 1.0 | |||||
| 200 | 96.66 ± 0.5 | |||||
| 250 | 100.00 ± 0. | |||||
| Pupa | Control | 0.00 | 8.364 (1.643–17.906) | 13.552 (3.516–25.583) | 0.465 | |
| 25 | 26.09 ± 0.7 | |||||
| 50 | 40.00 ± 0.5 | |||||
| 100 | 55.00 ± 0.5 | |||||
| 150 | 70.00 ± 2.5 | |||||
| 200 | 80.00 ± 1.0 | |||||
| 250 | 88.33 ± 0.5 |
| S. No. | Retention time (min) | Compound Name | Molecular Weight | Formula | Area (%) | Biological Activity |
|---|---|---|---|---|---|---|
| 1 | 16.695 | N-Hexadecanoic Acid | 256 | C16H32O2 | 13.640 | Pesticidal activity |
| 2 | 21.016 | (Z,Z)-9,12 Octadecadienoic Acid | 280 | C18H32O2 | 33.747 | Anti-inflammatory activity |
| 3 | 21.466 | 9-Eicosyne | 278 | C20H38 | 10.832 | No activity reported |
| 4 | 22.081 | cis-9,10-Epoxyoctadecan-1-ol | 280 | C18H32O2 | 1.352 | No activity reported |
| 5 | 22.136 | N-[Bromo-N-butyl]-2-piperidinone | 233 | C9H16ONBr | 2.612 | No activity reported |
| 6 | 22.986 | Hexatriacontane | 506 | C36H74 | 2.133 | No activity reported |
| 7 | 23.757 | 1-Bromo-2-methyldecane | 234 | C11H23Br | 3.121 | No activity reported |
| 8 | 24.442 | Tritetracontane | 604 | C43H88 | 3.647 | No activity reported |
| 9 | 25.117 | Heptacosane | 380 | C27H56 | 5.148 | Antibacterial |
| 10 | 25.778 | Tritetracontane | 618 | C44H90 | 5.801 | Anti-bacterial, Anti-fungal |
| 11 | 26.468 | 7-Hexyleicosane | 366 | C26H54 | 5.723 | No activity reported |
| 12 | 27.143 | Nonacosane | 408 | C29H60 | 5.408 | Anti-bacteria, |
| 13 | 27.888 | 1-Chloroheptacosane | 414 | C27H55CI | 3.866 | No activity reported |
| 14 | 28.749 | 9-Octyleicosane | 394 | C28H58 | 2.971 | No activity reported |
| Observed Wave Numbers (cm−1) | Peak Assignment | Visible Intensity | Functional Group |
|---|---|---|---|
| 3226.91 | N–H stretching | Broad shape | Aliphatic |
| 2927.94 | C–H bending | Medium | Alkane |
| 2858.51 | C–H stretching | Medium | Alkane |
| 1595.13 | C=O Stretching | Medium | Alkane |
| 1404.18 | C–H bending | Medium | Alkane |
| 1247.94 | C–O Stretching | Medium | Alkane, Ether |
| 1078.21 | S=O Stretching | Sharp | Sulfone |
| 1018.41 | C–O Stretching | Sharp | Alkane |
| 929.69 | N–O Stretching | Sharp | Aliphatic |
| 871.82 | C=C bending | Sharp | Alkane |
| 503.42 | C–Br Stretching | Medium | Alkane |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivekanandhan, P.; Kavitha, T.; Karthi, S.; Senthil-Nathan, S.; Shivakumar, M.S. Toxicity of Beauveria bassiana-28 Mycelial Extracts on Larvae of Culex quinquefasciatus Mosquito (Diptera: Culicidae). Int. J. Environ. Res. Public Health 2018, 15, 440. https://doi.org/10.3390/ijerph15030440
Vivekanandhan P, Kavitha T, Karthi S, Senthil-Nathan S, Shivakumar MS. Toxicity of Beauveria bassiana-28 Mycelial Extracts on Larvae of Culex quinquefasciatus Mosquito (Diptera: Culicidae). International Journal of Environmental Research and Public Health. 2018; 15(3):440. https://doi.org/10.3390/ijerph15030440
Chicago/Turabian StyleVivekanandhan, Perumal, Thangaraj Kavitha, Sengodan Karthi, Sengottayan Senthil-Nathan, and Muthugoundar Subramanian Shivakumar. 2018. "Toxicity of Beauveria bassiana-28 Mycelial Extracts on Larvae of Culex quinquefasciatus Mosquito (Diptera: Culicidae)" International Journal of Environmental Research and Public Health 15, no. 3: 440. https://doi.org/10.3390/ijerph15030440






